
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 11 March 2025
Sec. Thoracic Surgery
Volume 12 - 2025 | https://doi.org/10.3389/fsurg.2025.1547048
Purpose: Microwave ablation (MWA) is a minimally invasive local treatment with demonstrated safety and efficacy, but its role in managing multiple primary lung cancer (MPLC) is not well-established. This study retrospectively evaluates the clinical effectiveness of MWA compared to video-assisted thoracoscopic surgery (VATS) in treating MPLC.
Materials and methods: A retrospective analysis was conducted using data from patients with non-small cell lung cancer (NSCLC) treated at Peking University Cancer Hospital Yunnan Hospital between January 2021 and April 2024. All patients had undergone surgical resection for their first primary lung cancer (FPLC) and subsequently received either MWA or VATS for second primary lung cancer (SPLC). After 1:1 propensity score matching (PSM), 202 patients per group were included. Study endpoints included progression-free survival (PFS), overall survival (OS), complications, and pulmonary function changes.
Results: Median follow-up was 24.47 months. Survival analysis revealed a statistically significant difference in PFS between MWA and VATS groups (HR = 2.74, 95% CI: 1.40–5.36, p = 0.006), while OS showed no difference (HR = 1.41, 95% CI: 0.45–4.36, p = 0.56). The incidence of grade ≥ II complications was significantly lower in the MWA group (p < 0.001). Pulmonary function tests indicated no significant changes in forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1%, maximal voluntary ventilation (MVV), and diffusion capacity of the lung for carbon monoxide%(DLCO%) before and 1–3 month post MWA (p > 0.05).
Conclusions: In MPLC patients with stage IA SPLC, VATS demonstrates a greater clinical efficacy advantage in terms of local tumor control compared to MWA. Additionally, MWA provided significant advantages in reducing complication severity and preserving pulmonary function. These findings suggest that the therapeutic approach combining surgery with MWA represents a safe and effective option for MPLC.
With the advent of the post-pandemic era, the application of chest CT in lung cancer screening has become increasingly widespread. Meanwhile, the detection rate of MPLC has risen significantly across various countries and regions, accounting for approximately 3%–13% of all lung cancer cases (1, 2). In 1924, Beyreuther first reported MPLC, which has since garnered clinical attention (3). MPLC refers to the simultaneous or sequential discovery of two or more primary cancer lesions in the lungs that are anatomically distinct and originate independently (4). In clinical practice, most MPLC cases are diagnosed as early-stage lung cancer (5). Therefore, pulmonary resection remains the gold standard treatment (6). However, for MPLC patients who have already undergone one surgical procedure, even sublobar resection inevitably sacrifices substantial lung tissue, potentially increasing perioperative morbidity and mortality and affecting long-term quality of life (7, 8). Therefore, stereotactic body radiotherapy (SBRT) and ablation therapy play a pivotal role.
SBRT has become the standard treatment for early-stage patients who are ineligible for surgery, offering comparable overall survival and local control rates to surgical interventions (9). Nevertheless, severe adverse effects such as pneumonitis and bone marrow suppression remain significant concerns, and its application in multiple lesions is limited (10). On the contrary, local ablation therapy offers substantial advantages in avoiding radiation-associated toxicity and managing various lesions (11). MWA uses microwave energy to rapidly heat target tissues to 60°C–150°C, causing irreversible damage and necrosis to cancer cells. Compared to radiofrequency ablation (RFA), MWA has a shorter ablation time, larger ablation zones, and reduced heat sink effects (12). Existing data suggest that MWA demonstrates unique advantages in the treatment of early-stage NSCLC (13–15).
This study aims to retrospectively compare the short-term efficacy, complication rates, and changes in pulmonary function between MWA and VATS in treating SPLC presenting as stage IA.
From January 2021 to April 2024, patients with lung cancer who underwent surgical resection for FPLC and received MWA or VATS for SPLC in Peking University Cancer Hospital Yunnan Hospital were retrospectively investigated. The study received approval from the hospital's ethics committee.
Before treatment, medical history collection, physical examination, and imaging studies were performed. The optimal treatment plan was determined after a multidisciplinary consultation involving thoracic surgeons, medical oncologists, radiologists, respiratory physicians, and pathologists. Inclusion criteria: (1) Patients with FPLC who underwent surgical resection and were diagnosed with stage I-IIIB NSCLC; (2) SPLC lesions with a diameter ≤3 cm as shown by CT or PET-CT; (3) Lesions located in bilateral lungs or different lobes of the same lung; (4) SPLC confirmed as NSCLC via surgery, bronchoscopy, or concurrent biopsy during ablation. Exclusion criteria: (1) Evidence of mediastinal lymph node or distant metastasis detected by CT, PET-CT, MRI, or ultrasound; (2) SPLC treated with open thoracotomy; (3) FVC or FEV1 less than 1.5l before the second treatment; (4) Incomplete medical records. Patients were matched at a 1:1 ratio using propensity score matching (PSM) based on sex, age, TNM stage of FPLC, FVC before the second treatment, FEV1 before the second treatment, and SPLC lesion size. The patient selection flowchart is shown in Figure 1. Detailed clinical data were collected from medical records, including sex, age, body mass index, smoking history, past medical history, TNM stage, lesion size and consolidation tumor ratio (CTR), histological type, complications, and pulmonary function indices.
This study utilized the KY-2000 microwave ablation system with a working frequency of 2,450 ± 50 MHz and an output power range of 0–100 W for ablation treatment. The ablation needles had an effective length of 100–180 mm and external diameters of 16G, 18G, and 19G. A water circulation cooling system maintained the surface temperature of the needles. The ablation process was guided by a Siemens Definition AS + spiral CT.
A personalized MWA treatment plan was developed preoperatively based on the patient's medical history and examination results. Patients were fully informed about the treatment process and associated risks, and written informed consent was obtained. The appropriate body position was determined according to the lesion's location, and the optimal puncture site was identified using a metal surface locator. All MWA procedures were performed under local anesthesia and sterile conditions. After confirming satisfactory anesthesia, the ablation needle was precisely positioned at the pre-planned location. Currently, the energy output power and duration of MWA have not been standardized. In clinical practice, two main ablation modes are commonly used: one is a low-power, long-duration mode, and the other is a high-power, short-duration mode. We preset the ablation parameters based on the size, location, lung quality, and proportion of solid components in the lesion. Additionally, the patient's tolerance and the size of the target area play crucial roles during the ablation process, and these factors are used to appropriately adjust the final ablation parameters. The needle's position was verified using CT guidance before ablation was performed according to the pre-set power and time parameters. All MWA procedures were performed under local anesthesia and sterile conditions. After confirming satisfactory anesthesia, the ablation needle was precisely positioned at the pre-planned location. The needle's position was verified using CT guidance before ablation was performed according to the pre-set power and time parameters. The criteria for technical success were defined as achieving an ablation zone that extended 5–10 mm beyond the lesion boundary and matched the preoperative plan.
During the entire MWA procedure, patients' ECG and vital signs were closely monitored. Symptoms such as breathing difficulties, pain, coughing, and hemoptysis were observed, and symptomatic treatments were provided as necessary. After safely returning to the ward, patients were monitored for an additional 24 h. A follow-up chest CT scan was performed 24–48 h postoperatively to reassess technical success and detect any complications.
VATS was executed using the IMAGE1 HD video system (Karl Storz, Inc., Germany) and the Harmonic ultrasonic surgical scalpel (Ethicon Endo-Surgery, LLC, Puerto Rico, USA).
All surgeries were performed by our experienced thoracic surgery team to ensure complete tumor resection with negative margins while maintaining adequate safety margins. VATS was conducted under general anesthesia with single-lung ventilation. Patients were positioned in a full lateral decubitus position with the mid-chest level slightly over-extended to widen the intercostal space. A single 5 cm incision is made at the fourth or fifth intercostal space along the anterior axillary line. Through this incision, the mediastinal pleura and interlobar fissures are dissected, and the corresponding blood vessels and bronchi are separated during lobectomy or segmentectomy, following the same procedural steps as open surgery. An endoscopic linear cutting stapler is used to transect lung tissue, blood vessels, and bronchi, and a specimen retrieval bag is inserted through the small incision for extraction. Mediastinal lymph node dissection or sampling is performed simultaneously, and the resected primary lesion and lymph nodes are routinely sent for frozen section examination to complete lobectomy or segmentectomy. During wedge resection, the location of the lung nodule is determined based on preoperative imaging. The lung tissue is gently retracted to expose the target lesion, and if necessary, the pleural layers and interlobar fissures are separated. An endoscopic linear cutting stapler is then used to completely excise the lesion along with the surrounding lung tissue to ensure an adequate margin, while taking care to avoid damaging the lung vasculature and bronchi. During the procedure, the resected primary lesion is routinely sent for frozen section examination. Saline was instilled into the pleural cavity to expand the lung tissue, enabling assessment of the reliability of bronchial stump stapling, and checking for active bleeding or air leaks. At the end of the surgery, a 20F chest tube was inserted at the thoracic apex and connected to a negative pressure drainage system, and an 8F soft catheter was placed in the lateral chest wall for fluid drainage. The 20F chest tube was removed if no air leaks were detected and lung expansion was satisfactory 48 h postoperatively. The 8F chest tube was retained until discharge and was removed only when the drainage volume was less than 150 ml/day (16).
Patients' vital signs were closely monitored during the surgery. A chest x-ray or CT scan was performed within 24–48 h postoperatively to assess for complications.
Chest CT scans were performed at 1, 3, and 6 months after MWA treatment, using the lesion at the first-month post-MWA as the baseline for comparison. Key assessments included whether the local lesion was completely ablated, recurrence of the local lesion, occurrence of new lesions in the lung, and incidence of complications. Subsequent CT scans were conducted every 6 months, transitioning to annual scans after 2 years (17). For patients who underwent VATS, follow-up intervals and outcomes were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) (18).
The primary endpoint of this study was PFS, and the secondary endpoint was OS. PFS was defined as the time from the initiation of MWA or VATS treatment to the occurrence of any form of tumor progression or death. OS was defined as the time from the initiation of MWA or VATS treatment to death from any cause.
The study included common perioperative complications in thoracic surgery, assessing and grading their severity using the Clavien-Dindo classification system (19) This system categorizes postoperative complications into five grades: Grade I complications include the need for analgesics, antipyretics, antiemetics, electrolyte supplementation, and physical therapy, without requiring surgical, endoscopic, or radiologic interventions, but include wound infections requiring open drainage; Grade II complications require blood transfusion, total parenteral nutrition, or medications beyond those used in Grade I; Grade IIIa complications require surgical, endoscopic, or radiologic interventions without general anesthesia; while Grade IIIb requires such interventions under general anesthesia; Grade IVa involves life-threatening single organ dysfunction; Grade IVb involves life-threatening multi-organ dysfunction; Grade V denotes patient death. According to the Clavien-Dindo classification, Grade I complications generally do not require specific treatment. Thus, complications were further stratified into two categories: none or Grade I, and ≥Grade II.
The study included several pulmonary function assessment indicators, including FVC, FEV1, FEV1%, MVV, and DLCO%. In the MWA group, pulmonary function data were recorded before the first lung resection surgery, pre-ablation, and post-ablation. In the VATS group, pulmonary function data were recorded before the first surgery and before the second.
Data analysis was performed using SPSS software version 26.0, while GraphPad Prism software (version 9) and the R software program were used to create charts. PSM was applied to balance potential confounding factors between the MWA and VATS groups. Categorical data were expressed as frequencies and percentages, with intergroup comparisons conducted using the χ2 test or Fisher's exact test. Continuous data were described using the mean ± standard deviation (x̅ ± s) or median (interquartile range) [M(Q1, Q3)] and compared between groups using the T-test or non-parametric tests. Kaplan–Meier methods were used to estimate PFS and OS, and survival outcomes were compared using the log-rank test. In univariate and multivariate survival analyses, Cox proportional hazards model were used to calculate hazard ratios (HR) and their 95% confidence intervals (CI). All statistical tests were two-sided, with a significance level set at p < 0.05.
Between January 2021 and April 2024, 618 and 8,634 lung cancer patients underwent MWA treatment and lung resection surgery, respectively, at Peking University Cancer Hospital Yunnan Hospital. Based on strict inclusion and exclusion criteria, 220 and 335 patients were finally selected for the MWA and VATS groups, respectively. To balance the baseline characteristics of the two groups, 1:1 PSM was performed based on six variables significantly affecting prognosis: sex, age, TNM stage of the first surgery, FVC before the second treatment, FEV1 before the second treatment and SPLC lesion size (Table 1). The standardized mean difference (SMD) after matching is less than 0.2 (Figure 2). After matching, 202 patients were included in each group, achieving good comparability of clinical characteristics (Table 2).
The mean age of patients in both groups was approximately 55 years, with the proportion of females significantly higher than males (MWA group: 62.87%, VATS group: 61.88%). The postoperative stage following the first lung resection was predominantly in the early stage, with over 90% of patients classified as stage IA. The mean diameter of SPLC lesions was 1.14 ± 0.51 cm in the MWA group and 1.17 ± 0.45 cm in the VATS group. Adenocarcinoma was the most common pathological type following both treatments. In the VATS group, 103 patients (50.9%) underwent lobectomy, and 99 patients (49.1%) underwent sublobar resection, including 50 patients (24.8%) who underwent segmentectomy and 49 patients (24.3%) who underwent wedge resection.
Median follow-up was 24.47 months, ranging from 5.23–44.72 months. A statistically significant difference in PFS was observed between the MWA and VATS groups (HR = 2.74, 95% CI 1.40–5.36, p = 0.006) (Figure 3a), while the difference in OS was not statistically significant (HR = 1.41, 95% CI 0.45–4.36, p = 0.56) (Figure 3b). Univariate and multivariate survival analysis results indicated that MWA treatment, advanced TNM stage of FPLC, and larger tumor diameter of SPLC were independent risk factors for shorter PFS (Figure 4). The advanced TNM stage of FPLC and older age were identified as independent risk factors for shorter OS, but treatment modality was not an independent factor for OS (Figure 5). These findings suggest that while MWA has certain limitations in local tumor control, it remains an effective and reliable treatment option.
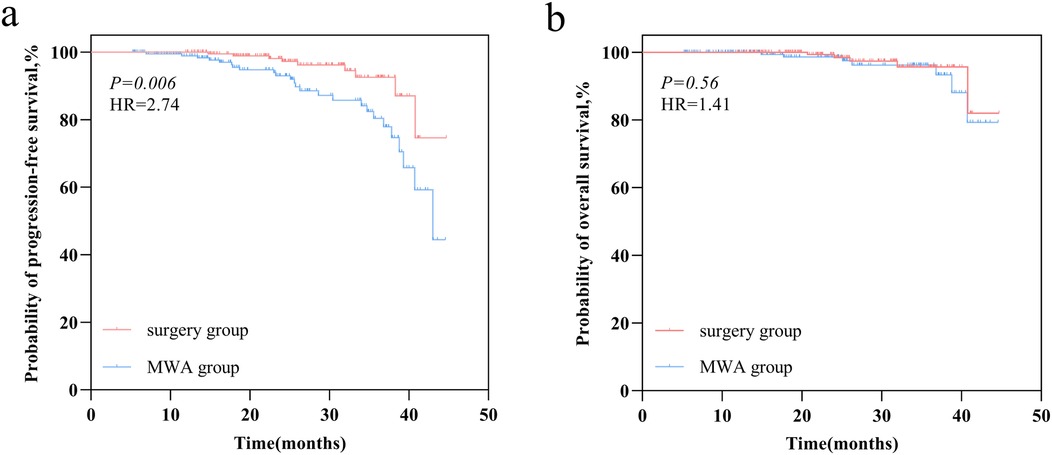
Figure 3. Survival outcomes comparison in the MWA group and VATS group. (a) Kaplan–Meier curve of progression-free survival (PFS). (b) Kaplan–Meier curve of overall survival (OS).

Figure 4. Univariate and multivariate Cox regression analyses of PFS in MPLC patients treated with MWA or surgery.
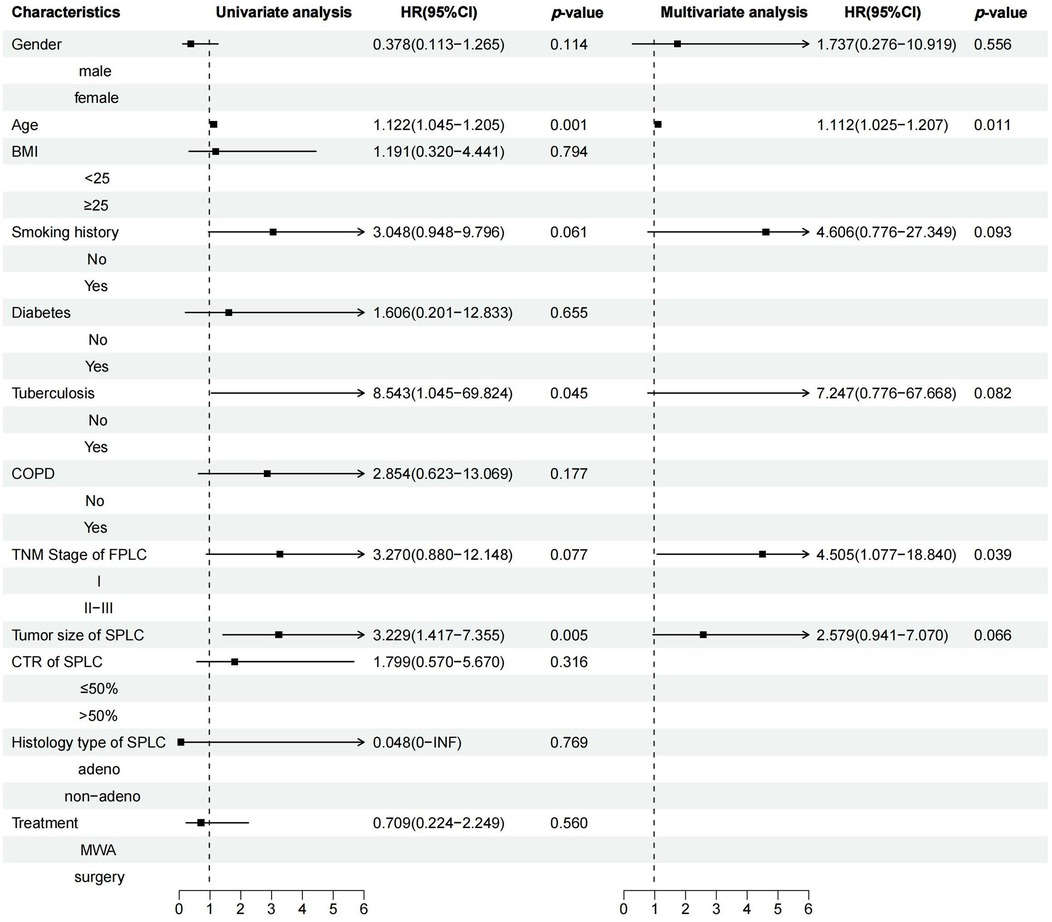
Figure 5. Univariate and multivariate Cox regression analyses of OS in MPLC patients treated with MWA or surgery.
All patients successfully underwent MWA and VATS. In the MWA group, 48 patients (23.76%) experienced postoperative complications. The three most common complications were pneumothorax (37/56, 66.07%), pleural effusion (15/56, 26.79%), and pneumonia (3/56, 5.36%). In the VATS group, 45 patients (22.28%) experienced postoperative complications, primarily including pleural effusion (21/70, 30%), pneumothorax (14/70, 20%), and arrhythmia (15/70, 21.43%). No cases of multi-organ dysfunction or perioperative mortality occurred in either group. Comparative analysis of postoperative complications showed no statistically significant difference in overall complication rates (P = 0.133); however, the incidence of ≥Grade II complications was significantly different (P < 0.001) (Table 3), indicating that MWA-related complications were relatively less severe compared to VATS.
To effectively control inter-individual variability, this study compared pulmonary function changes in the MWA group at three-time points: before the first lung resection surgery, pre-MWA, and 1–3 months after MWA. The results showed that the five pulmonary function indicators: FVC, FEV1, FEV1%, MVV, and DLCO% had statistically significant overall differences across the three time points (p < 0.001) However, further group comparisons revealed no statistically significant difference between the pre-MWA and post-MWA measurements (Figure 6). These findings suggest that compared to surgery, pulmonary function remains relatively stable after MWA, demonstrating its protective effect on lung function.
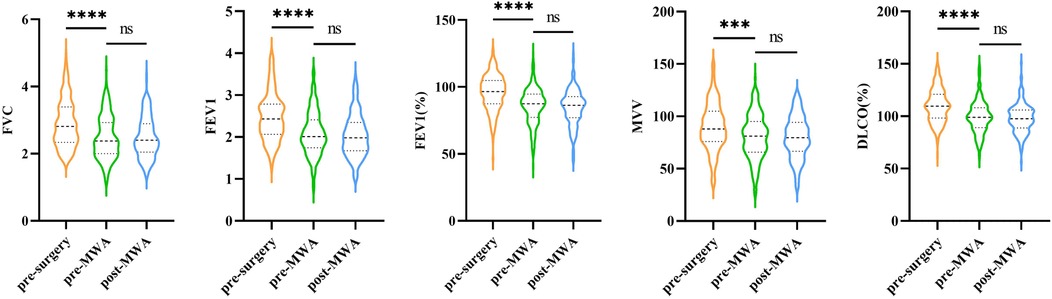
Figure 6. Changes in five pulmonary function parameters measured at three distinct time points (pre-surgery, pre-MWA, and post-MWA).
In the current study, we initially employed PSM to compare patients undergoing MWA and VATS. Subsequently, we conducted a systematic evaluation of the two groups, focusing on short-term efficacy, incidence of complications, and changes in pulmonary function. The results showed a statistically significant difference in PFS between the MWA and VATS groups (HR = 2.74, 95% CI 1.40–5.36, p = 0.006), with survival analysis indicating that MWA was an independent risk factor for shorter PFS. However, no statistically significant difference in OS was observed between the groups (HR = 1.41, 95% CI 0.45–4.36, p = 0.56), and MWA was not an independent factor for OS.
Regarding complications, pneumothorax was the most common complication in both groups. The proportion of patients with ≥Grade II complications was significantly lower in the MWA group, indicating better safety with MWA. there were no statistically significant differences in the five pulmonary function indicators before and after MWA treatment (p > 0.05), indicating a minimal impact on patients' pulmonary function.
With innovations in surgical techniques and approaches, VATS has become the mainstream treatment for early-stage NSCLC (20, 21). Meanwhile, the incidence of MPLC is increasing annually (22), but repeated surgeries may lead to high complication risks and restricted pulmonary function, posing clinical challenges (23). Fortunately, SBRT and ablation therapy have become important treatments for lung cancer (24). However, since MPLC typically involves multiple lesions with varying intervals of occurrence, combined with limitations in cumulative radiation doses and potential tumor resistance, these factors restrict the application of radiotherapy in MPLC (10, 25). In contrast, ablation therapy has demonstrated certain advantages in treating MPLC. Existing literature largely focuses on patients who opt for MWA due to poor cardiopulmonary function or other contraindications for surgery (26, 27). Currently, there is a lack of systematic comparative studies on MWA vs. surgical treatment for SPLC. The findings of studies would provide critical insights for optimizing treatment strategies for these patients.
Local ablation therapy has demonstrated good clinical efficacy and tolerability in clinical practice, making it a valuable treatment option for specific populations (28). MWA, in particular, has gained increasing research interest due to its high heating efficiency and low heat sink effect (14). A retrospective comparative study of MWA and lobectomy showed comparable 2-year OS and disease-free survival (DFS) between the two groups (27). Another study comparing MWA and sublobar resection for early subpleural NSCLC found no significant difference in 3-year OS but a slightly inferior recurrence-free survival (RFS) with MWA (23). Additionally, Han et al. (26). conducted a comparative analysis of 204 patients (101 of whom underwent MWA) with ground-glass opacity adenocarcinoma. The results showed comparable 3-year OS between the two groups, with no deaths observed and excellent local progression-free survival (LPFS). The results of this study showed no significant difference in OS between MWA and VATS, but VATS demonstrated superior PFS. The prognostic data in this study fell within the intermediate range of previous findings, being higher than that of patients with stage IA disease and severe cardiopulmonary dysfunction but lower than studies including only ground-glass nodules. The study also indicated that advanced TNM stage and larger lesion size are independent risk factors for MPLC progression, consistent with previous prognostic studies on lung cancer (29). The similarity in OS outcomes between VATS and MWA treatment may be related to the median follow-up time of 24.47 months in this study. Considering the relatively favorable prognosis of stage I lung cancer patients, this follow-up period may be too short to reveal potential differences between the two treatments in terms of long-term survival. The differences between the two treatment methods in terms of local tumor control may be attributed to the following factors. Firstly, VATS helps minimize the likelihood of residual disease by directly excising the tumor and confirming the surgical margins. In contrast, MWA is influenced by the heat sink effect and the tumor's anatomical location, which makes it difficult to precisely control the extent of thermal damage. This can potentially lead to incomplete ablation in certain areas, forming “sub-ablation zones” and increasing the risk of local recurrence. Secondly, compared to ablation, one significant advantage of surgery is the ability to perform systematic lymph node dissection or sampling, which not only helps identify and treat potential lymph node metastasis but also makes tumor staging more precise. In contrast, the staging in the MWA group primarily relies on imaging assessments (such as CT or PET-CT), with bronchoscopic biopsy or mediastinal/hilar lymph node sampling conducted in only a few suspicious cases. Therefore, some patients assessed as stage I by preoperative imaging and treated with ablation might actually be at stage II or higher, thus affecting the prognosis in the MWA group (30). Lastly, compared to surgical resection, MWA has certain limitations in assessing postoperative disease recurrence, particularly during early post-ablation imaging follow-up. The ablation target area is typically larger than the original tumor volume, as it includes both the tumor itself and the surrounding lung tissue affected by thermal damage. In a previous study, after 3 months of ablation, some patients may show increased fluorine 18 fluorodeoxyglucose (FDG) uptake on PET-CT scans, which in 15%–20% of cases was considered a response to treatment-induced inflammation rather than actual disease recurrence (31). Thus, this imaging change could lead to an overestimation of local recurrence rates and potentially affect the accurate assessment of MWA's therapeutic efficacy. In summary, for stage IA MPLC patients, surgical resection remains the first-line treatment. However, for patients with multiple, dispersed lesions or those who cannot tolerate or refuse lung resection, despite MWA having a higher risk of local recurrence compared to surgery, it remains an effective and viable alternative treatment option.
SBRT has become the standard treatment for early-stage inoperable NSCLC. However, its application in MPLC still faces several challenges. First, patients undergoing SBRT typically require an additional non-therapeutic invasive procedure to obtain a pathological diagnosis, whereas ablation therapy can acquire pathological results through simultaneous biopsy, which reduces patient discomfort and risk to some extent. Second, the lesions in MPLC present with complex characteristics, including synchronous and asynchronous onset, same-side same lung lobe, same-side different lung lobes, and bilateral lung lobes. These lesions are often located close together, exceeding the tolerance threshold for radiotherapy, which limits the clinical application of SBRT. Finally, radiation-induced lung toxicity (such as radiation pneumonitis and pulmonary fibrosis) remains a significant safety concern in thoracic radiotherapy and poses a major challenge for lung function preservation (32).
In terms of complications, the overall incidence of complications was similar between MWA and VATS (23.76% vs. 22.28%). Stratified analysis revealed that ≥Grade 2 complications were significantly more common in the VATS group, and the complication rate in the VATS group was consistent with post-MPLC surgery rates reported by other centers (15%–25.5%) (33, 34). It is worth noting that due to the lack of comparative data on Clavien-Dindo graded complications between MWA and surgical treatment in other studies, an effective comparison at specific grades could not be performed. However, Wang et al. (27). compared the overall complication rates of MWA and VATS, showing that complications in the VATS group were more severe than those in the MWA group, consistent with our findings. Pneumothorax, a primary complication of interest across studies, showed significant variation in incidence (15%–55%) (27, 28). In this study, the incidence of pneumothorax was 18.3%, which was well controlled, possibly due to strict pre-ablation planning and limitations on the number of puncture needles. Common postoperative complications, such as pneumothorax and arrhythmia, are easily detected through routine post-surgical examinations and clinical signs. The findings of this study suggest that most complications following MWA are self-limiting and do not require additional treatments such as medication or drainage. Therefore, based on previous research findings, patients who undergo MWA may experience shorter hospital stays and lower hospitalization costs (26, 27), while demonstrating a greater advantage in terms of safety.
Pulmonary function is an important indicator for evaluating the long-term quality of life in postoperative patients (35). Studies have shown that pulmonary function declines to some extent after VATS, and sublobar resection preserves pulmonary function better than lobectomy (34). In this study, all five pulmonary function indicators showed varying degrees of decline after VATS, consistent with previous research findings. Regarding MWA, Wu et al.'s study demonstrated that among 35 patients with pulmonary ground-glass nodules, pulmonary function experienced only a temporary decline after MWA and returned to baseline within six months (36). A retrospective study involving 133 MWA patients showed that pulmonary function could return to baseline levels within one month after ablation (37). The findings of this study showed no significant changes in pulmonary function indicators 1–3 months after MWA, aligning with previous literature. MPLC is a clinically complex and challenging subtype of lung cancer. However, there is currently no standardized surgical treatment guideline for MPLC or for residual and recurrent lesions following lung cancer surgery. Traditional surgical treatment often requires the resection of a significant portion of lung tissue, which inevitably increases perioperative complications and mortality rates. As the extent of surgical resection increases—from wedge resection to segmentectomy, and ultimately to lobectomy—progressive loss of lung function becomes inevitable. In particular, patients undergoing total pneumonectomy have a significantly higher likelihood of requiring home oxygen therapy postoperatively, which considerably impacts their quality of life. In contrast, a combined approach of surgery and MWA offers the advantage of preserving lung function while maintaining the potential for curative treatment. Therefore, MWA serves as a crucial therapeutic modality, offering enhanced lung parenchyma preservation and presenting a novel treatment option for MPLC patients who may otherwise suffer from substantial lung tissue loss due to multiple surgical interventions.
Our study had certain limitations that should be mentioned. First, the retrospective design of this study inevitably introduces potential biases, particularly in patient selection and data collection. Although baseline characteristics were balanced using propensity score matching (PSM), confounding effects from unobserved variables may still exist. To reduce such biases, future studies should prioritize prospective designs or randomized group assignments. Second, this study analyzed disease progression and survival times of patients after two treatments. However, as the data inclusion began in January 2021 and the follow-up time was relatively short, this may limit the comprehensive assessment of long-term outcomes. To further validate the long-term efficacy of microwave ablation (MWA), the research team plans to extend the follow-up period to provide more reliable and comprehensive efficacy data. Third, as a single-center study, the representativeness of the sample in this study may be limited, affecting the external validity of the results. To enhance the generalizability and reliability of the findings, future studies plan to conduct multi-center research to validate and extend the conclusions. Finally, The lack of post-second treatment pulmonary function data in the surgical group limits the comprehensiveness of the inter-group comparison, which may have a certain impact on the accuracy of the results.
In conclusion, for MPLC patients with stage IA SPLC, VATS demonstrates superior local tumor control compared to MWA and offers greater clinical efficacy. Additionally, MWA offers significant advantages over VATS in terms of complication severity and lung function preservation. These findings highlight the significant clinical value of MWA in the treatment of MPLC. Therefore, we recommend that clinicians consider MWA as a key adjunctive therapy in the treatment of MPLC when developing treatment plans, and optimize the strategy based on the individual needs of patients, particularly for those who require lung tissue preservation and seek to minimize surgical risk. Although this study is a single-center retrospective analysis, future multi-center studies and long-term follow-up will further validate and enhance the generalizability of these results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Third Affiliated Hospital of Kunming Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
BL: Writing – original draft, Writing – review & editing. SG: Writing – original draft, Writing – review & editing. JM: Data curation, Visualization, Writing – review & editing. ZY: Data curation, Methodology, Writing – review & editing. YC: Formal Analysis, Methodology, Writing – review & editing. XW: Supervision, Writing – review & editing. YH: Methodology, Supervision, Writing – original draft, Writing – review & editing.
The authors declare that this study, author identity, and/or publication were supported by funding. The research was funded by the following grants: National Natural Science Foundation of China (82360562 and 82272980), Yunnan Provincial Department of Science and Technology Key Research Projects (202403AC100015 and 202405AS350015), and National Cancer Center (NCC201925B02).
I sincerely extend my heartfelt gratitude to my colleagues in the department for their support and guidance throughout this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tie H, Luo J, Shi R, Li Z, Chen D, Wu Q. Characteristics and prognosis of synchronous multiple primary lung cancer after surgical treatment: a systematic review and meta-analysis of current evidence. Cancer Med. (2020) 10(2):507–20. doi: 10.1002/cam4.3614
2. Niu N, Zhou L, Zhao J, Ma X, Yang F, Qi W. Sublobar resection versus lobectomy in the treatment of synchronous multiple primary lung cancer. World J Surg Oncol. (2023) 21(1):135. doi: 10.1186/s12957-023-02996-w
3. Beyreuther H. Multipicate von carcinomen bei einem fall von sog. Schenecberger Lungenkrebs mit Tuberkulose. (1924) 250(1-2):13. doi: 10.1007/bf01891568
4. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. (1975) 70(4):606–12. doi: 10.1016/s0022-5223(19)40289-4
5. de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume Ct screening in a randomized trial. N Engl J Med. (2020) 382(6):503–13. doi: 10.1056/NEJMoa1911793
6. Chen T-F, Xie C-Y, Rao B-Y, Shan S-C, Zhang X, Zeng B, et al. Surgical treatment to multiple primary lung cancer patients: a systematic review and meta-analysis. BMC Surg. (2019) 19(1):185. doi: 10.1186/s12893-019-0643-0
7. Chang ATC, Ng CSH, Nezami N. Treatment strategies for malignant pulmonary nodule: beyond lobectomy. Point-counterpoint. Curr Opin Pulm Med. (2024) 30(1):35–47. doi: 10.1097/mcp.0000000000001027
8. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (Jcog0802/Wjog4607l): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. (2022) 399(10335):1607–17. doi: 10.1016/s0140-6736(21)02333-3
9. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non–small cell lung cancer, version 3.2022, Nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network. (2022) 20(5):497–530. doi: 10.6004/jnccn.2022.0025
10. Vlaskou Badra E, Baumgartl M, Fabiano S, Jongen A, Guckenberger M. Stereotactic radiotherapy for early stage non-small cell lung cancer: current standards and ongoing research. Transl Lung Cancer Res. (2021) 10(4):1930–49. doi: 10.21037/tlcr-20-860
11. Huang G, Yang X, Li W, Wang J, Han X, Wei Z, et al. A feasibility and safety study of computed tomography-guided percutaneous microwave ablation: a novel therapy for multiple synchronous ground-glass opacities of the lung. Int J Hyperthermia. (2020) 37(1):414–22. doi: 10.1080/02656736.2020.1756467
12. Shang Y, Li G, Zhang B, Wu Y, Chen Y, Li C, et al. Image-guided percutaneous ablation for lung malignancies. Front Oncol. (2022) 12:1020296. doi: 10.3389/fonc.2022.1020296
13. Shao C, Zhi X, Mao S, Wu L, Yu J, Yang S, et al. Efficacy and safety of local ablative therapy for patients with Nsclc and coexisting interstitial lung disease. Thorac Cancer. (2024) 15(10):778–87. doi: 10.1111/1759-7714.15258
14. Quirk MT, Lee S, Murali N, Genshaft S, Abtin F, Suh R. Alternatives to surgery for early-stage non–small cell lung cancer: thermal ablation. Clin Chest Med. (2020) 41(2):197–210. doi: 10.1016/j.ccm.2020.02.002
15. Yang Q, Lc L, Fm L, Yi Q, Luo W. Survival outcomes of radiofrequency ablation compared with surgery in patients with early-stage primary non-small-cell lung cancer: a meta-analysis. Respir Investig. (2022) 60(3):337–44. doi: 10.1016/j.resinv.2022.01.002
16. Wang H, Zhou X, Xie D, Jiang S, Ding H, Gonzalez D, et al. Uniportal video-assisted thoracic surgery—the experiences of Shanghai pulmonary hospital. Journal of Visualized Surgery. (2016) 2:56. doi: 10.21037/jovs.2016.03.06
17. Ye X, Fan W, Wang Z, Wang J, Wang H, Wang J, et al. Expert consensus for thermal ablation of pulmonary subsolid nodules (2021 edition). Zhongguo Fei Ai Za Zhi. (2021) 24(5):305–22. doi: 10.3779/j.issn.1009-3419.2021.101.14
18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1). Eur J Cancer. (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
19. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
20. Wei S, Chen M, Chen N, Liu L. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol. (2017) 15(1):1–9. doi: 10.1186/s12957-017-1168-6
21. Cilleruelo-Ramos A, Cladellas-Gutiérrez E, de la Pinta C, Quintana-Cortés L, Sosa-Fajardo P, Couñago F, et al. Advances and controversies in the management of early stage non-small cell lung cancer. World J Clin Oncol. (2021) 12(12):1089–100. doi: 10.5306/wjco.v12.i12.1089
22. Zuin A, Andriolo LG, Marulli G, Schiavon M, Nicotra S, Calabrese F, et al. Is lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer? Eur J Cardiothorac Surg. (2013) 44(2):e120–e5. doi: 10.1093/ejcts/ezt219
23. Liu J, Wang C, Yi W, Zheng H, Zheng A. A retrospective comparative study of microwave ablation and sublobectomy in the treatment of early subpleural nonsmall cell lung cancer. Int J Hyperthermia. (2022) 39(1):1379–86. doi: 10.1080/02656736.2022.2136410
24. Laeseke P, Ng C, Ferko N, Naghi A, Wright GWJ, Zhang Y, et al. Stereotactic body radiation therapy and thermal ablation for treatment of Nsclc: a systematic literature review and meta-analysis. Lung Cancer. (2023) 182(5):107259–67. doi: 10.1016/j.lungcan.2023.107259
25. Wu C, Cao B, He G, Li Y, Wang W. Stereotactic ablative brachytherapy versus percutaneous microwave ablation for early-stage non-small cell lung cancer: a multicenter retrospective study. BMC Cancer. (2024) 24(1):304–14. doi: 10.1186/s12885-024-12055-6
26. Han X, Wei Z, Zhao Z, Yang X, Ye X. Cost and effectiveness of microwave ablation versus video-assisted thoracoscopic surgical resection for ground-glass nodule lung adenocarcinoma. Front Oncol. (2022) 12:962630. doi: 10.3389/fonc.2022.962630
27. Wang Y, Liu B, Cao P, Wang W, Wang W, Chang H, et al. Comparison between computed tomography-guided percutaneous microwave ablation and thoracoscopic lobectomy for stage I non-small cell lung cancer. Thorac Cancer. (2018) 9(11):1376–82. doi: 10.1111/1759-7714.12842
28. Hu X, Hu Q, He Y, Yi X, Wu Z, Hu H, et al. Efficacy and safety of microwave ablation and its synergistic potential in the treatment of early-stage non-small cell lung cancer. Clin Imaging. (2024) 107(11):110070–82. doi: 10.1016/j.clinimag.2023.110070
29. Zhang H, Chen L, Mao F, Li J, Hamaji M, Shimada Y, et al. Long-term prognosis analysis of surgical therapy for bilateral synchronous multiple primary lung cancer: a follow-up of 293 cases. J Thorac Dis. (2024) 16(2):1450–62. doi: 10.21037/jtd-23-1940
30. Chan MV, Huo YR, Cao C, Ridley L. Survival outcomes for surgical resection versus Ct-guided percutaneous ablation for stage I non-small cell lung cancer (Nsclc): a systematic review and meta-analysis. Eur Radiol. (2021) 31(7):5421–33. doi: 10.1007/s00330-020-07634-7
31. Deandreis DLS, Dromain C, Auperin A, Coulot J, Lumbroso J, Deschamps F, et al. Role of Fdg Pet/Ct and chest Ct in the follow-up of lung lesions treated with radiofrequency ablation. Radiology. (2011) 258(1):270–6. doi: 10.1148/radiol.10092440
32. Niezink A, de Jong RA, Muijs CT, Langendijk JA, Widder J. Pulmonary function changes after radiotherapy for lung or esophageal cancer: a systematic review focusing on dose-volume parameters. Oncologist. (2017) 22(10):1257–64. doi: 10.1634/theoncolo-gist.2016-0324
33. Suzuki K, Saji H, Aokage K, Watanabe S-i, Okada M, Mizusawa J, et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. (2019) 158(3):895–907. doi: 10.1016/j.jtcvs.2019.03.090
34. Nomori H, Yamazaki I, Machida Y, Otsuki A, Cong Y, Sugimura H, et al. Lobectomy versus segmentectomy: a propensity score-matched comparison of postoperative complications, pulmonary function and prognosis. Interact Cardiovasc Thorac Surg. (2022) 34(1):57–65. doi: 10.1093/icvts/ivab212
35. Watanabe T, Tanahashi M, Suzuki E, Yoshii N, Tsuchida H, Yobita S, et al. Surgical treatment for synchronous multiple primary lung cancer: is it possible to achieve both curability and preservation of the pulmonary function? Thorac Cancer. (2021) 12(22):2996–3004. doi: 10.1111/1759-7714.14164
36. Wu W, Peng J, Gao H, Lin Y, Lin Q, Weng Z. Factors associated with pulmonary function changes in patients undergoing microwave ablation for pulmonary ground-glass nodules. Technol Cancer Res Treat. (2022) 21(1):1–8. doi: 10.1177/15330338221094429
Keywords: multiple primary lung cancer, video-assisted thoracoscopic surgery, microwave ablation, complication, pulmonary function
Citation: Li B, Gao S, Mao J, Yang Z, Chen Y, Wang X and Huang Y (2025) A retrospective study of microwave ablation and thoracoscopic surgery for multiple primary lung cancer: a propensity score matching analysis. Front. Surg. 12:1547048. doi: 10.3389/fsurg.2025.1547048
Received: 17 December 2024; Accepted: 26 February 2025;
Published: 11 March 2025.
Edited by:
Yo Kawaguchi, Shiga University of Medical Science, JapanReviewed by:
Mohamed Rahouma, Weill Cornell Medical Center, NewYork-Presbyterian, United StatesCopyright: © 2025 Li, Gao, Mao, Yang, Chen, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunchao Huang, ZG9jeXVuY2hhb2h1YW5nQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.