
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 28 March 2025
Sec. Reconstructive and Plastic Surgery
Volume 12 - 2025 | https://doi.org/10.3389/fsurg.2025.1548597
This article is part of the Research Topic Craniomaxillofacial Reconstructive and Regenerative Surgery View all articles
 Martin Kauke-Navarro1*†
Martin Kauke-Navarro1*† Leonard Knoedler2*†
Leonard Knoedler2*† Helena Baecher3
Helena Baecher3 Khalil Sherwani4
Khalil Sherwani4 Samuel Knoedler1
Samuel Knoedler1 Omar Allam1
Omar Allam1 Fortunay Diatta1
Fortunay Diatta1 Michael Alperovich1
Michael Alperovich1 Ali-Farid Safi5*
Ali-Farid Safi5*
Background: Assessing facial plastic surgery techniques is essential for improving patient safety and outcomes through evidence-based practices. Despite the extensive use of facial implants, there is a scarcity of thorough research on their long-term effects and safety profiles.
Methods: A systematic review was conducted following PRISMA guidelines, analyzing studies from 1970 to 2024 on various implant materials for facial reconstruction and augmentation. The databases searched for this review included PubMed, Web of Science, Google Scholar, and EMBASE. Inclusion criteria were full-text articles in English, focusing on alloplastic materials for craniofacial skeleton replacement or augmentation.
Results: We included 117 studies with 4,273 patients and a mean follow-up of 34 months (range: 15 days to 25 years). Of these, 56% focused on reconstruction, 33% on aesthetics, and 10% on both. Patient ages ranged from 6 months to 85 years, with most studies addressing the orbital (29%), chin (22%), and malar (19%) regions. 67% of studies evaluated potential complications and found an overall rate of 4.4%. Nerve injuries (2.1%) and infections (1.0%) were the most frequent issues, with hematoma, implant displacement, and bone resorption rates at 1.4%, 0.59%, and 0.68%, respectively. Patient-specific implants (PSIs) showed promise in reducing complications such as infections, suggesting that customization to patient anatomy may provide benefits. The highest rate of complication-free postoperative recovery was observed with polyethylene facial implants.
Conclusion: This review highlights variability in implant performance. The increased use of PSI suggests improved outcomes, warranting further investigation. Standardized outcome reporting and further research are needed to enhance comparability and guide clinical practice.
Systematic Review Registration: PROSPERO, identifier (CRD42024501754).
Alloplastic facial implants are routinely used to correct facial asymmetries, defects, and deformities. Esthetic balancing surgeries are increasingly performed using alloplastic facial implants (1–3). A wide range of alloplastic implant materials have been used for these purposes of which titanium, porous polyethylene (MedPor), polyether-ether-ketone (PEEK), silicone and poly-methyl methacrylate (PMMA) are among the most used materials. Each material possesses physicochemical properties and biological profiles, with associated advantages and risks (1, 4, 5).
Facial implants are used to address bony defects, for example resulting from trauma, oncologic resections and congenital deficiencies (1–3). Among these, oncologic resections account for a relevant portion of cases requiring facial reconstruction. In 2020, an estimated 930,000 new cases of head and neck cancers were reported worldwide, including cancers of the lip and oral cavity, salivary glands, oropharynx, nasopharynx, hypopharynx, and larynx, according to GLOBOCAN 2020 estimates from the International Agency for Research on Cancer (IARC) (4). These estimates underscore the substantial burden of head and neck malignancies globally. Other patients who may need implant-based reconstruction are facial trauma patients (5). In 2017 alone, there were an estimated 7.5 million new cases of facial fractures globally, based on data from the Global Burden of Disease Study (6). In addition to reconstructive indications, implants are frequently used for aesthetic facial augmentations, such as chin and midfacial enhancements (2, 3). According to the 2023 ASPS procedural statistics report the number of cheek implants increased by 7% to 8,825 procedures in 2023, while chin implant procedures rose by 1% to 5,484 cases, reflecting the rising interest in facial augmentation (7).
To date, there is insufficient evidence to establish the superiority of one specific material for use in facial implantology. Outcome reports are often limited to case series with short follow-ups. Additionally, patient-specific implants are increasingly used as opposed to standard “off-the-shelf” implants. The added benefit of anatomical customization has not been systematically evaluated.
This systematic review of the literature comprehensively summarizes the experience with facial alloplastic implants over the last 54 years (1970–2024), aiming to provide an update on the risk profile of selected implant materials and to help guide evidence-based treatment decision making.
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (6). This study should be viewed as a descriptive review, as we did not perform a meta-analysis due to the heterogeneity observed in outcome parameters. This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42024501754). A detailed description of the search strategy and search string can be found in the Supplementary Digital Content; Figure 1 (including Prisma 2020 flowchart). In terms of inclusion and exclusion criteria, we only included studies that used an alloplastic material to permanently replace missing parts of the splanchnocranium (reconstruction) or for augmentation for aesthetic purposes. Other alloplastic materials/implant materials used for skeletal fixation (e.g., titanium plates or absorbable plates made from polyglycolic acid), injectable fillers (e.g., dermal injectable hydroxyapatite fillers such as Radiesse) and implants used for the reconstruction of the neurocranium were excluded. Quality assessment of the included studies was conducted using the Newcastle Ottawa Score (NOS) and the Level of Evidence (LOE) (Supplementary Tables 2, 3).
The following data points were extracted: Digital object identifier (DOI), first author, journal, publication year, study population size, study design, implant material, material costs [USD], mechanical material properties, printing technology, facial area targeted, clinical indication for implantation (i.e., reconstruction, aesthetic facial contouring), length of follow-up, acute/longer-term complications, surgical revision rate, implant acceptance rate, functional/aesthetic outcome, patient satisfaction, time from implant design to implantation [days], implant measurements [mm], antibiotic/anti-infectious prophylaxis. FIs were defined as PSIs when their manufacturing technique involved CT-based design and CAD/CAM or 3D printing (7).
117 articles met the inclusion criteria totaling 4,273 patients (Table 1). Mean follow-up was 34 months (15 days to 25 years) (81, 101). In general, 66 articles (56%) investigated FIs for reconstructive indications, whereas 39 studies (33%) studied FIs to improve the patient's facial aesthetics. Overall, 12 studies (10%) investigated FI in both reconstructive and aesthetic procedures. Figure 2 illustrates the trend in the number of publications on facial implantology over time.
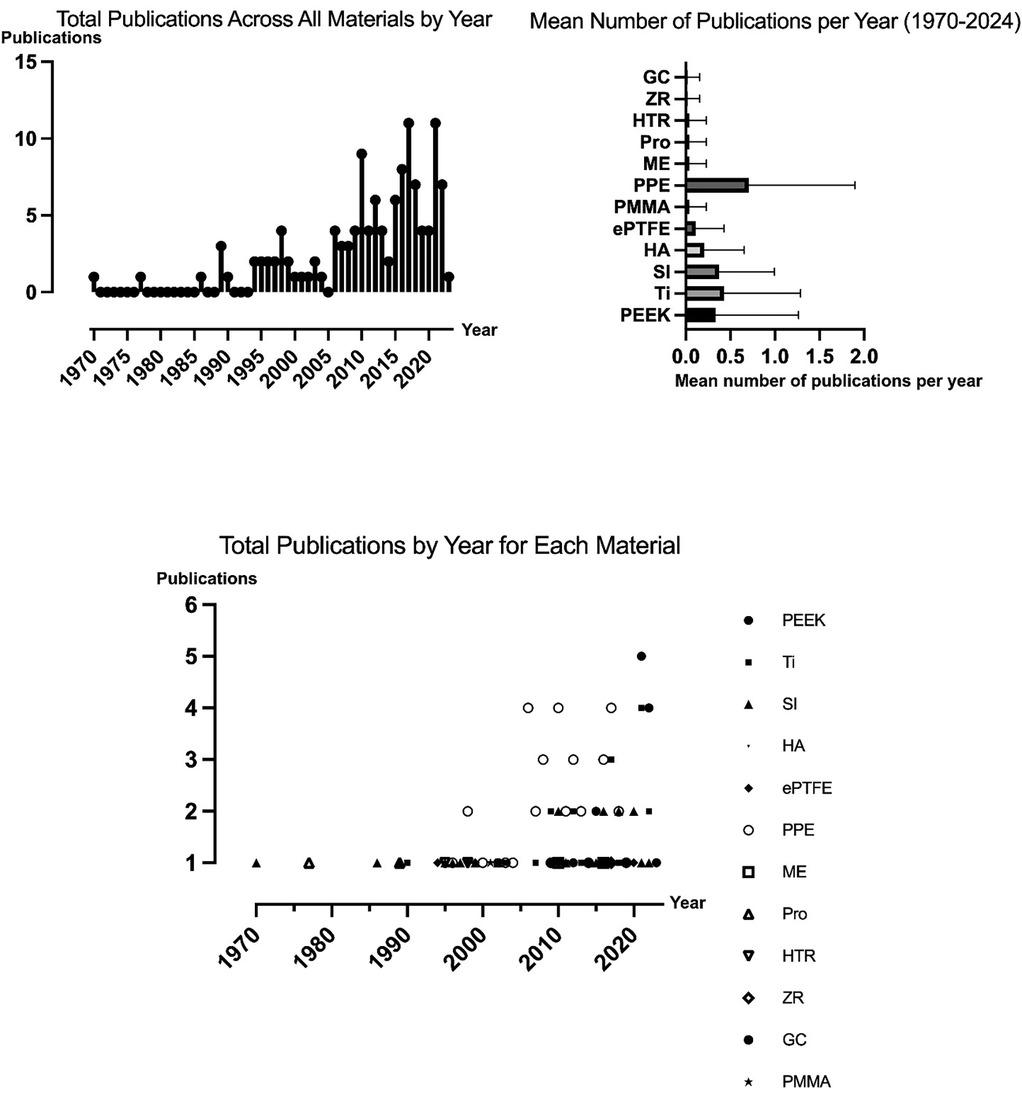
Figure 2. Trends in facial implantology publications: total publications and mean publications per material per year.
Of the 117 studies included, 34 studies (29%) focused on FIs made of porous polyethylene, 18 (15%) on FIs made of titanium, 18 (15%) on FIs made of polyetheretherketone (PEEK), and 15 (13%) on articles investigating silicone facial implants. Eight studies (6.8%) investigated FIs made of hydroxylapatite (HA), of which six (5.1%) addressed hydroxylapatite/poly-l-lactide (HA/PLLA) FIs. Four studies (3.4%) investigated polytetrafluoroethylene (ePTFE) implants, respectively, while two studies (1.7%) studied FIs made of polymethylmethacrylate (PMMA). One article (0.85%) each focused on Proplast implants, hard tissue replacement (HTR) implants, mersilene and glass ceramic. Additionally, 14 articles (12%) included multiple implant materials, of which titanium and polyethylene were the most common combination (n = 6; 5.1%).
Patient ages ranged from 6 months to 85 years. Most studies focused on the orbital (n = 34; 29%), chin (n = 26, 22%), and malar region (n = 22; 19%) (Table 1; Figure 3).
In 34 articles (29%), authors described manufacturing techniques of FIs based on preoperative scans [i.e., patient-specific implants (PSIs)] (7, 9–25). In contrast, 43 studies (36%) investigated the utilization of prefabricated implants (i.e., off-the-shelf implants) (29–115). Of all patients receiving off-the-shelf implants, 6.5% (175/2,684) showed complications, while the total complication rate of PSI-treated patients was 1.6% (7/435). Detailed complication rates ordered by fabrication technique are shown in Table 2.
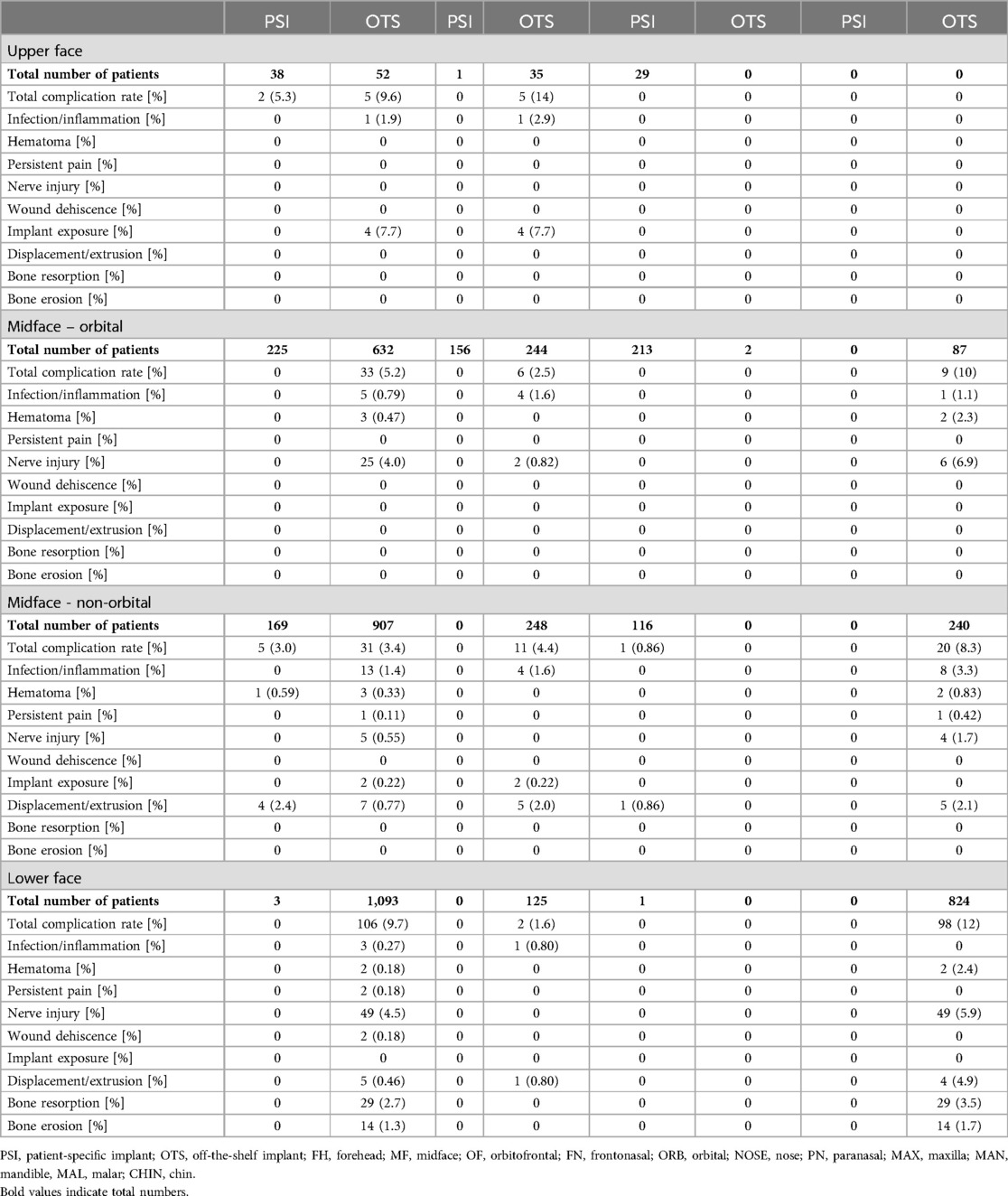
Table 2. Complication rates of patient-specific implants (PSIs) and off-the-shelf (OTS) implants per patient in the upper face (FH, OF, FN), the orbital region (ORB), the midface (MAL, NOSE, PN, MAX, TEMP), and the lower face (CHIN, MAN).
Out of 117 articles, 50 (43%) did not report any intraoperative or postoperative complications during the follow-up period. The mean follow-up period of studies reporting no complications was 25 months. The majority of articles without any complications included polyethylene implants (n = 18; 15%), followed by PEEK implants (n = 12; 10%) and titanium implants (n = 11; 9.3%) (8–11, 15, 16, 18–21, 24, 25, 29, 30, 39, 41, 42, 47, 51, 58, 61, 65, 67, 69, 83, 84, 86–88, 90–95, 109, 113, 119). When excluding case reports and case series with less than five patients (ECR), the highest complication rate was 58% (54). Revision rates ranged from 0% to 26%, while 35 articles (30%) reported revision surgeries (ECR) (3, 11, 19, 31, 32, 36, 39, 43, 49, 50, 58, 66, 70, 71, 75, 78, 80, 82, 87, 89, 95, 96, 98, 99, 101, 102, 104–107, 112, 114–116, 121, 122, 125, 126). In total, 190 patients showed complications, yielding a complication rate per patient of 4.4% [(190/4,273) for details see Tables 3A–D].
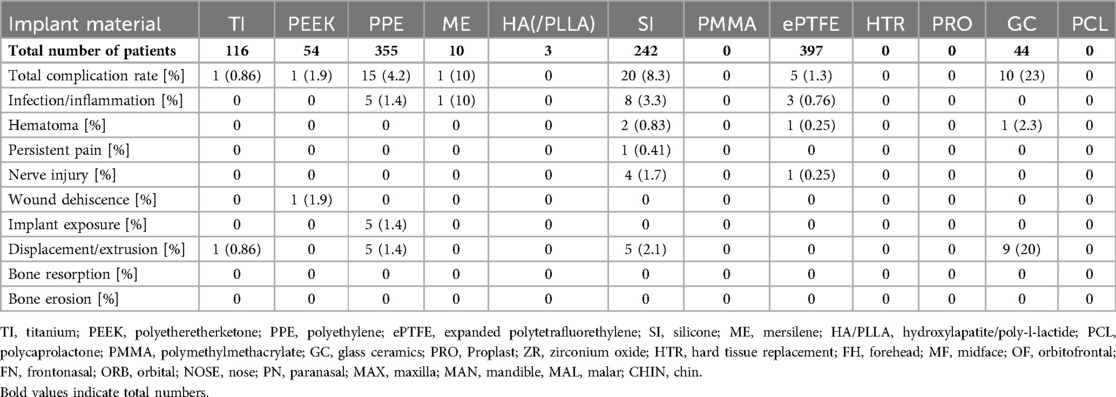
Table 3C. Complication rates per patient for FIs of the midface except for the orbital area (MAL, NOSE, PN, MAX, TEMP).
This analysis excludes case reports and series with fewer than five patients to provide more robust assessment of complication rates across various implant materials. The rate of complications was highest (48%) in a study investigating polyethylene FIs for mental reconstruction (71). The highest infection rate was found for temporal implants made of mersilene (10%) (75). Hematomas rates peaked at 5.3% in a study that examining PMMA FIs for fronto-orbital reconstruction (101). Rates of persistent pain following FI were reported to be as high as 10% in one study using titan and polyethylene implants for the reconstruction of orbital floor fractures (72). A study on aesthetic chin surgery using polyethylene FI revealed transient nerve injuries in 48% of cases (71). The rate of wound dehiscence was highest (20%) in an article investigating Proplast implants for chin augmentation (123). Implant exposure rates reached up to 12% in a study using polyethylene implants for reconstruction of facial deformities, respectively (110). The rate of implant displacement was as high as 8.3% in a study on silicone implants for the malar region (106). Further, the rate of implant extrusion was as high as 20% in a study reporting on the use of glass ceramics (99). One study on aesthetic chin augmentation using silicone FI revealed bone resorption in 53% of cases (100). Another study on aesthetic genioplasty reported bone erosion in up to 93% (37).
Notably, 50 of 117 studies (43%) that reported on postoperative complications found no adverse events. The rate of complication-free postoperative recovery was highest for polyethylene FIs. Overall, 18 of 33 polyethylene studies (55%), that reported postoperative complication rates, had complication-free recovery. However, it is worth noting that for various implant materials (e.g., PCL) only one study looked into complications and did not report any complications (25–27, 117). Detailed complication rates ordered by implant material are listed in Table 4.
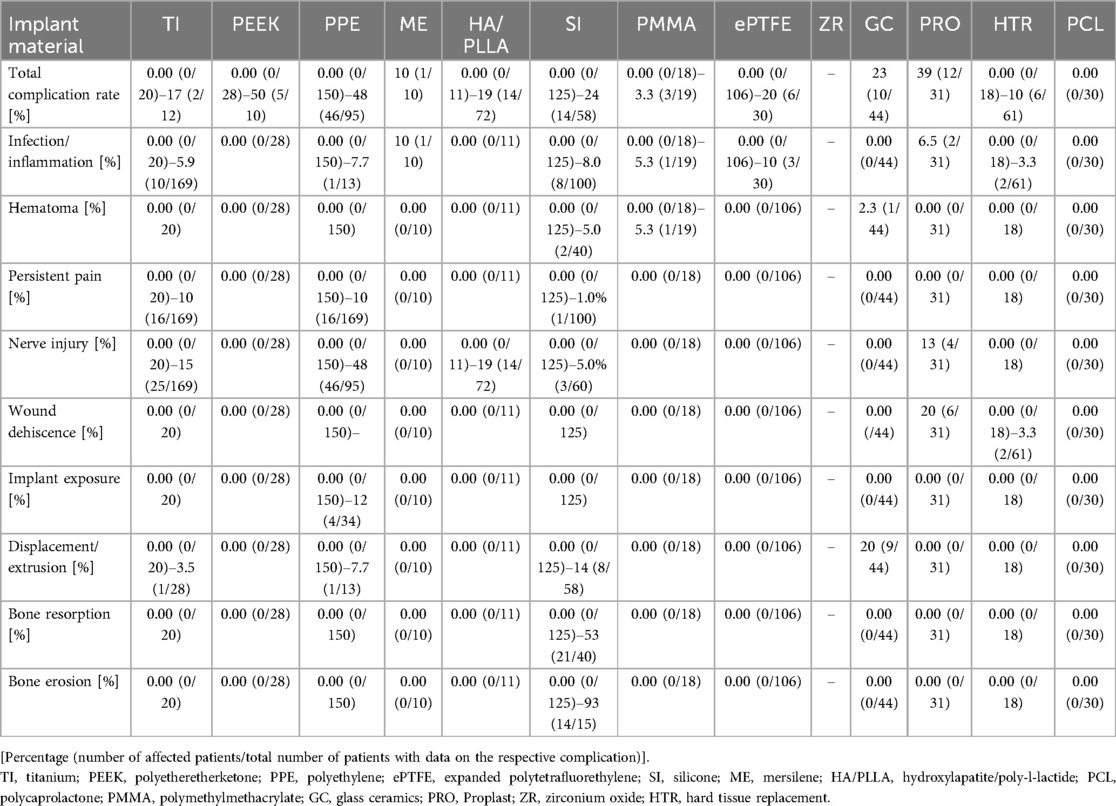
Table 4. Postoperative complications in various implant materials (ECR). [Percentage (number of affected patients/total number of patients with data on the respective complication)].
Overall, 16 articles (14%) reported implant infections or inflammation as postoperative complications in 1.4%–10% of study cases (ECR) (36, 70, 72, 75, 78, 80, 81, 85, 98, 101, 103, 107, 110, 121, 123, 124, 127). In these studies, polyethylene (n = 7; 6.0%) and silicone (n = 4, 3.4%) were the most common implant materials, with infection rates between 1.4% and 8.0% (ECR) (36, 70, 78, 80, 81, 98, 103, 110, 121, 124). Postoperative cutaneous fistulas were described in two case reports using PPE with one patient each (1.7%) (43, 49, 64). In total, 44 patients exhibited infections, yielding a mean infection rate per patient of 1.0% (44/4,273).
Seven studies (6.0%) reported postoperative hematoma, of which two articles (1.7%) addressed FIs made of silicone. One study (0.85%) each investigated FIs made of PEEK, HA/PLLA, HA, PTFE, glass ceramic, and PMMA. Rates of postoperative hematomas ranged between 1.0% and 5.0% (ECR) (17, 32, 38, 40, 50, 80, 89, 99, 101, 105, 114). 61 patients presented with postoperative hematoma, resulting in a cumulative hematoma rate per patient of 1.4% (61/4,273).
Persistent pain following implantation was reported in four studies (3.4%) (72, 80, 112, 115). The complication rates related to persistent pain ranged from 1.0% to 10%. Across all reviewed studies, 0.47% of patients (20/4,273) experienced persistent postoperative pain.
Eleven studies (9.4%) reported postoperative neuropraxia with paresthesia/hypesthesia, of which four articles (3.4%) used FIs made of PPE. Silicone was utilized in three studies (2.6%) and HA/PLLA in two studies (1.7%). Titanium, ePTFE, HA, HA/PLLA, and Proplast were each addressed in one study (0.85%) (17, 23, 31, 34, 38, 68, 71–73, 105, 106, 123). In total, 90 patients showed any nerve injury, yielding an overall rate of nerve injury per patient of 2.1% (90/4,273). Most nerve injuries affected branches of the trigeminal nerve (V3: 4 studies, V2: 6 studies). One study reported a case of temporal nerve paresis following temporal FI.
In three studies (2.6%), the authors reported postoperative wound dehiscence, while four articles (3.4%) revealed postoperative implant exposure, of which all occurred following the use of polyethylenic FIs. Implant exposure and wound dehiscence rates ranged between 3.1%–12% and 3.3%–19% (ECR), respectively (78, 81, 82, 102, 110, 115, 123). In sum, 0.21% (9 of 4,273 patients) patients with wound dehiscence were identified.
Nine studies (7.7%) reported on postoperative implant displacement or implant extrusion, ranging between 0.66% and 20% (ECR). Of these, four studies (3.4%) involved FIs made of polyethylene and three involved FIs made of silicone (2.6%) (36, 70, 72, 81, 103, 106, 122). Collectively, 25 patients showed postoperative implant displacement, yielding a total displacement rate per patient of 0.59% (25/4,273).
Six articles (5.1%) reported bone resorption (n = 2, 1.7%) or bone erosion (n = 4, 3.4%). All articles reported on silicone implants (37, 53, 100, 112, 114, 116). Bone resorption occurred in 29 out of 4,273 patients (0.68%) and bone erosion in 23 out of 4,273 patients (0.54%).
Supplementary Table 4 presents detailed information on aesthetic outcomes. In brief, description of the aesthetic outcome varied widely but was most commonly described as “good” (n = 9; 7.7%), “excellent” (n = 7; 6.0%), or “improved” (n = 6; 5.1%). Reporting was heterogenous, with one study utilized an aesthetic outcome score ranging from 1 to 4 (1: unsatisfactory aesthetic outcome; 4: excellent aesthetic outcome) to assess the postoperative aesthetic outcomes (48). Two other studies used an analog visual scale (VAS), ranging from 0 to 10 (1: unsatisfactory outcome; 10: satisfactory outcome) to evaluate functional and aesthetic outcomes (11, 106). A different study compared implant projection to the mirrored contralateral side using Adobe Photoshop (Adobe Systems Incorporated, United States) to determine the side-to-side differences (60). 19 studies (16%) reported on poor aesthetic outcomes. Most of these studies investigated polyethylene FIs (n = 11; 9.4%). Again, there were no standardized outcome measurements (32, 39, 55, 70–72, 76, 80, 96, 98, 104, 106, 112, 114).
The authors revealed improved functional outcomes in 27 articles (23%), while eleven studies (9.4%) found poor functional outcomes. While improved functional outcomes encompassed a wide array of different functional parameters (e.g., improved airway function), all studies with unsatisfying functional results reported on eye bulb dysfunctions such as diplopia, persisting enophthalmos, or binocular vision loss (27, 40, 60, 62, 68, 72, 85, 91, 118, 126).
29 (25%) articles assessed patient satisfaction. Results were reported as “satisfied” (n = 13; 11%), “pleasing” (n = 5; 4.3%), or “excellent” (n = 1; 0.85%). Ten studies (8.5%) noted poor patient-reported outcomes, with dissatisfaction rates ranging from 0.16% to 16%. Most articles reporting satisfactory patient-reported outcomes used FIs made of polyethylene (n = 6; 5.1%) and silicone (n = 6; 5.1%) (9, 10, 24, 29, 39, 47, 74, 87, 91, 95, 100, 113). Further details on patient satisfaction are provided in Table 5.
This systematic review examines alloplastic materials used in facial reconstructive and aesthetic surgery over the past 54 years, including data from 4,279 patients and 13 different materials. Consistent with prior publications, most outcomes reported over the last 54 years focus on PPE, titanium, PEEK, and silicone (3, 5, 128–130). Recent years have seen more reports on PEEK and PPE, while those on silicone, HTR, and ePTFE have decreased (3, 5). PEEK implants have been used to reconstruct complex bony defects, likely due to their intrinsic mechanical stability (8). The increased use of PEEK may be attributed to the ease of handling, improved availability and cost-effectiveness of 3D printing technology for this material in recent years. PPE was the most commonly used material for nearly all facial regions, except the lower face where silicone predominated. However, recent publication trends indicate that PPE has been the most studied material in recent years, while reports on silicone implants have decreased. This suggests a shift in preference from silicone to PPE. Additionally, the increased use of hydroxyapatite-based materials points to a trend toward more natural and biocompatible options.
In this context, the reporting of outcomes differed between studies, and the duration of follow-up varied significantly (5, 131, 132). Notably, 42% of articles did not report any complication in this complex population, with an average follow-up of 25 months. Those studies with longer follow-ups (38 months) noted any complication. Patient-reported outcomes vary significantly between studies, making it challenging to draw definitive conclusions. Despite these variations and limitations, we present fundamental outcome metrics for all implant materials and anatomical locations, which could inform and guide future research.
A significant portion of the studies (44%) reported positive aesthetic results, predominantly with PEEK, titanium, and polyethylene implants. However, the lack of standardized outcome parameters poses a challenge in comparing results across studies. The use of scales such as an aesthetic outcome score or analog visual scales (VAS) in a few studies suggests potential pathways for standardization (Supplementary Table 4). While a substantial number of studies indicated favorable aesthetic results, 16% reported poor outcomes, primarily associated with polyethylene implants. This disparity underscores the need for more consistent reporting and possibly the exploration of other factors influencing aesthetic satisfaction. In terms of functional outcomes, 23% of studies reported improvements, particularly in airway function. However, a notable subset identified ocular issues, such as diplopia and enophthalmos.
No single material consistently outperformed other materials across multiple criteria such as biocompatibility, safety profile, and patient satisfaction. This finding aligns with prior reports on complication rates for different alloplastic materials (3, 5). An example is porous polyethylene (MedPor), which is widely used due to its simplicity, ease of handling, and low complication rate (87, 133). Our review corroborates the generally safe risk-profile of PPE for numerous facial augmentation and reconstruction procedures.
Titanium and PEEK implants are used when structural rigidity is needed (1, 133–135). Other materials, such as hydroxyapatite-based implants, are increasingly used (27, 35). The choice of material seems to be contingent on surgical goals, anatomical considerations; patient wishes, which underscores the need for patient-individualized treatment planning. The highest rate of complication-free postoperative recovery was observed with polyethylene facial implants (FIs). Of the 33 studies on polyethylene, 18 (55%) reported complication-free outcomes, highlighting the favorable safety profile of PPE.
The overall complication rate in the upper face was low, with PPE showing a 13% complication rate, primarily due to implant exposure. Hydroxyapatite-based implants showed no complications among 17 patients. For orbital reconstruction in the midface, PPE, titanium, and hydroxyapatite were the most frequently used implants. This area had higher complication rates, with titanium at 23%, PPE at 13%, hydroxyapatite at 11%, and silicone implants (SI) at 10%. The main complications involved nerve injuries and implant dysfunctions, reflecting the complexity of these cases of orbital reconstruction.
In the non-orbital midface, ePTFE (n = 397) was most used often in the setting of rhinoplasty for dorsal augmentation, followed by PPE (n = 355) and silicone (n = 242). Glass ceramics had the highest complication rate at 23%, while silicone implants had an 8.3% rate. In the lower face, silicone and PPE were the most reported materials, with the region showing the highest overall complication rates: 17% for PPE implants and a substantial 39% for Proplast implants. Many complications were due to hematoma (17% for PPE) and wound dehiscence (19% for Proplast). Silicone implants had a 7.8% complication rate, with bone resorption/erosion reported in about 6% of cases, a complication only noted for silicone in the lower facial region.
We analyzed the range of complication rates per implant type in larger studies, explicitly excluding data from case reports and series (Table 4). Using this methodology, unusual or particularly challenging cases, often highlighted in case reports, are omitted. In the previous literature, conclusions are largely based on the summary of case reports which may negatively skew complication rates.
PEEK implants demonstrated the highest complication rate of up to 50% (5 out of 10), followed closely by PPE with up to 48% complication rate (46 out of 95) and Titanium at 35% (9 out of 26). Infection rates varied, with a maximum of 10% reported for ePTFE implants and the lowest at 1% for ME implants. Titanium, PPE, and Silicone showed infection rates up to 5.9%, 7.7%, and 8%, respectively. Wound dehiscence occurred in up to 12% of cases with PPE implants and 20% with GC Proplast. Displacement was most frequent in the Silicone group, reaching up to 14%. Additionally, nerve injuries were most associated with PPE, occurring in 48% of cases.
None of the studies reported on implant related malignancy. In other types of alloplastic implants, specifically macro-textured breast implants, there is a theoretical risk of developing breast implant associated anaplastic large cell lymphoma (BIA-ALCL) (101). This form of malignancy is a type of T-cell non-Hodgkin lymphoma which can develop around an implant within the capsule and is thought to be induced by the implant texture leading to chronic inflammation (81). It is however recognized that this pathologic entity can occur in any part of the body where an implant with a rough surface is implanted (rough implant associated anaplastic large cell lymphoma (RIA-ALCL) (9). For example, the occurrence of ALCL has recently been reported in case of a gluteal implant (81). Although this type of malignancy has not been reported with the use of facial implants, it is a possibility that such an outcome might come to light in the future. Other malignancies such as implant associated squamous cell carcinoma have been associated with various types of implants, including subperiosteal implant of the maxilla and is simply thought to be related to chronic inflammation and stress to squamous epithelium (54).
In recent years, the use of patient individualized implants (PSI) has become increasingly popular (136, 137). However, there is a lack of comprehensive studies evaluating their actual benefits in facial implantology and compare it to OTS implants. Current evidence does not consistently demonstrate a significant advantage of PSIs over OTS implants in the upper face and non-orbital midface regions. In orbital midface cases, some studies have reported fewer complications with PSIs compared to OTS implants, which may suggest that customization offers advantages in complex reconstructions, such as orbital repairs (138, 139). However, these findings require further validation. Notably, no infections were reported for PSIs across all regions analyzed, and there were no documented cases of nerve injuries or wound dehiscence, while hematomas were rarely seen. While these findings may indicate potential benefits related to improved surgical planning, shorter operative times, and a better anatomical fit, they should be interpreted with caution. The absence of reported complications does not necessarily imply superiority, as reporting biases, study heterogeneity, and lack of randomized controlled trials limit definitive conclusions. Further prospective studies are necessary to rigorously assess whether PSI implants provide measurable clinical advantages over OTS implants in terms of safety and long-term outcomes.
This systematic review has inherent limitations. The included studies exhibit heterogeneity in outcome reporting, including differences in study design, patient populations, implant types, surgical techniques, and follow-up durations, making direct comparisons challenging and precluding a meta-analysis. Furthermore, publication bias must be considered as positive outcomes are more likely to be submitted for publication. Similarly, in some cases, one must assume underreporting of complications and inconsistencies in complication definitions which introduce data inconsistencies. Lastly, the lack of randomized controlled trials limits the ability to establish causality and the effectiveness of different implant types.
This systematic review offers a comprehensive analysis of alloplastic materials used in facial reconstructive and aesthetic surgery over the past 54 years. It stresses the need for personalized treatment planning and highlights the need for additional research to better understand each material's safety and efficacy. The review advocates for standardized outcome reporting to enhance comparability and guide future clinical practices. Although some studies suggest that PSIs may help reduce complications such as infections and nerve injuries, the current evidence remains limited. Customization to patient anatomy may offer potential advantages, but further long-term investigations are required to assess the durability, complication rates, and overall clinical impact of these implants. Continued research will be essential in guiding evidence-based treatment decisions.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
LK: Conceptualization, Data curation, Methodology, Supervision, Validation, Visualization, Writing – original draft. MK-N: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft. HB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. KS: Data curation, Investigation, Software, Writing – review & editing. SK: Data curation, Methodology, Resources, Validation, Visualization, Writing – review & editing. OA: Resources, Visualization, Writing – review & editing. FD: Writing – review & editing. MA: Investigation, Supervision, Writing – review & editing. A-FS: Project administration, Resources, Supervision, Validation, Writing – original draft.
The author(s) declare that no financial support was received for the research and/or publication of this article.
MA consults for Johnson & Johnson.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1548597/full#supplementary-material
1. Kauke-Navarro M, Knoedler L, Knoedler S, Deniz C, Safi A-F. Surface modification of PEEK implants for craniofacial reconstruction and aesthetic augmentation—fiction or reality? Front Surg. (2024) 11:1351749. doi: 10.3389/fsurg.2024.1351749
2. Kauke-Navarro M, Knoedler L, Knoedler S, Deniz C, Stucki L, Safi AF. Balancing beauty and science: a review of facial implant materials in craniofacial surgery. Front Surg. (2024) 11:1348140. doi: 10.3389/fsurg.2024.1348140
3. Rojas YA, Sinnott C, Colasante C, Samas J, Reish RG. Facial implants: controversies and criticism. A comprehensive review of the current literature. Plast Reconstr Surg. (2018) 142:991–9. doi: 10.1097/PRS.0000000000004765
4. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Intl J Cancer. (2021) 149:778–89. doi: 10.1002/ijc.33588
5. Suresh V, Anolik R, Powers D. The utility of polyether-ether-ketone implants adjacent to sinus cavities after craniofacial trauma. J Oral Maxillofac Surg. (2018) 76:2361–9. doi: 10.1016/j.joms.2018.05.002
6. Lalloo R, Lucchesi LR, Bisignano C, Castle CD, Dingels ZV, Fox JT, et al. Epidemiology of facial fractures: incidence, prevalence and years lived with disability estimates from the global burden of disease 2017 study. Inj Prev. (2020) 26:i27–35. doi: 10.1136/injuryprev-2019-043297
7. American Society of Plastic Surgeons (ASPS). 2023 Plastic Surgery Statistics Report. (2023). Available online at: https://www.plasticsurgery.org/documents/news/statistics/2023/plastic-surgery-statistics-report-2023.pdf
8. Saponaro G, Todaro M, Barbera G, Scivoletto G, Foresta E, Gasparini G, et al. Patient-Specific facial implants in polyetheretherketone and their stability: a preliminary study. Ann Plast Surg. (2023) 90(6):564–7. doi: 10.1097/sap.0000000000003527
9. Santanelli Di Pompeo F, Paolini G, Firmani G, Sorotos M. From breast implant to rough implant associated–anaplastic large cell lymphoma (RIA-ALCL). Aesth Surg J. (2022) 42:NP445–6. doi: 10.1093/asj/sjac005
10. Shi H, Yin X, Hu Y. Solitary neurofibroma of the zygoma: three-dimensional virtual resection and patient-specific polyetheretherketone implant reconstruction. J Craniofac Surg. (2022) 33(8):e781–3. doi: 10.1097/scs.0000000000008526
11. Lim HK, Choi YJ, Choi WC, Song IS, Lee UL. Reconstruction of maxillofacial bone defects using patient-specific long-lasting titanium implants. Sci Rep. (2022) 12(1):7538. doi: 10.1038/s41598-022-11200-0
12. Ha SH, Lee H, Choi JY. Correction of midface deficiency in patient with crouzon syndrome by orthognathic surgery and patient specific facial implant: case report. J Craniofac Surg. (2022) 33(2):e191–4. doi: 10.1097/scs.0000000000008287
13. Mayo W, Mohamad AH, Zazo H, Zazo A, Alhashemi M, Meslmany A, et al. Facial defects reconstruction by titanium mesh bending using 3D printing technology: a report of two cases. Ann Med Surg. (2022) 78:103837. doi: 10.1016/j.amsu.2022.103837
14. Kim MW, Kim SH, Nam SB, Lee JW, Jeong DK, Kim YH. Reconstruction of temporal hollowing deformities using silicone implants made using a toy-clay model: a report of three cases. Arch Craniofac Surg. (2022) 23(1):34–8. doi: 10.7181/acfs.2021.00479
15. Hamsho R, Mahardawi B, Assi H, Alkhatib H. Polyetheretherketone (PEEK) implant for the reconstruction of severe destruction in the maxilla: case report. Plast Reconstr Surg Glob Open. (2022) 10(8):e4473. doi: 10.1097/gox.0000000000004473
16. Darwich K, Ismail MB, Al-Mozaiek MYA, Alhelwani A. Reconstruction of mandible using a computer-designed 3D-printed patient-specific titanium implant: a case report. Oral Maxillofac Surg. (2021) 25(1):103–11. doi: 10.1007/s10006-020-00889-w
17. Watanabe A, Yamanaka Y, Rajak SN, Nakayama T, Ueda K, Sotozono C. Assessment of a consecutive series of orbital floor fracture repairs with the hess area ratio and the use of unsintered hydroxyapatite particles/poly l-lactide composite sheets for orbital fracture reconstruction. J Oral Maxillofac Surg. (2021) 79(2):420–8. doi: 10.1016/j.joms.2020.09.019
18. Ramieri V, Maffìa F, Vellone V, Marianetti S, Marianetti TM. The pyramid chin augmentation: a new technique. J Craniofac Surg. (2021) 32(2):738–9. doi: 10.1097/scs.0000000000007074
19. Antúnez-Conde R, Salmerón JI, Navarro C. Intraosseous venous malformation of the zygomatic bone: virtual surgical planning and reconstruction with customized CAD-CAM titanium implant. J Craniofac Surg. (2021) 32(8):e757–9. doi: 10.1097/scs.0000000000007751
20. Olate S, Huetequeo-Molina C, Requena R, Uribe F. Patient specific implants to solve structural facial asymmetry after orthognathic surgery. J Craniofac Surg. (2021) 32(3):e269–71. doi: 10.1097/scs.0000000000007113
21. Sesqué A, Dang NP, Coste A, Barthélémy I, Depeyre A. Orbitofrontal reconstruction with a three-dimensional titanium patient-specific implant after intraosseous haemangioma resection. J Craniofac Surg. (2021) 32(1):e69–72. doi: 10.1097/scs.0000000000006935
22. Yashin KS, Ermolaev AY, Ostapyuk MV, Kutlaeva MA, Rasteryaeva MV, Mlyavykh SG, et al. Case report: simultaneous resection of bone tumor and CAD/CAM titanium cranioplasty in fronto-orbital region. Front Surg. (2021) 8:718725. doi: 10.3389/fsurg.2021.718725
23. Khashaba MM, Shaheen HA, Ibrahim WH, AlDainy DG. Accuracy of patient-specific temporal implants using PEKK. J Craniomaxillofac Surg. (2021) 49(10):943–9. doi: 10.1016/j.jcms.2021.05.001
24. Narciso R, Basile E, Bottini DJ, Cervelli V. PEEK implants: an innovative solution for facial aesthetic surgery. Case Rep Surg. (2021) 2021:5518433. doi: 10.1155/2021/5518433
25. Yang M, Wu Z, Yu H, Cheng J. Reconstruction for diverse fronto-orbital defects with computer-assisted designed and computer-assisted manufactured PEEK implants in one-stage operation: case reports. Medicine (Baltimore). (2021) 100(40):e27452. doi: 10.1097/md.0000000000027452
26. Bai SS, Li D, Xu L, Duan HC, Yuan J, Wei M. A novel method to enhance dynamic rhinoplasty outcomes: double “V” carving for alloplastic grafts. Ear Nose Throat J. (2020) 99(4):262–7. doi: 10.1177/0145561319840110
27. Jang HU, Kim SY. Biodegradable implants for orbital wall fracture reconstruction. Arch Craniofac Surg. (2020) 21(2):99–105. doi: 10.7181/acfs.2020.00010
28. Mrad MA, Shah Mardan QNM, Mahabbat NA. Radicular cysts and chin implants; an unexpected complication prompting explantation—case report. Int J Surg Case Rep. (2020) 77:766–8. doi: 10.1016/j.ijscr.2020.11.117
29. Doh G, Eo S, Hong KY. Temporal hollowing augmentation with polyetheretherketone patient-specific implant. J Craniofac Surg. (2019) 30(7):2131–3. doi: 10.1097/scs.0000000000005629
30. Scofield-Kaplan SM, Patel SY, Mancini R. Orbital floor and rim reconstruction with a titanium orbital implant and acellular dermis. Ophthalmic Plast Reconstr Surg. Jan/. (2019) 35(1):e19–21. doi: 10.1097/iop.0000000000001285
31. Tsumiyama S, Umeda G, Ninomiya K, Miyawaki T. Use of unsintered hydroxyapatite and poly-l-lactic acid composite sheets for management of orbital wall fracture. J Craniofac Surg. (2019) 30(7):2001–3. doi: 10.1097/scs.0000000000005734
32. Suh SY, Yeom JA, Ahn JH. Porous polyethylene implant associated with delayed orbital complications and osteomyelitis after orbital reconstruction. J Craniofac Surg. (2018) 29(7):1910–1. doi: 10.1097/scs.0000000000004985
33. Woo JM, Baek SH, Kim JC, Choi JY. Contour restoration of over-resected mandibular angle and lower border by reduction mandibuloplasty using three-dimensional planning and computer-aided design and manufacturing custom-made titanium implants. J Craniofac Surg. (2018) 29(4):e340–3. doi: 10.1097/scs.0000000000004283
34. Findikcioglu K, Sibar S, Gulsen A. Treatment approach to severe microgenia cases: combined use of osseous and implant genioplasty. J Craniofac Surg. (2018) 29(2):e175–9. doi: 10.1097/scs.0000000000004245
35. Kanazawa S, Kiya K, Kubo T, Hosokawa K. Hydroxyapatite implantation for the repair of a congenital nasal anomaly: 10 years follow-up. J Surg Case Rep. (2018) 2018(6). doi: 10.1093/jscr/rjy146
36. Al-Jandan B, Marei HF. Mandibular angle augmentation using solid silicone implants. Dent Med Probl. (2018) 55(4):367–70. doi: 10.17219/dmp/99531
37. Sciaraffia CE, Ahumada MF, Parada FJ, Gonzalez E, Prado A. Bone resorption after use of silicone chin implants, long-term follow-up study with lateral chin radiography. Plast Reconstr Surg Glob Open. (2018) 6(7):e1850. doi: 10.1097/gox.0000000000001850
38. Kohyama K, Morishima Y, Arisawa K, Arisawa Y, Kato H. Immediate and long-term results of unsintered hydroxyapatite and poly L-lactide composite sheets for orbital wall fracture reconstruction. J Plast Reconstr Aesthet Surg. (2018) 71(7):1069–75. doi: 10.1016/j.bjps.2018.03.006
39. Franco J, Harris MS, Vernon D, Shipchandler TZ. Reconstruction of midface defect from idiopathic destructive process using medpor implant. Am J Otolaryngol. (2017) 38(3):351–3. doi: 10.1016/j.amjoto.2017.01.007
40. Zieliński R, Malińska M, Kozakiewicz M. Classical versus custom orbital wall reconstruction: selected factors regarding surgery and hospitalization. J Craniomaxillofac Surg. (2017) 45(5):710–5. doi: 10.1016/j.jcms.2017.02.008
41. Callahan AB, Campbell AA, Petris C, Kazim M. Low-cost 3D printing orbital implant templates in secondary orbital reconstructions. Ophthalmic Plast Reconstr Surg. (2017) 33(5):376–80. doi: 10.1097/iop.0000000000000884
42. Sainsbury DC, George A, Forrest CR, Phillips JH. Bilateral malar reconstruction using patient-specific polyether ether ketone implants in Treacher-Collins syndrome patients with absent zygomas. J Craniofac Surg. (2017) 28(2):515–7. doi: 10.1097/scs.0000000000003351
43. Cho WK, Ko AC, Korn BS, Kikkawa DO. Orbitocutaneous fistula secondary to buried polyethylene mesh implant 12 years after injury. Ophthalmic Plast Reconstr Surg. (2017) 33(5):e107–8. doi: 10.1097/iop.0000000000000822
44. Lee EI. Aesthetic alteration of the chin. Semin Plast Surg. (2013) 27(03):155–60. doi: 10.1055/s-0033-1357113
45. Ghosh S, Pramanick D, Ray A, Burman R, Saha A. Fronto-orbital reconstruction using polymethyl methacrylate implant. Natl J Maxillofac Surg. (2017) 8(2):153–6. doi: 10.4103/njms.NJMS_10_17
46. Kanno T, Karino M, Yoshino A, Koike T, Ide T, Tatsumi H, et al. Feasibility of single folded unsintered hydroxyapatite particles/poly-L-lactide composite sheet in combined orbital floor and medial wall fracture reconstruction. J Hard Tissue Biol. (2017) 26(2):237–44. doi: 10.2485/jhtb.26.237
47. Hosseini SN, Alizadeh A, Zahedi A. Reconstructing a giant frontal osteoma with porex. J Craniofac Surg. (2016) 27(8):2078–80. doi: 10.1097/scs.0000000000003059
48. Joo YH, Jang YJ. Comparison of the surgical outcomes of dorsal augmentation using expanded polytetrafluoroethylene or autologous costal cartilage. JAMA Facial Plast Surg. (2016) 18(5):327–32. doi: 10.1001/jamafacial.2016.0316
49. Timoney PJ, Clark JD, Frederick PA, Krakauer M, Compton C, Horbinski C, et al. Foreign body granuloma following orbital reconstruction with porous polyethylene. Ophthalmic Plast Reconstr Surg. (2016) 32(6):e137–8. doi: 10.1097/iop.0000000000000328
50. Hussain RN, Clark M, Berry-Brincat A. The use of a polyetheretherketone (PEEK) implant to reconstruct the midface region. Ophthalmic Plast Reconstr Surg. (2016) 32(6):e151–3. doi: 10.1097/iop.0000000000000345
51. de Menezes JD, Moura LB, Martins RP, Hochuli-Vieira E. Porous polyethylene implant as aesthetic complement in orthognathic surgery. J Craniofac Surg. (2016) 27(8):e790–1. doi: 10.1097/scs.0000000000003131
52. Park YW. Frontal augmentation as an adjunct to orthognathic or facial contouring surgery. Maxillofac Plast Reconstr Surg. (2016) 38(1):37. doi: 10.1186/s40902-016-0084-y
53. Polo M. Bone resorption under chin implants: the orthodontist’s role in its diagnosis and management. Am J Orthod Dentofacial Orthop. (2017) 151(1):201–8. doi: 10.1016/j.ajodo.2016.06.035
54. Santanelli Di Pompeo F, et al. Breast implants and the risk of squamous cell carcinoma of the breast: a systematic literature review and epidemiologic study. Aesthetic Surg J. (2024) 44:757–68. doi: 10.1093/asj/sjae023
55. Nahumi N, Shohet MR, Bederson JB, Elahi E. Frontorbital fibrous dysplasia resection and reconstruction with custom polyetherlatone alloplast. J Craniofac Surg. (2015) 26(8):e720–2. doi: 10.1097/scs.0000000000002225
56. Gander T, Essig H, Metzler P, Lindhorst D, Dubois L, Rücker M, et al. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J Craniomaxillofac Surg. (2015) 43(1):126–30. doi: 10.1016/j.jcms.2014.10.024
57. Yim HW, Nguyen A, Kim YK. Facial contouring surgery with custom silicone implants based on a 3D prototype model and CT-scan: a preliminary study. Aesthetic Plast Surg. (2015) 39(3):418–24. doi: 10.1007/s00266-015-0482-z
58. Rotaru H, Schumacher R, Kim SG, Dinu C. Selective laser melted titanium implants: a new technique for the reconstruction of extensive zygomatic complex defects. Maxillofac Plast Reconstr Surg. (2015) 37(1):1. doi: 10.1186/s40902-015-0001-9
59. Park H, Kim HS, Lee BI. Medial wall orbital reconstruction using unsintered hydroxyapatite particles/poly L-lactide composite implants. Arch Craniofac Surg. (2015) 16(3):125–30. doi: 10.7181/acfs.2015.16.3.125
60. Jalbert F, Boetto S, Nadon F, Lauwers F, Schmidt E, Lopez R. One-step primary reconstruction for complex craniofacial resection with PEEK custom-made implants. J Craniomaxillofac Surg. (2014) 42(2):141–8. doi: 10.1016/j.jcms.2013.04.001
61. Atherton D, Haers P. Midfacial augmentation in teenage cleft patients using malar and paranasal medpor implants. Int J Oral Maxillofac Surg. (2014) 43(7):824–6. doi: 10.1016/j.ijom.2014.01.015
62. Kozakiewicz M, Szymor P. Comparison of pre-bent titanium mesh versus polyethylene implants in patient specific orbital reconstructions. Head Face Med. (2013) 9(32). doi: 10.1186/1746-160x-9-32
63. Kozakiewicz M, Elgalal M, Walkowiak B, Stefanczyk L. Technical concept of patient-specific, ultrahigh molecular weight polyethylene orbital wall implant. J Craniomaxillofac Surg. (2013) 41(4):282–90. doi: 10.1016/j.jcms.2012.10.007
64. Alonso N, de Pochat VD, de Barros AR, Tavares LS. Long-term complication after rhinoplasty using porous polyethylene implant: cutaneous fistula of the forehead. J Craniofac Surg. (2013) 24(6):2176–8. doi: 10.1097/SCS.0b013e3182a2de02
65. Hatamleh MM, Cartmill M, Watson J. Management of extensive frontal cranioplasty defects. J Craniofac Surg. (2013) 24(6):2018–22. doi: 10.1097/SCS.0b013e3182a41bcc
66. Hayashi M, Muramatsu H, Sato M, Tomizuka Y, Inoue M, Yoshimoto S. Surgical treatment of facial fracture by using unsintered hydroxyapatite particles/poly l-lactide composite device (OSTEOTRANS MX®): a clinical study on 17 cases. J Cranio Maxillofac Surg. (2013) 41(8):783–8. doi: 10.1016/j.jcms.2013.01.026
67. Guo J, Tian W, Long J, Gong H, Duan S, Tang W. A retrospective study of traumatic temporal hollowing and treatment with titanium mesh. Ann Plast Surg. (2012) 68(3):279–85. doi: 10.1097/SAP.0b013e3181ff76a1
68. Kim CY, Jeong BJ, Lee SY, Yoon JS. Comparison of surgical outcomes of large orbital fractures reconstructed with porous polyethylene channel and porous polyethylene titan barrier implants. Ophthalmic Plast Reconstr Surg. (2012) 28(3):176–80. doi: 10.1097/IOP.0b013e3182467c4a
69. Scolozzi P. Maxillofacial reconstruction using polyetheretherketone patient-specific implants by “mirroring” computational planning. Aesthetic Plast Surg. (2012) 36(3):660–5. doi: 10.1007/s00266-011-9853-2
70. Niechajev I. Facial reconstruction using porous high-density polyethylene (medpor): long-term results. Aesthetic Plast Surg. (2012) 36(4):917–27. doi: 10.1007/s00266-012-9911-4
71. Lin J, Chen X. Modified technique of chin augmentation with MEDPOR for Asian patients. Aesthetic Surg J. (2012) 32(7):799–803. doi: 10.1177/1090820X12455191
72. Kirby EJ, Turner JB, Davenport DL, Vasconez HC. Orbital floor fractures: outcomes of reconstruction. Ann Plast Surg. (2011) 66(5):508–12. doi: 10.1097/SAP.0b013e31820b3c7a
73. Kim YH, Kim TG, Lee JH, Nam HJ, Lim JH. Inlay implanting technique for the correction of medial orbital wall fracture. Plast Reconstr Surg. (2011) 127(1):321–6. doi: 10.1097/PRS.0b013e3181f95cfd
74. Aynehchi BB, Burstein DH, Parhiscar A, Erlich MA. Vertical incision intraoral silicone chin augmentation. Otolaryngol Head Neck Surg. (2012) 146(4):553–9. doi: 10.1177/0194599811434889
75. Atherton DD, Joshi N, Kirkpatrick N. Augmentation of temporal fossa hollowing with mersilene mesh. J Plast Reconstr Aesthet Surg. (2010) 63(10):1629–34. doi: 10.1016/j.bjps.2009.09.022
76. Park JY, Kim SG, Baik SM, Kim SY. Comparison of genioplasty using medpor and osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2010) 109(2):e26–30. doi: 10.1016/j.tripleo.2009.10.007
77. Li M, Lin X, Xu Y. The application of rapid prototyping technique in chin augmentation. Aesthetic Plast Surg. (2010) 34(2):172–8. doi: 10.1007/s00266-009-9397-x
78. Chen CT, Hu TL, Lai JB, Chen YC, Chen YR. Reconstruction of traumatic nasal deformity in orientals. J Plast Reconstr Aesthet Surg. (2010) 63(2):257–64. doi: 10.1016/j.bjps.2008.10.006
79. Tang W, Guo L, Long J, Wang H, Lin Y, Liu L, et al. Individual design and rapid prototyping in reconstruction of orbital wall defects. J Oral Maxillofac Surg. (2010) 68(3):562–70. doi: 10.1016/j.joms.2009.04.042
80. Hopping SB, Joshi AS, Tanna N, Janjanin S. Volumetric facelift: evaluation of rhytidectomy with alloplastic augmentation. Ann Otol Rhinol Laryngol. (2010) 119(3):174–80. doi: 10.1177/000348941011900305
81. Shauly O, Gould DJ, Siddiqi I, Patel KM, Carey J. The first reported case of gluteal implant-associated anaplastic large cell lymphoma (ALCL). Aesthetic Surg J. (2019) 39:NP253–8. doi: 10.1093/asj/sjz044
82. Kim MM, Boahene KD, Byrne PJ. Use of customized polyetheretherketone (PEEK) implants in the reconstruction of complex maxillofacial defects. Arch Facial Plast Surg. Jan. (2009) 11(1):53–7. doi: 10.1001/archfaci.11.1.53
83. Stringer D, Brown B. Correction of mandibular asymmetry using angled titanium mesh. J Oral Maxillofac Surg. (2009) 67(8):1619–27. doi: 10.1016/j.joms.2008.12.068
84. Jirman R, Horák Z, Mazánek J, Reznícek J. Individual replacement of the frontal bone defect: case report. Prague Med Rep. (2009) 110(1):79–84.19591381
85. Guo L, Tian W, Feng F, Long J, Li P, Tang W. Reconstruction of orbital floor fractures: comparison of individual prefabricated titanium implants and calvarial bone grafts. Ann Plast Surg. (2009) 63(6):624–31. doi: 10.1097/SAP.0b013e3181999df3
86. Emsen IM, Benlier E. A new approach on reconstruction of frontonasal encephalomeningocele assisted with medpor. J Craniofac Surg. (2008) 19(2):537–9. doi: 10.1097/SCS.0b013e318163e194
87. Gui L, Huang L, Zhang Z. Genioplasty and chin augmentation with medpore implants: a report of 650 cases. Aesthetic Plast Surg. (2008) 32(2):220–6. doi: 10.1007/s00266-007-9106-6
88. Coban YK, Kabalci SK. Surgical treatment of posttraumatic enophthalmos with diced medpor implants through mini-lateral canthoplasty incision. J Craniofac Surg. (2008) 19(2):539–41. doi: 10.1097/SCS.0b013e318163e1a9
89. Garibaldi DC, Iliff NT, Grant MP, Merbs SL. Use of porous polyethylene with embedded titanium in orbital reconstruction: a review of 106 patients. Ophthalmic Plast Reconstr Surg. Nov. (2007) 23(6):439–44. doi: 10.1097/IOP.0b013e31815a1235
90. Scholz M, Wehmöller M, Lehmbrock J, Schmieder K, Engelhardt M, Harders A, et al. Reconstruction of the temporal contour for traumatic tissue loss using a CAD/CAM-prefabricated titanium implant-case report. J Craniomaxillofac Surg. (2007) 35(8):388–92. doi: 10.1016/j.jcms.2007.06.006
91. Eski M, Sengezer M, Turegun M, Deveci M, Isik S. Contour restoration of the secondary deformities of zygomaticoorbital fractures with porous polyethylene implant. J Craniofac Surg. (2007) 18(3):520–5. doi: 10.1097/scs.0b013e318053432c
92. Ozturk S, Acarturk TO, Yapici K, Sengezer M. Treatment of “en coup de sabre” deformity with porous polyethylene implant. J Craniofac Surg. (2006) 17(4):696–701. doi: 10.1097/00001665-200607000-00016
93. Romo T 3rd, Kwak ES. Difficult revision case: overaggressive resection. Facial Plast Surg Clin North Am. (2006) 14(4):411–5. viii. doi: 10.1016/j.fsc.2006.06.009
94. Gürlek A, Ersoz-Ozturk A, Celik M, Firat C, Aslan S, Aydogan H. Correction of the crooked nose using custom-made high-density porous polyethylene extended spreader grafts. Aesthetic Plast Surg. Mar. (2006) 30(2):141–9. doi: 10.1007/s00266-005-0152-7
95. Thornton MA, Mendelsohn M. Total skeletal reconstruction of the nasal dorsum. Arch Otolaryngol Head Neck Surg. (2006) 132(11):1183–8. doi: 10.1001/archotol.132.11.1183
96. Menderes A, Baytekin C, Topcu A, Yilmaz M, Barutcu A. Craniofacial reconstruction with high-density porous polyethylene implants. J Craniofac Surg. (2004) 15(5):719–24. doi: 10.1097/00001665-200409000-00004
97. Ellis E 3rd, Tan Y. Assessment of internal orbital reconstructions for pure blowout fractures: cranial bone grafts versus titanium mesh. J Oral Maxillofac Surg. (2003) 61(4):442–53. doi: 10.1053/joms.2003.50085
98. Yaremchuk MJ. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. (2003) 111(6):1818–27. doi: 10.1097/01.Prs.0000056866.80665.7a
99. Dusková M, Smahel Z, Vohradník M, Tvrdek M, Mazánek J, Kozák J, et al. Bioactive glass-ceramics in facial skeleton contouring. Aesthetic Plast Surg. (2002) 26(4):274–83. doi: 10.1007/s00266-002-1032-z
100. Saleh HA, Lohuis PJFM, Vuyk HD. Bone resorption after alloplastic augmentation of the mandible. Clin Otolaryngol Allied Sci. (2002) 27(2):129–32. doi: 10.1046/j.1365-2273.2002.00546.x
101. Cordeiro PG, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesth Surg. (2020) 73:841–6. doi: 10.1016/j.bjps.2019.11.064
102. Cordeiro PG, Ghione P, Ni A, Hu Q, Ganesan N, Galasso N, et al. Exposure of high-density porous polyethylene (medpor) used for contour restoration and treatment. Br J Oral Maxillofac Surg. (2000) 38(1):44–9. doi: 10.1054/bjom.1998.0038
103. Ramirez OM. Mandibular matrix implant system: a method to restore skeletal support to the lower face. Plast Reconstr Surg. (2000) 106(1):176–89. doi: 10.1097/00006534-200007000-00034
104. Yaremchuk MJ. Mandibular augmentation. Plast Reconstr Surg. (2000) 106(3):697–706. doi: 10.1097/00006534-200009010-00030
105. Fedok FG, van Kooten DW, Levin RJ. Temporal augmentation with a layered expanded polytetrafluoroethylene implant. Otolaryngol Head Neck Surg. (1999) 120(6):929–33. doi: 10.1016/s0194-5998(99)70340-2
106. Metzinger SE, McCollough EG, Campbell JP, Rousso DE. Malar augmentation: a 5-year retrospective review of the silastic midfacial malar implant. Arch Otolaryngol Head Neck Surg. (1999) 125(9):980–7. doi: 10.1001/archotol.125.9.980
107. Mendelsohn M, Dunlop G. Gore-tex augmentation grafting in rhinoplasty–is it safe? J Otolaryngol. (1998) 27(6):337–41.9857319
108. Karras SC, Wolford LM. Augmentation genioplasty with hard tissue replacement implants. J Oral Maxillofac Surg. (1998) 56(5):549–52. doi: 10.1016/s0278-2391(98)90449-9
109. Yaremchuk MJ, Israeli D. Paranasal implants for correction of midface concavity. Plast Reconstr Surg. (1998) 102(5):1676–84; discussion 1685. doi: 10.1097/00006534-199810000-00055
110. Frodel JL, Lee S. The use of high-density polyethylene implants in facial deformities. Arch Otolaryngol Head Neck Surg. (1998) 124(11):1219–23. doi: 10.1001/archotol.124.11.1219
111. Hirano S, Shoji K, Kojima H, Omori K. Use of hydroxyapatite for reconstruction after surgical removal of intraosseous hemangioma in the zygomatic bone. Plast Reconstr Surg. (1997) 100(1):86–90. doi: 10.1097/00006534-199707000-00015
112. Abrahams JJ, Caceres C. Mandibular erosion from silastic implants: evaluation with a dental CT software program. AJNR Am J Neuroradiol. (1998) 19(3):519–22.9541311
113. Semergidis TG, Migliore SA, Sotereanos GC. Alloplastic augmentation of the mandibular angle. J Oral Maxillofac Surg. (1996) 54(12):1417–23. doi: 10.1016/s0278-2391(96)90256-6
114. Vuyk HD. Augmentation mentoplasty with solid silicone. Clin Otolaryngol Allied Sci. (1996) 21(2):106–18. doi: 10.1111/j.1365-2273.1996.tb01312.x
115. Eppley BL, Sadove AM, Holmstrom H, Kahnberg KE. HTR polymer facial implants: a five-year clinical experience. Aesth Plast Surg. (1995) 19(5):445–50. doi: 10.1007/bf00453878
116. Matarasso A, Elias AC, Elias RL. Labial incompetence: a marker for progressive bone resorption in silastic chin augmentation. Plast Reconstr Surg. (1996) 98(6):1007–14. doi: 10.1097/00006534-199611000-00012
117. Owsley TG, Taylor CO. The use of gore-tex for nasal augmentation: a retrospective analysis of 106 patients. Plast Reconstr Surg. (1994) 94(2):241–8. discussion 249–50. doi: 10.1097/00006534-199408000-00003
118. Ono I, Gunji H, Suda K, Kaneko F, Yago K. Orbital reconstruction with hydroxyapatite ceramic implants. Scand J Plast Reconstr Surg Hand Surg. (1994) 28(3):193–8. doi: 10.3109/02844319409015980
119. Blake GB, MacFarlane MR, Hinton JW. Titanium in reconstructive surgery of the skull and face. Br J Plast Surg. (1990) 43(5):528–35. doi: 10.1016/0007-1226(90)90115-g
120. Moenning JE, Wolford LM. Chin augmentation with various alloplastic materials: a comparative study. Int J Adult Orthodon Orthognath Surg. (1989) 4(3):175–87.2561746
121. Epker BN, Stella JP. Reconstruction of frontal and frontal-nasal deformities with prefabricated custom implants. J Oral Maxillofac Surg. (1989) 47(12):1272–6. doi: 10.1016/0278-2391(89)90722-2
122. Pitanguy I, Martello L, Caldeira AML, Alexandrino A. Augmentation mentoplasty: a critical analysis. Aesth Plast Surg. (1986) 10(1):161–9. doi: 10.1007/BF01575287
123. Dann JJ, Epker BN. Proplast genioplasty: a retrospective study with treatment recommendations. Angle Orthod. (1977) 47(3):173–85. doi: 10.1043/0003-3219(1977)047
124. Laub DR, Spohn W, Lash H, Weber J, Chase RA. Accurate reconstruction of traumatic bony contour defects of periorbital area with prefabricated silastic. J Trauma. (1970) 10(6):472–80. doi: 10.1097/00005373-197006000-00005
125. Huang GJ, Zhong S, Susarla SM, Swanson EW, Huang J, Gordon CR. Craniofacial reconstruction with poly(methyl methacrylate) customized cranial implants. J Craniofac Surg. (2015) 26(1):64–70. doi: 10.1097/scs.0000000000001315
126. Lee GH, Ho SY. Orbital adherence syndrome following the use of titanium precontoured orbital mesh for the reconstruction of posttraumatic orbital floor defects. Craniomaxillofac Trauma Reconstr. (2017) 10(1):77–83. doi: 10.1055/s-0036-1584398
127. Yu Y, Liu W, Chen J, Quan L, Zheng X, Liu L. No need to routinely remove titanium implants for maxillofacial fractures. J Oral Maxillofac Surg. (2019) 77(4):783–8. doi: 10.1016/j.joms.2018.10.022
128. Kauke-Navarro M, Knoedler L, Knoedler S, Deniz C, Stucki L, Safi A-F. Balancing beauty and science: a review of facial implant materials in craniofacial surgery. Mini review. Front Surg. (2024) 2024:11. doi: 10.3389/fsurg.2024.1348140
129. Oliver JD, Eells AC, Saba ES, Boczar D, Restrepo DJ, Huayllani MT, et al. Alloplastic facial implants: a systematic review and meta-analysis on outcomes and uses in aesthetic and reconstructive plastic surgery. Aesthetic Plast Surg. (2019) 43(3):625–36. doi: 10.1007/s00266-019-01370-0
131. Rojas YA, Sinnott C, Colasante C, Samas J, Reish RG. Facial implants: controversies and criticism. A comprehensive review of the current literature. Plast Reconstr Surg. (2018) 142(4):991–9. doi: 10.1097/prs.0000000000004765
132. Quatela VC, Chow J. Synthetic facial implants. Facial Plast Surg Clin North Am. (2008) 16(1):1–10. doi: 10.1016/j.fsc.2007.09.002
133. Ahmad AF, Yaakob H, Khalil A, Georges P. Evaluating patients’ satisfaction level after using 3D printed PEEK facial implants in repairing maxillofacial deformities. Ann Med Surg (Lond). (2022) 79:104095. doi: 10.1016/j.amsu.2022.104095
134. Anabtawi M, Thomas M, Lee NJ. The use of interlocking polyetheretherketone (PEEK) patient-specific facial implants in the treatment of facial deformities. A retrospective review of ten patients. J Oral Maxillofac Surg. (2021) 79(5):1145.e1–9. doi: 10.1016/j.joms.2020.12.009
135. Berrone M, Aldiano C, Pentenero M, Berrone S. Correction of a mandibular asymmetry after fibula reconstruction using a custom-made polyetheretherketone (PEEK) onlay after implant supported occlusal rehabilitation. Acta Otorhinolaryngol Ital. (2015) 35(4):285–8.26824216
136. Systermans S, Cobraiville E, Camby S, Meyer C, Louvrier A, Lie SA, et al. An innovative 3D hydroxyapatite patient-specific implant for maxillofacial bone reconstruction: a case series of 13 patients. J Craniomaxillofac Surg. (2024) 52(4):420–31. doi: 10.1016/j.jcms.2024.02.026
137. Atef M, Mounir M, Shawky M, Mounir S, Gibaly A. Polyetheretherketone patient-specific implants (PPSI) for the reconstruction of two different mandibular contour deformities. Oral Maxillofac Surg. (2022) 26(2):299–309. doi: 10.1007/s10006-021-00984-6
138. Ducic Y. Three-dimensional alloplastic orbital reconstruction in skull base surgery. Laryngoscope. (2001) 111(7):1306–12. doi: 10.1097/00005537-200107000-00031
139. Bachelet JT, Cordier G, Porcheray M, Bourlet J, Gleizal A, Foletti JM. Orbital reconstruction by patient-specific implant printed in porous titanium: a retrospective case series of 12 patients. J Oral Maxillofac Surg. (2018) 76(10):2161–7. doi: 10.1016/j.joms.2018.04.006
Keywords: facial implants, facial implantology, face design, facial reconstruction, aesthetic facial surgery, craniomics
Citation: Kauke-Navarro M, Knoedler L, Baecher H, Sherwani K, Knoedler S, Allam O, Diatta F, Alperovich M and Safi A-F (2025) A systematic review of implant materials for facial reconstructive and aesthetic surgery. Front. Surg. 12:1548597. doi: 10.3389/fsurg.2025.1548597
Received: 19 December 2024; Accepted: 3 March 2025;
Published: 28 March 2025.
Edited by:
Robert Alexander Sader, Goethe University Frankfurt, GermanyReviewed by:
Andreas George Manios, University Hospital of Heraklion, GreeceCopyright: © 2025 Kauke-Navarro, Knoedler, Baecher, Sherwani, Knoedler, Allam, Diatta, Alperovich and Safi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Kauke-Navarro, a2F1a2UtbmF2YXJyby5tYXJ0aW5AeWFsZS5lZHU=; Ali-Farid Safi, c2FmaUBjcmFuaW9sb2dpY3VtLmNo; YWxpLWZhcmlkLnNhZmlAZmFjdWx0eS51bmliZS5jaA==; Leonard Knoedler, TGVvbmFyZC5rbm9lZGxlckBjaGFyaXRlLmRl
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.