- 1Faculty of Medicine, Bogomolets National Medical University (NMU), Kyiv, Ukraine
- 2Faculty of Medicine, Alborz University of Medical Sciences (ABZUMS), Karaj, Iran
Postprandial hypoglycemia (PPH) is a challenging and significant complication that can occur following bariatric and metabolic surgery. Symptoms of PPH are typical of hypoglycemia, such as sweating, weakness, disorientation, palpitation, etc. The complex nature of PPH is essential to achieve accurate diagnosis and effective management. This review aims to give extensive coverage of the intricate nature of PPH common with bariatric and metabolic surgery, outlining its pathogenesis, risk factors, clinical presentation, diagnostic strategies, and treatment options. The study explores various clinical forms and pathogenic mechanisms behind PPH while discussing diagnostic tools like continuous glucose monitoring or mixed meal tolerance tests. Furthermore, it considers possible interventions, including dietary changes, pharmaceutical therapies, and surgeries, to relieve symptoms and improve patient's quality of life. It aims to comprehensively understand how healthcare professionals can effectively manage this disorder for patients undergoing bariatric and metabolic surgery.
1 Introduction
Hypoglycemia after meals is known as postprandial hypoglycemia (PPH) and can induce symptoms including perspiration, weakness, disorientation, and palpitations due to low blood glucose levels (1, 2). It is a serious complication, particularly in patients who have undergone upper gastrointestinal (GI) tract surgery, such as bariatric and metabolic surgery, because it can have a significant impact on GI physiological functions, recovery, quality of life, and patients’ health. This disorder involves elevated insulin production during meals, which causes modest to severe hypoglycemia. Appropriate management and treatment options are required to improve patient outcomes and quality of life (3–6).
In recent years, there has been growing recognition of the prevalence and significant impact of PPH in patients who have undergone bariatric and metabolic surgery (4). Despite its clinical importance, PPH must still be better understood and under-researched. With the increasing incidence of upper GI disorders, the challenges in diagnosis, and the negative impact on patient well-being, PPH represents a significant concern in gastroenterology. This led to increased interest in understanding its underlying mechanisms, identifying those most at risk, and determining practical management approaches. However, the available information could be more cohesive and consistent, making it easier for healthcare providers to synthesize and apply it effectively.
This review seeks to provide a thorough summary of PPH following bariatric and metabolic surgeries, covering its frequency, symptoms, diagnostic techniques, and treatment choices. By consolidating this information, the objective is to enhance comprehension, improve clinical decision-making, and ultimately achieve better outcomes for patients grappling with this complex condition.
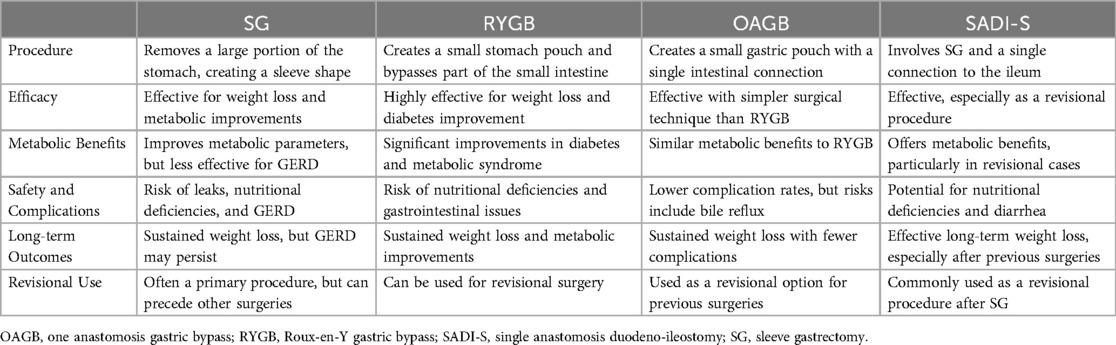
2 Review of bariatric and metabolic surgeries
Bariatric surgery remains the most effective long-term treatment for morbid obesity type-2 diabetes and metabolic syndrome, with recent updates expanding eligibility and improving safety and outcomes (7). In this section, we review the four main types of these surgeries.
2.1 Sleeve gastrectomy (SG)
Sleeve gastrectomy (SG) is one of the most commonly performed bariatric surgical procedures aimed at inducing body mass loss and improving metabolic profile in patients with morbid obesity. In this procedure, 75%–80% of the stomach is laparoscopically removed, forming a sleeve-like organ that limits food consumption and satiety-inducing changes in gut hormones (8). SG is highly effective for achieving clinically significant weight loss, with an average total body weight loss of 23% in one year and 16% following five years postoperatively (9, 10). While SG is technically less complex than RYGB and safer in general, it may be inferior to RYGB for treating some comorbid conditions, including gastroesophageal reflux disease (GERD). Despite its benefits, SG is associated with hazards such as leaks, nutritional inadequacies, and the development of GERD, emphasizing the significance of long-term follow-up and lifestyle changes for long-term success (11–10).
2.2 Roux-en-Y gastric bypass (RYGB)
Roux-en-Y Gastric Bypass (RYGB) is an established bariatric procedure that delivers weight loss and improves metabolic health, particularly for patients with obesity and its comorbidities, such as type 2 diabetes (12). This procedure involves creating a small stomach pouch and attaching it directly to the lower part of the small intestine, bypassing most of your stomach and upper duodenum and reducing calorie absorption (13). RYGB is an effective operation that results in substantial weight loss and superior glycemic control compared to intensive lifestyle management. It has broader metabolic effects that reduce the risk of long-term renal impairment and cardiovascular risk factors. While RYGB may be superior to other bariatric procedures, including sleeve gastrectomy for glycemic control and triglyceride reduction, the SG might lead to a more significant BMI (14, 15). RYGB is associated with long-term weight loss and co-morbidity resolution but carries the risks of malnutrition and gastrointestinal adverse events, necessitating judicious patient selection in conjunction with life-long follow-up for optimal outcomes (16).
2.3 One anastomosis gastric bypass (OAGB)
One Anastomosis Gastric Bypass (OAGB) is a bariatric surgery that promotes weight loss and improves metabolic health, serving as a simpler alternative to the traditional RYGB (17, 18). This procedure involves creating a small gastric pouch and connecting it directly to the small intestine, bypassing a portion of the stomach and initial small intestine. OAGB is effective in achieving significant weight loss and addressing obesity-related conditions like type 2 diabetes and hypertension. It is generally safer with fewer complications than other bariatric surgeries, though risks include nutritional deficiencies, bile reflux, and marginal ulcers (19, 20). Additionally, OAGB can be used as a revisional surgery for patients with inadequate results from previous procedures. Compared to RYGB, OAGB offers similar outcomes with a simpler surgical technique, making it a viable option for those seeking bariatric surgery, provided there is careful patient selection and diligent postoperative management (20).
2.4 Single anastomosis duodeno-ileostomy with sleeve gastrectomy (SADI-S)
Single Anastomosis Duodeno-ileostomy with Sleeve Gastrectomy (SADI-S) is a bariatric surgery that effectively combines restrictive and malabsorptive techniques to promote significant weight loss and metabolic improvement. By creating a single connection between the duodenum and ileum, this procedure bypasses a large portion of the small intestine, reducing nutrient absorption (21, 22). SADI-S is especially beneficial as a revisional surgery for patients who have experienced insufficient weight loss or regain after an initial sleeve gastrectomy, showing favorable results compared to other bariatric procedures. While effective, SADI-S requires careful postoperative management due to potential complications such as nutritional deficiencies and gastrointestinal issues (23).
2.5 Comparison
Table 1 summarizes and compares the four main types of bariatric and metabolic surgeries. The choice of procedure depends on individual patient needs, comorbidities, and surgical goals. Long-term follow-up and lifestyle changes are crucial for the success of any bariatric surgery.
3 Incidence and prevalence of PPH
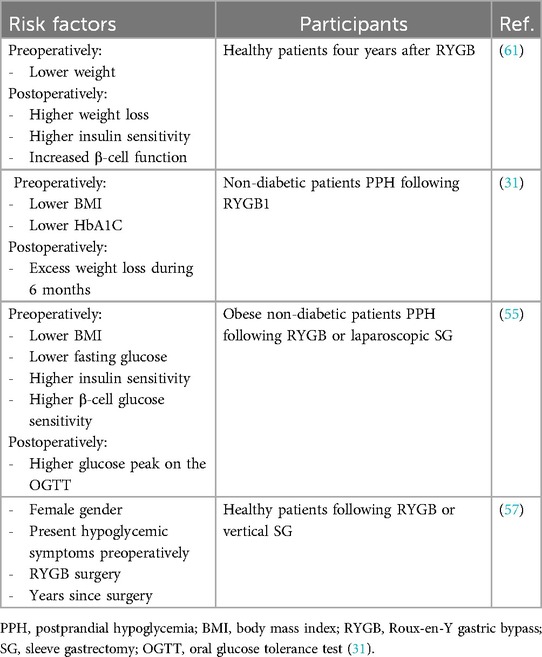
Different studies have reported widely varying incidence rates for PPH, ranging from 10% to 72%. The wide range is due to the differences in study populations, diagnostic criteria, follow-up durations, and timing of assessment, as well as evaluation tools used (24–26). These figures are based on patients’ admission notes or self-reports concerning related symptoms (27). Except for some case studies, there are no published incidence rates of hypoglycemia following bariatric and metabolic surgery (4, 28). Studies have reported that the incidence of PPH varies depending on the type of surgery undergone (25, 29). Over five years, a study found that the occurrence of hyper-insulinemic PHH after RYGB surgery started at 0.5% before the surgery. It then increased to 9.1% at 12 months post-surgery and slightly decreased to 7.9% at 60 months (5 years) after the surgery (30).
A randomized trial comparing SG to RYGB found that 14% of SG patients had reactive hypoglycemia (blood glucose <3.1 mmol/L after 75-g oral glucose load) one year after surgery. This implies that, while hypoglycemia can occur after SG, it may be less common than after RYGB (29). A retrospective clinical study found that the incidence of dumping syndrome after OAGB (42.9%) was lower than that observed after RYGB (56.4%) but significantly higher than after SG (15.6%) (29). A study indicated that over time, revealing that the cumulative occurrence of RYGB hypoglycemia rose from 2.7% to 13.3% between the first- and fifth years post-surgery. The PPH following bariatric and metabolic surgery might probably be associated with a lower preoperative body mass index (BMI), reduced levels of HbA1c, and a higher percentage of excess weight loss (31).
4 Pathophysiological mechanisms of PPH
Previous studies have suggested some theories that the basic pathophysiological mechanisms of PPH after upper bariatric and metabolic surgeries may include hypersecretion of incretin, sensitivity or resistance to insulin, dysregulation of the “intrapancreatic axis,” and alpha-cell dysfunction. However, the exact pathophysiological mechanism of PPH is unclear (25, 32–34).
The exact mechanisms of PPH following bariatric and metabolic surgeries are not fully understood, but several hypotheses have been proposed:
1) Incretin hypersecretion: One of the leading hypotheses is the exaggerated secretion of incretin hormones, primarily glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), in response to the accelerated delivery of nutrients to the small intestine after these surgeries (25, 35, 36). GLP-1 secretion significantly increases after RYGB and, to a lesser extent, after SG compared to non-operated individuals (36). GIP secretion is lowest after RYGB but remains elevated after SG (36). The exaggerated incretin response, especially GLP-1, leads to an inappropriate and excessive stimulation of insulin secretion, resulting in postprandial hyperinsulinemic hypoglycemia (25, 35).
2) Changes in insulin kinetics, including increased insulin secretion, decreased hepatic insulin clearance, and altered insulin sensitivity, have been implicated in PPH (37, 38). Increased beta-cell glucose sensitivity and insulin secretion have been observed after both RYGB and SG (36, 39). Diminished hepatic insulin extraction may contribute to higher circulating insulin levels and hypoglycemia risk (37). Rapid weight loss and changes in insulin sensitivity may also play a role in the development of PPH (25).
3) Dysregulation of Other Hormones. Disturbances in regulating other hormones, such as glucagon, glicentin, and ghrelin, have been proposed as potential contributors to PPH. Alpha-cell dysfunction and impaired glucagon secretion may exacerbate PPH. Increased postprandial secretion of glicentin, a marker of PPH risk, has been observed in some studies. Disrupted negative feedback between insulin and ghrelin may contribute to the pathogenesis of PPH (25, 35, 40).
4) Anatomical and Physiological Changes. The altered anatomy and physiological changes following bariatric and metabolic surgeries, such as accelerated gastric emptying, intestinal transit time, and nutrient absorption, are thought to play a role in developing PPH. Rapid gastric emptying and nutrient delivery to the small intestine after RYGB may contribute to the exaggerated incretin and insulin responses. Changes in gut hormone secretion patterns and intestinal adaptation after surgery may also be involved (41, 42).
The rapid influx of carbohydrates into the small intestine following bariatric surgery increases the release of incretin hormones such as GLP-1. This excessive secretion of insulin by the pancreas in reaction to the incretins is a significant contributor to postprandial hyperinsulinemic hypoglycemia (43). Increased tissue sensitivity to insulin, mediated by factors like insulin-like growth factor-1, can also promote the development of hypoglycemia (25). Besides the incretin effect, nutrient passage through the GI tract may trigger harmful feedback mechanisms (anti-incretins) to counterbalance the effects of glucose-lowering. Alterations in this balance, resulting from bypassing the duodenum, jejunum, and a portion of the ileum during bariatric procedures, can induce postprandial hyperinsulinemic hypoglycemia (33).
Postoperative metabolic hypoglycemia is partly caused by altered gastric emptying of ingested food, resulting in rapid glucose absorption in the intestine and extreme postprandial secretion of GI peptides, particularly GLP-1 (4). Mismatches between the time of insulin secretion and glucose absorption (44) or insulin over-secretion (45) are the main known reasons, which seem to be multifactorial, but the primary regulator is interleukin 1-β (46). The rise of incretin hormones such as GIP (44) and GLP-1 was reported in many studies after gastric bariatric surgery (34, 44, 47–49) and vagotomy subjects with pyloroplasty (50).
Rapid weight loss and regression of insulin resistance after bariatric surgery may lead to a slower normalization of insulin production, contributing to hypoglycemia. Changes in the activity of pancreatic alpha cells, responsible for glucagon secretion, may also contribute to the onset of PPH (25).
GLP-1 is hypersecretion from L cells (50) and hypertrophies β cells via enhanced expression of the transcription factor of islet cells and duodenal homeobox-1 protein (47). The process of hypertrophy and hyperplasia of β cells has been named nesidioblastosis (51, 52) which has been known to have a role in hyperinsulinemia (51–53). However, this finding has not been seen in most reported cases (49, 54).
Figure 1 illustrates a summary of the etiological mechanisms of hypoglycemia that can occur following bariatric and metabolic surgeries.
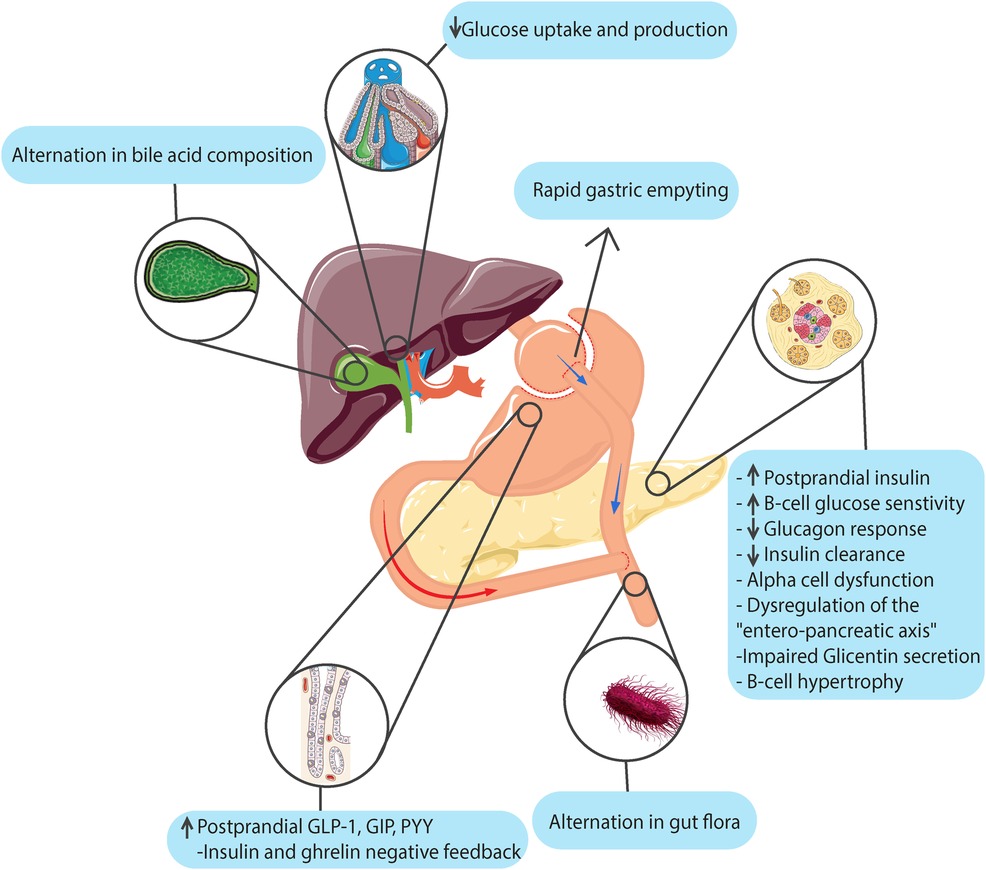
Figure 1. Etiological mechanisms of hypoglycemia following bariatric and metabolic surgeries. (This image is designed and generated by the authors).
5 Risk factors for PPH following bariatric and metabolic surgery
Preoperative factors include lower BMI, higher insulin sensitivity, preoperative hypoglycemic symptoms, female sex (34, 55, 56). Studies have found that patients with a lower preoperative BMI are at a higher risk of developing PPH after RYGB and SG. This association may be related to higher insulin sensitivity in leaner individuals (55, 56). Patients with higher preoperative insulin sensitivity, measured by indices like the oral glucose insulin sensitivity index, are more likely to experience PPH after RYGB and SG (55). The presence of preoperative symptoms suggestive of hypoglycemia has been identified as a significant risk factor for developing PPH after bariatric surgery (31). Several studies have reported a higher prevalence of PPH symptoms in female patients after RYGB and SG (34, 56).
Surgical factors include the bariatric procedure type and the time since surgery. RYGB has been consistently associated with a higher risk of PPH compared to SG. The incidence of severe hypoglycemic episodes requiring hospitalization is also higher after RYGB. A longer duration since the bariatric surgery has been linked to an increase (31, 34, 57)
Overall, risk factors associated with PPH following bariatric and metabolic surgeries as identified in retrospective epidemiological studies comprise female gender, younger age, absence of diabetes diagnosis before surgery, history of pre-surgery hypoglycemia unrelated to diabetes or diabetes medications, lower pre-surgery hemoglobin A1C (HbA1C) levels, and increased excess weight loss after the operation (31, 58). Patients with reduced BMI after bariatric surgery are at increased risk of postprandial hyperinsulinemic hypoglycemia, particularly those with high insulin secretion and beta-cell function pre-surgery. Younger individuals and those undergoing upper bariatric and metabolic surgery are more susceptible. Rapid weight loss, improved insulin sensitivity post-surgery, and faster carbohydrate absorption and incretin hormone imbalance contribute to this risk (38, 59, 60).
It has been shown that elevated pre-surgery plasma glucose levels, increased insulin sensitivity, and heightened beta-cell glucose sensitivity are significant predictors of spontaneous self-reported PPH following RYGB and laparoscopic SG (55). Younger age, lower preoperative BMI, and high postprandial beta-cell activity are associated with a higher risk of developing PPH (58).
Table 2 presents the risk factors for PPH following bariatric and metabolic surgeries.
6 Clinical manifestations and complications of PPH following bariatric and metabolic surgery
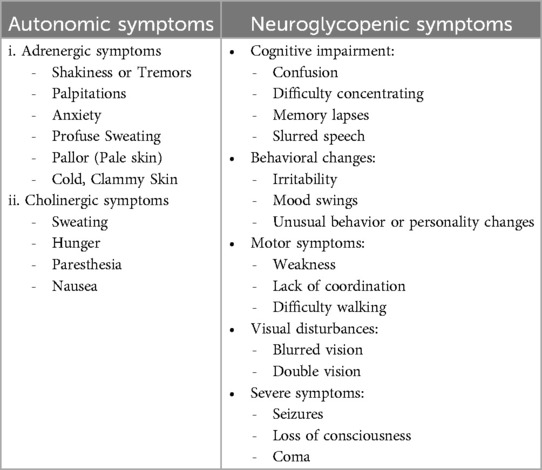
Clinical manifestations in PPH following bariatric and metabolic surgeries depend on the severity of hypoglycemia. Heart palpitations, anxiety and disorientation, hunger, perspiration, excitation, tremors, and paresthesia are among the unsettling symptoms that mild to moderate hypoglycemia can produce. In contrast, severe hypoglycemia may manifest as drowsiness, delirium, disorientation, seizures, and comes (62, 63). Early dumping symptoms, which might include diarrhea, palpitations, lightheadedness, extreme weariness, nausea, and vomiting, usually appear 10–30 min after a meal. Usually, glucose levels are not low at the onset of these symptoms (48). These symptoms can be seen when the blood glucose is less than 55 mg/dl. However, this scale can be shifted lower when a person currently has hypoglycemia (52).
Hypoglycemia symptoms are nonspecific, making differential diagnosis crucial. These symptoms fall into two categories: neuroglycopenic and autonomic. Neuroglycopenic symptoms arise from central nervous system deprivation and range from mild (e.g., blurred vision, dizziness, flushing, drowsiness, fatigue, weakness) to severe (e.g., seizures, loss of consciousness, confusion, difficulty speaking). Autonomic symptoms result from the activation of the autonomic nervous system and are divided into adrenergic (e.g., shakiness, heart-pounding, anxiety) and cholinergic (e.g., sweating, hunger, paresthesia) (64) (Table 3).
Severe PPH can lead to severe neuroglycopenic symptoms such as seizures, disorientation, loss of consciousness, and even hypoglycemic coma. These neuroglycopenia symptoms often occur with fainting spells, especially after large meals, and can be mistaken for other conditions, necessitating careful diagnostic workup (65). Severe hypoglycemia also may cause motor vehicle accidents, falls, and even death. Associated disability and loss of quality of life and this situation cannot be healed during this time (66).
6.1 Dumping syndrome
Dumping syndrome is a disorder that may develop following bariatric and metabolic surgery, which occurs when food, particularly sugar, passes from the stomach to the small colon too rapidly. There are two forms of dumping syndrome: early and late dumping syndrome (67–69).
Early dumping syndrome occurs 10 to 30 min after eating. It presents with symptoms like nausea, vomiting, abdominal cramps, diarrhea, dizziness, and rapid heart rate, resulting from the rapid movement of food into the small intestine, causing fluid shifts and blood pressure changes. It is commonly seen after surgeries like gastrectomy or gastric bypass, which disrupt normal stomach function and emptying. Management typically involves dietary adjustments, such as eating smaller, more frequent meals, avoiding high-sugar foods, and increasing fiber intake, with medications used in some cases to slow gastric emptying (67, 69, 70).
Late dumping syndrome, which occurs 1 to 3 h after eating, is characterized by hypoglycemia, weakness, sweating, dizziness, and disorientation caused by an increased insulin response owing to fast sugar absorption in the small intestine (71–73). It is associated with procedures that alter stomach function, similar to early dumping syndrome, but the essential distinction is in time and the insulin-related mechanism (72). Management includes dietary changes such as preferring complex carbs, increasing protein intake, and avoiding fluids with meals. In some situations, medications such as acarbose or diazoxide may be needed. Both early and late dumping syndromes may have a major impact on quality of life, but with adequate therapy, symptoms are typically efficiently managed (74).
7 Diagnosis of PPH following bariatric and metabolic surgeries
7.1 Clinical considerations
Currently, there are no established clinical guidelines for diagnosing PPH. Collecting a detailed disease history is crucial, and provocative tests have been proposed for detection. A comprehensive clinical history and physical examination can help identify the underlying reason and guide further diagnostic tests. Non-diabetic hypoglycemia should be evaluated and managed individually depending on clinical symptoms and probable diagnosis (75). Patients show postoperative episodes of hypoglycemia with adrenergic, cholinergic, and neuroglycopenic signs and symptoms (45, 76). Whipple's triad is a diagnostic tool with specific parameters to identify hypoglycemia. The three components of hypoglycemia are symptoms, hypoglycemia, and relief after rising plasma glucose concentration (77).
Besides venous blood glucose testing, several other diagnostic techniques can be useful to diagnose PPH, such as continuous glucose monitoring (CGM), Glycemic pattern, Histopathology, Selective arterial calcium stimulation test, and Radiological investigation (78).
Based on specific blood glucose level thresholds, the seriousness of hypoglycemia is classified as (79, 80):
i. Mild hypoglycemia: Blood glucose levels between 54 and 70 mg/dl (3.0–3.9 mmol/L)
ii. Moderate hypoglycemia: Blood glucose levels between 40 and 54 mg/dl (2.2–3.0 mmol/L)
iii. Severe hypoglycemia: Blood glucose levels ≤40 mg/dl (2.2 mmol/L)
7.2 Blood glucose testing
Blood glucose testing is essential for diagnosing and managing this condition. Standard tests in actual practice include the oral glucose tolerance test (OGTT), the mixed meal tolerance test (MMTT) (63), and continuous glucose monitoring (CGM) (81, 82).
The OGTT is commonly used to diagnose hypoglycemia. It involves administering a 75-g glucose load and measuring blood glucose levels at various intervals. However, this test can sometimes lead to over-diagnosing improved glucose tolerance due to postprandial hyper-insulinemic hypoglycemia observed in many post-surgery patients (83).
Another method is the Mixed Meal Tolerance Test (MMTT), which uses a meal containing carbohydrates and fats equivalent to 75 g of glucose. It is considered more reflective of real-life conditions than the OGTT. The MMTT has shown that post-surgery patients often do not exhibit hypoglycemia, indicating any improvement in glucose tolerance compared to pre-surgery data (83, 84).
Continuous glucose monitoring (CGM) is increasingly used to diagnose and manage PPH. It provides continuous data on glucose levels, capturing fluctuations that might be missed with intermittent point-of-care (POC) blood glucose checks. CGM has been essential in detecting asymptomatic hypoglycemia and glycemic excursions in pediatric and adult patients’ post-surgery (81, 82, 84).
Studies showed that the MMTT effectively detects PPH and severe hypoglycemic events, particularly in patients with persistent post-bariatric hypoglycemia during long-term follow-up. CGM complements the MMTT by identifying asymptomatic hypoglycemia, fasting hypoglycemia, and glucose variability over an extended period. Combining both tests may provide the most comprehensive assessment for diagnosing persistent post-bariatric hypoglycemia (84, 85). Maia et al. showed that the CGMS effectively detects PPH and improves therapeutic management but has low sensitivity to detect unrecognized hypoglycemia in type 1 diabetes patients (86). Baseline parameters, such as HbA1c and weight loss, can help predict PPH in patients after gastric bypass surgery, aiding in screening and selecting those requiring further evaluation (46).
Venous samples are recommended for testing glucose concentration because capillary blood glucose can falsely be lower in the setting of relative hypotension and Raynaud’s disease (78).
7.3 Diagnostic medical imaging
Diagnostic medical imaging plays a crucial role in evaluating and differentially diagnosing PPH after upper GI surgery, particularly in ruling out other causes of hypoglycemia, like insulinoma, as the underlying cause (87).
The diameter of the gastroenterostomy has a considerable impact on quick stomach emptying, which is a crucial determinant in the development of PPH (88). This connection must be considered while doing medical imaging since a bigger diameter may result in faster food transit into the jejunum. This expedited process might cause an excessive insulin response, which contributes to the symptoms of PPH (89). Understanding the gastroenterostomy's features during imaging examinations can help effectively identify and treat individuals with postprandial hypoglycemia.
CT volumetry is a diagnostic imaging technique used to evaluate anatomical changes after bariatric and metabolic surgery (90). It primarily assesses gastric reservoir capacity and its association with clinical outcomes such as weight loss and problems. However, according to current literature, its direct relevance in identifying PPH after bariatric surgery is not well-established (87, 90).
Computed Tomography (CT) Scan is often the initial imaging modality to assess the pancreas and surrounding structures for potential insulinomas or other pancreatic lesions. However, CT scans may not detect small insulinomas, limiting their diagnostic utility (91, 92).
Endoscopic ultrasound (EUS) is the most sensitive imaging technique for detecting small pancreatic lesions, including insulinomas. It allows for high-resolution visualization of the pancreas and can guide fine-needle aspiration (FNA) for cytological evaluation if a suspicious lesion is identified (92).
Selective arterial calcium stimulation test (SACST) is an invasive procedure that involves injecting calcium gluconate into the arteries, supplying the pancreas with insulin to stimulate insulin release from potential insulinomas. It can help localize the source of excessive insulin production when imaging is inconclusive (93).
8 Management and treatment of PPH after upper GI surgery
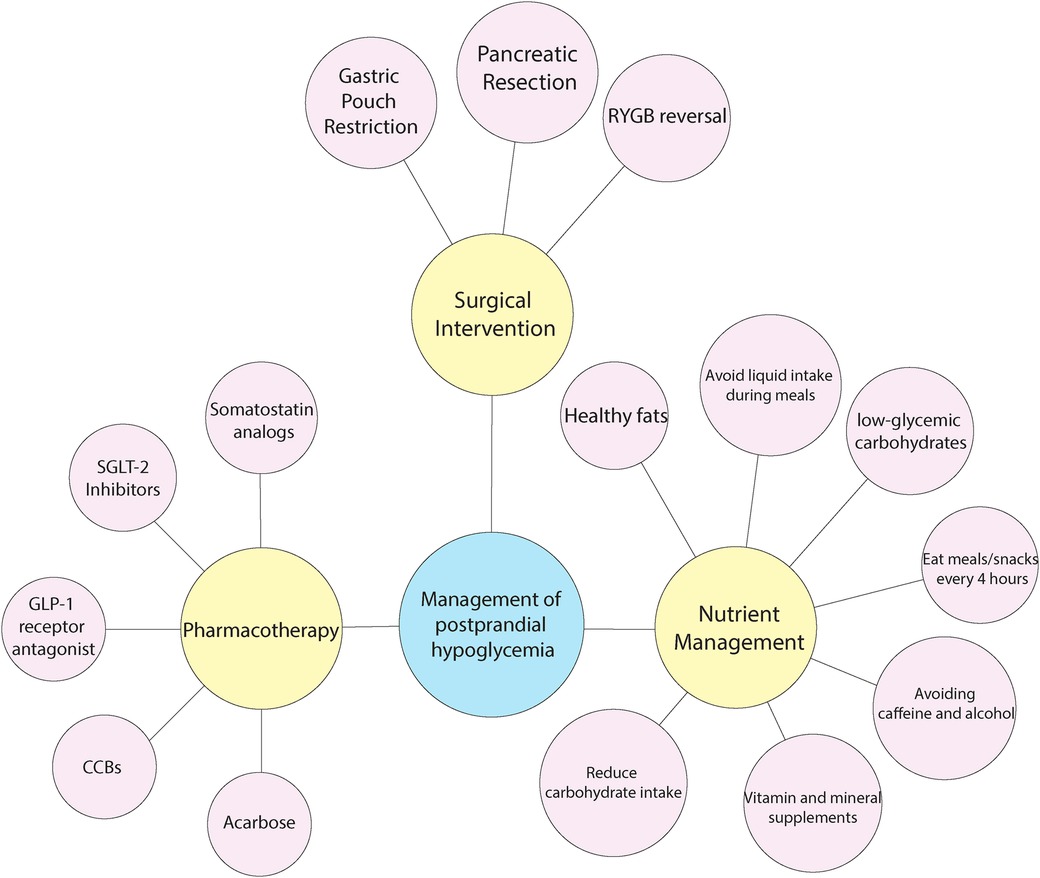
Managing PPH after upper GI surgery is challenging, aimed at stabilizing blood sugar levels and preventing sharp drops that lead to hypoglycemia. Practical strategies for managing PPH, especially in individuals who have undergone bariatric surgery, include a combination of lifestyle changes, exercise, medication, and surgical interventions. A personalized, multidisciplinary strategy tailored to each patient's specific requirements is crucial for successfully managing PPH (53, 94). (see Figure 2).
8.1 Lifestyle modifications and exercise
Postprandial exercise has been studied for its effects on glucose levels. A study by Ternhamar et al. concluded that moderate-intensity exercise shortly after meal intake did not significantly lower plasma nadir glucose levels in RYGB patients. However, replacing high-glycemic-index meals with low-glycemic-index meals showed some benefit in reducing glucose excursions (95).
8.2 Nutrition management
Nutritional management is defined as “Adjusting the quantity and quality of food intake to improve an individual's health status (96). Accordingly, nutritional therapy for post-bariatric hypoglycemic patients aims to reduce rapid glucose rise after meal consumption (97). Patients are often advised to consume small, frequent meals with low glycemic index carbohydrates combined with proteins and fats to manage PPH (32). It is known that high-carbohydrate, low-protein meals cause hypoglycemia more strongly (98). Therefore, hypoglycemia is associated with the type of food consumed, and dietary modifications could be a possible treatment for post-prandial hypoglycemia. Eating small but numerous meals is an appropriate option also for pregnant patients (99). It is proved that reducing the amount of carbohydrates, combined with a higher protein consumption, lowers the risk of hypoglycemia by decreasing insulin secretion (100). Generally, most hypoglycemic patients (mild to moderate cases) are supposed to be cured with regimen adjustments (55, 101, 102). However, severe cases do not respond to diet modifications (103).
Suhl et al. (97) studied medical nutrition therapy in post-prandial hypoglycemic patients. They indicate 10 points for nutritional management, including consumption of low-glycemic carbohydrates, healthy fats, high but calculated amounts of protein, and avoiding caffeine and alcohol. They emphasize vitamin and mineral supplements. In addition, patients should avoid liquid intake during meals and eat meals/snacks every 4 h. A case series by Abrahamsson et al. (47) approves this point. They also suggest a low-carbohydrate, protein-rich diet before starting pharmacotherapy. Other studies focus mainly on the amount of carbohydrates. It is known that limiting carbohydrate intake and eating multiple meals is a successful dietary modification (104).
Applying nutritional management for hypoglycemic patients has some difficulties; for example, hyperinsulinemic hypoglycemic patients’ tendency towards carbohydrate consumption increases (105). Furthermore, most patients need better nutritional knowledge and undesirable food habits. These factors affect the success of nutritional management and should be considered for the patients’ management (94, 97, 106).
8.3 Pharmacological therapy
Pharmacotherapy is essential for managing PPH following bariatric and metabolic surgery when lifestyle modifications and dietary changes are insufficient. It modulates insulin secretion, delays carbohydrate absorption, stabilizes blood glucose levels, and alleviates symptoms (107). Pharmacotherapy offers several options for managing PPH. The choice of medication depends on the specific pathophysiological mechanism and the patient's overall health. Combining medication with dietary changes and continuous glucose monitoring can effectively manage this condition. Close monitoring by a healthcare professional is essential to adjust treatment plans and manage any potential side effects.
Numerous studies showed that these pharmacological groups have potential therapeutic effects on PPH. They are SGLT2 Inhibitors and IL-1 Antagonists (108–110)., GLP-1 Receptor Antagonists (47, 111, 112), GLP-1 Receptor Agonists (47, 111) Calcium Channel Blockers, and Acarbose (113), Somatostatin Analogs (114), and Diazoxide (115).
- SGLT2 Inhibitors and IL-1 Antagonists: Empagliflozin, an SGLT2 inhibitor, and Anakinra, an IL-1 receptor antagonist, both significantly reduce postprandial insulin release and prevent hypoglycemia in patients after gastric bypass surgery (108–110).
- GLP-1 Receptor Antagonists: GLP-1 receptor antagonists, such as exendin (11–39), correct hypoglycemia by reducing postprandial insulin secretion and stabilizing glucose levels in patients with hyperinsulinemic hypoglycemia after gastric bypass (47, 111, 112).
- GLP-1 Receptor Agonists: GLP-1 receptor agonists have shown potential in managing PPH by stabilizing glucose levels without causing hypoglycemia, although more controlled studies are needed to confirm their efficacy (47, 111).
- Calcium Channel Blockers and Acarbose: Verapamil, a calcium channel blocker, and acarbose, an alpha-glucosidase inhibitor, have been used to reduce the frequency and severity of hypoglycemic episodes in patients with non-insulinoma pancreatogenous hypoglycemic syndrome (NIPHS) after bariatric surgery (113)
- Somatostatin Analogs: Octreotide, a somatostatin analog, has effectively managed postprandial hyperinsulinemic hypoglycemia by attenuating the exaggerated postprandial insulin and incretin response, leading to significant symptom relief (114).
- Diazoxide: Diazoxide, a KATP channel opener, has successfully managed severe PPH in patients after RYGBby reducing insulin secretion (115).
Hepprich et al. showed that SGLT2-inhibitors and IL-1 antagonism may improve PPH after gastric bypass surgery by reducing glucose-induced IL-1 and preventing hypoglycemia (108).
The PID algorithm accurately and safely adjusts glucose infusion rate for post-prandial hypoglycemic clamps in both healthy and bariatric surgery patients, ensuring standardized results (116).
8.4 Surgical interventions
Surgical interventions for PPH, such as gastric bypass reversal, partial gastrectomy, or pancreatic resection, are typically considered only when non-surgical treatments like dietary modifications, pharmacotherapy, and continuous glucose monitoring have failed. These surgeries aim to address severe cases by altering the GI anatomy or managing excessive insulin production, but they carry significant risks and are usually reserved for the most refractory cases (53, 117). Surgical procedures will slow the gastric reserve's rapid transit to the intestine or restore the GI system to its typical structure (118).
Surgical intervention for severe post-RYGB hypoglycemia includes pancreatic resection, RYGB reversal, and gastric pouch restriction, with resolution of symptoms in 67%, 76%, and 82% of patients, respectively (119, 120).
Gastric pouch restriction is the most commonly performed surgical treatment for PPH after RYGB. Treatment options include procedures like pouch banding and/or pouch resection, which aim to control the size of the gastric pouch and reduce the severity of hypoglycemic episodes (121).
In rare circumstances, a partial gastrectomy may be done to lower the size of the stomach pouch, which can assist delay gastric emptying and minimize the risk of hypoglycemic episodes. However, this technique is more intrusive and has serious dangers (53).
Partial pancreatic resection, though controversial and typically reserved for severe cases, has been considered in managing PPH linked to hyperinsulinemia caused by nesidioblastosis. However, this approach is not recommended since PPH is primarily due to alterations in digestive anatomy rather than pancreatic β-cell proliferation. Despite some success in symptom resolution, partial or complete pancreatectomy carries significant risks, including high postoperative morbidity, mortality, and a high likelihood of symptom recurrence.
Partial pancreatic resection, though controversial and typically reserved for severe cases, has been considered in managing PPH linked to hyperinsulinemia caused by nesidioblastosis (53, 119). However, this approach is not recommended since PPH is primarily due to alterations in digestive anatomy rather than pancreatic β-cell proliferation. Despite some success in symptom resolution, partial or complete pancreatectomy carries significant risks, including high postoperative morbidity, mortality, and a high likelihood of symptom recurrence (118, 119).
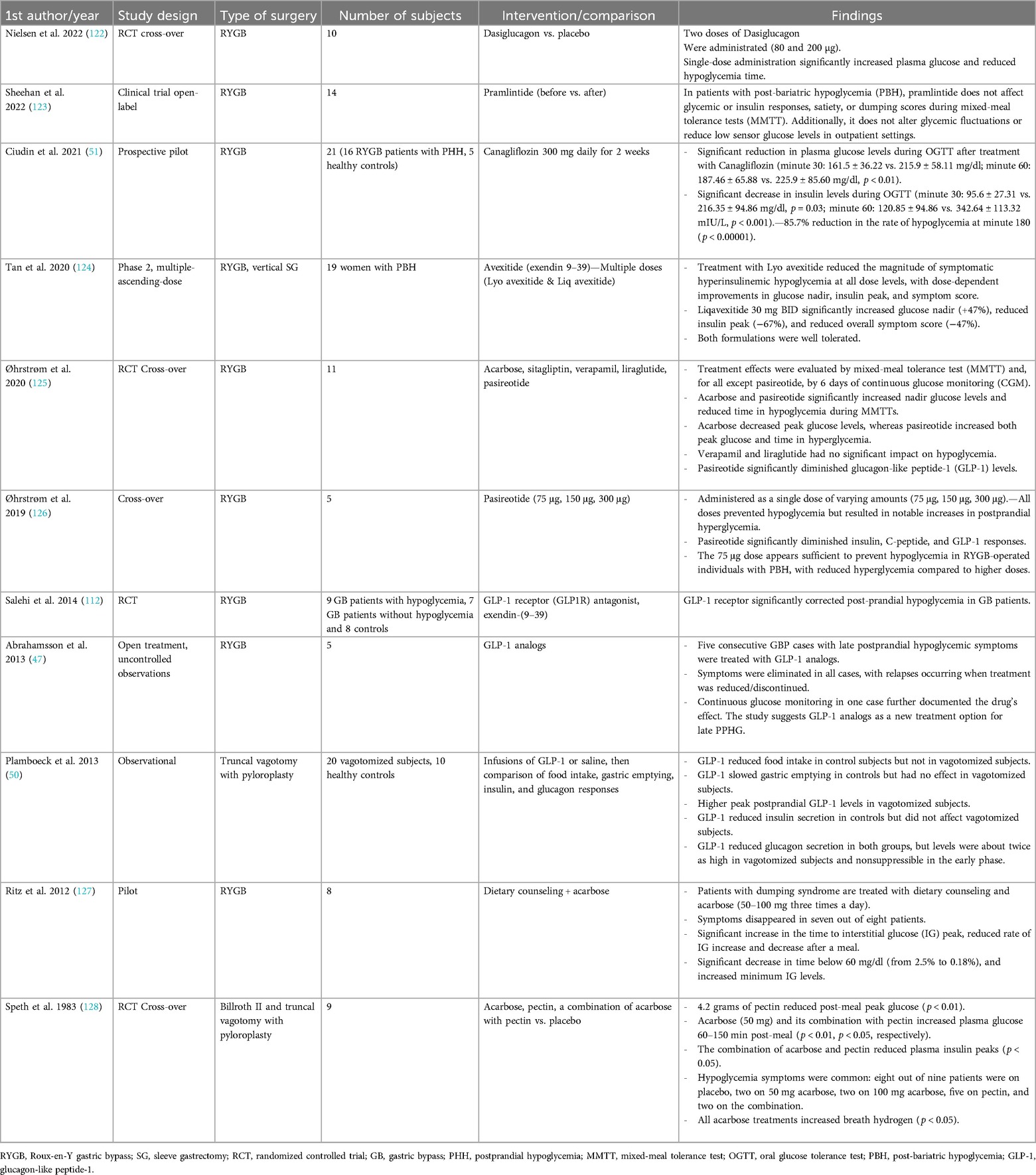
A summary of various studies on the treatment of PPH is exhibited in Table 4.
9 Conclusion
In summary, PPH following bariatric and metabolic surgery presents with a range of neuroglycopenic and adrenergic symptoms, and its diagnosis remains challenging due to the lack of standardized clinical guidelines. The primary approaches to managing PPH following bariatric and metabolic surgery include Lifestyle Modifications, Exercise, and Nutrition Management. The literature recommends implementing dietary changes, such as limiting carbohydrates, avoiding high glycemic index foods, opting for heart-healthy fats and sufficient protein, refraining from alcohol and liquids during meals, and adjusting meal timing. Pharmacotherapy is essential when lifestyle modifications and dietary changes are insufficient. Surgical interventions are considered a last resort for patients who do not respond sufficiently to dietary, medical, or other non-surgical treatments. Further investigations into predictive markers, optimal treatment strategies, and long-term outcomes will be pivotal in refining our approach to mitigating the impact of this challenging complication on postoperative patients. These efforts will enhance our ability to effectively manage PPH and improve the quality of life for those affected.
Author contributions
MK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. OG: Data curation, Investigation, Resources, Software, Validation, Visualization, Writing – original draft, Conceptualization, Funding acquisition, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We sincerely thank all contributors to this comprehensive literature review. We especially appreciate colleagues’ insights and research assistants’ assistance and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PPH, postprandial hypoglycemia; GI, gastrointestinal; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; OAGB, one anastomosis gastric bypass; SADI-S, single anastomosis duodeno-ileostomy with sleeve gastrectomy; PBH, post-bariatric hypoglycemia; GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide; BMI, body mass index; OGTT, oral glucose tolerance test; MMTT, mixed meal tolerance Test; CGM, continuous glucose monitoring.
References
1. Mathew P, Thoppil D. Hypoglycemia. StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC (2024).
2. Rossini G, Risi R, Monte L, Sancetta B, Quadrini M, Ugoccioni M, et al. Postbariatric surgery hypoglycemia: nutritional, pharmacological and surgical perspectives. Diabetes Metab Res Rev. (2024) 40(2):e3750. doi: 10.1002/dmrr.3750
3. Coolen M, Broadley M, Hendrieckx C, Chatwin H, Clowes M, Heller S, et al. The impact of hypoglycemia on quality of life and related outcomes in children and adolescents with type 1 diabetes: a systematic review. PLoS One. (2021) 16(12):e0260896. doi: 10.1371/journal.pone.0260896
4. Sheehan A, Patti ME. Hypoglycemia after upper gastrointestinal surgery: clinical approach to assessment, diagnosis, and treatment. Diabetes Metab Syndr Obes. (2020) 13:4469–82. doi: 10.2147/DMSO.S233078
5. Carpentieri GB, Gonçalves SEAB, Mourad WM, Pinto LGC, Zanella MT. Hypoglycemia post bariatric surgery: drugs with different mechanisms of action to treat a unique disorder. Arch Endocrinol Metab. (2023) 67(3):442–9. doi: 10.20945/2359-3997000000598
6. Abrahamsson N, Edén Engström B, Sundbom M, Karlsson FA. Hypoglycemia in everyday life after gastric bypass and duodenal switch. Eur J Endocrinol. (2015) 173(1):91–100. doi: 10.1530/EJE-14-0821
7. Hsu JL, Farrell TM. Updates in bariatric surgery. Am Surg. (2024) 90(5):925–33. doi: 10.1177/00031348231220576
8. Brethauer SM, Schauer PR. Sleeve gastrectomy. Bariatric Surgery. (2024):89–100. doi: 10.1201/9781003522737-9
9. Arterburn DE, Johnson E, Coleman KJ, Herrinton LJ, Courcoulas AP, Fisher D, et al. Weight outcomes of sleeve gastrectomy and gastric bypass compared to nonsurgical treatment. Ann Surg. (2021) 274(6):e1269–e76. doi: 10.1097/SLA.0000000000003826
10. Mouawad C, Aoun R, Dahboul H, El Feghali E, Kassar S, Alkassis M, et al. Quality of life after laparoscopic sleeve gastrectomy: pre-operative, 1-year and 5-year results. J Minim Access Surg. (2023) 19(4):459–65. doi: 10.4103/jmas.jmas_193_22
11. Varvoglis DN, Lipman JN, Li L, Sanchez-Casalongue M, Zhou R, Duke MC, et al. Gastric bypass versus sleeve gastrectomy: comparison of patient outcomes, satisfaction, and quality of life in a single-center experience. J Laparoendosc Adv Surg Tech A. (2023) 33(2):155–61. doi: 10.1089/lap.2022.0127
12. Simonson DC, Halperin F, Foster K, Vernon A, Goldfine AB. Clinical and patient-centered outcomes in obese patients with type 2 diabetes 3 years after randomization to Roux-en-Y gastric bypass surgery versus intensive lifestyle management: the SLIMM-T2D study. Diabetes Care. (2018) 41(4):670–9. doi: 10.2337/dc17-0487
13. Johnson WH, Fernanadez AZ, Farrell TM, MacDonald KG, Grant JP, McMahon RL, et al. Surgical revision of loop (“mini”) gastric bypass procedure: multicenter review of complications and conversions to Roux-en-Y gastric bypass. Surg Obes Relat Dis. (2007) 3(1):37–41. doi: 10.1016/j.soard.2006.09.012
14. Chen Z, Cai J, Lei D, Zhang H. Roux-en-Y gastric bypass surgery for the prevention of long-term renal function damage in type 2 diabetes: a clinical study. Altern Ther Health Med. (2024) AT10184.38702168
15. Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. (2016) 59(5):945–53. doi: 10.1007/s00125-016-3903-x
16. Borges L, de Carvalho KMB, da Costa THM. Usual dietary intake, physical activity, weight loss, and body composition after five years of Roux-en-Y gastric bypass. Int J Obes (Lond). (2023) 47(4):263–72. doi: 10.1038/s41366-023-01256-x
17. Aleman R, Lo Menzo E, Szomstein S, Rosenthal RJ. Efficiency and risks of one-anastomosis gastric bypass. Ann Transl Med. (2020) 8(Suppl 1):S7. doi: 10.21037/atm.2020.02.03
18. Rheinwalt KP, Plamper A, Rückbeil MV, Kroh A, Neumann UP, Ulmer TF. One anastomosis gastric bypass–mini-gastric bypass (OAGB-MGB) versus Roux-en-Y gastric bypass (RYGB)—a mid-term cohort study with 612 patients. Obes Surg. (2020) 30:1230–40. doi: 10.1007/s11695-019-04250-3
19. Ding Z, Jin L, Song Y, Feng C, Shen P, Li H. Comparison of single-anastomosis gastric bypass and sleeve gastrectomy on type 2 diabetes mellitus remission for obese patients: a meta-analysis of randomized controlled trials. Asian J Surg. (2023) 46(10):4152–60. doi: 10.1016/j.asjsur.2023.03.062
20. Balamurugan G, Leo SJ, Sivagnanam ST, Balaji Prasad S, Ravindra C, Rengan V, et al. Comparison of efficacy and safety between Roux-en-Y gastric bypass (RYGB) vs one anastomosis gastric bypass (OAGB) vs single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S): a systematic review of bariatric and metabolic surgery. Obes Surg. (2023) 33(7):2194–209. doi: 10.1007/s11695-023-06602-6
21. Cottam D, Cottam S, Surve A. Single-anastomosis duodenal ileostomy with sleeve gastrectomy “continued innovation of the duodenal switch”. Surg Clin North Am. (2021) 101(2):189–98. doi: 10.1016/j.suc.2020.12.010
22. Hage K, Teixeira AF, Surve A, Lind R, Jawad MA, Ghanem M, et al. Single anastomosis duodenal switch versus Roux-en-Y gastric bypass in patients with BMI≥ 50 kg/m2: a multi-centered comparative analysis. Surg Endosc. (2024) 38(5):2657–65. doi: 10.1007/s00464-024-10765-3
23. Esparham A, Roohi S, Ahmadyar S, Dalili A, Moghadam HA, Torres AJ, et al. The efficacy and safety of laparoscopic single-anastomosis duodeno-ileostomy with sleeve gastrectomy (SADI-S) in mid- and long-term follow-up: a systematic review. Obes Surg. (2023) 33(12):4070–9. doi: 10.1007/s11695-023-06846-2
24. Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. (2010) 53(11):2307–11. doi: 10.1007/s00125-010-1798-5
25. Yukina MY, Chernova MO, Troshina EA, Evdoshenko VV, Platonova NM. Postprandial hypoglycemia after upper gastrointestinal tract surgery: prevalence and pathophysiology (part 1). Almanac Clin Med. (2021) 49(4):285–96. doi: 10.18786/2072-0505-2021-49-029
26. Belligoli A, Sanna M, Serra R, Fabris R, Pra CD, Conci S, et al. Incidence and predictors of hypoglycemia 1 year after laparoscopic sleeve gastrectomy. Obes Surg. (2017) 27(12):3179–86. doi: 10.1007/s11695-017-2742-2
27. Davis DB, Khoraki J, Ziemelis M, Sirinvaravong S, Han JY, Campos GM. Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: confirming a non-pancreatic etiology. Mol Metab. (2018) 9:15–27. doi: 10.1016/j.molmet.2017.12.011
28. Kubota T, Shoda K, Ushigome E, Kosuga T, Konishi H, Shiozaki A, et al. Utility of continuous glucose monitoring following gastrectomy. Gastric Cancer. (2020) 23(4):699–706. doi: 10.1007/s10120-019-01036-5
29. Capristo E, Panunzi S, De Gaetano A, Spuntarelli V, Bellantone R, Giustacchini P, et al. Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: a randomized trial. J Clin Endocrinol Metab. (2018) 103(6):2136–46. doi: 10.1210/jc.2017-01695
30. Raverdy V, Baud G, Pigeyre M, Verkindt H, Torres F, Preda C, et al. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: a five year longitudinal study. Ann Surg. (2016) 264(5):878–85. doi: 10.1097/SLA.0000000000001915
31. Lee CJ, Wood GC, Lazo M, Brown TT, Clark JM, Still C, et al. Risk of post-gastric bypass surgery hypoglycemia in nondiabetic individuals: a single center experience. Obesity (Silver Spring). (2016) 24(6):1342–8. doi: 10.1002/oby.21479
32. Yukina MY, Chernova MO, Troshina EA, Evdoshenko VV, Platonova NM. Postprandial hypoglycemia after upper gastrointestinal tract surgery: diagnosis and treatment (part 2). Almanac Clin Med. (2021) 49(5):305–14. doi: 10.18786/2072-0505-2021-49-030
33. Kamvissi V, Salerno A, Bornstein SR, Mingrone G, Rubino F. Incretins or anti-incretins? A new model for the “entero-pancreatic axis”. Horm Metab Res. (2015) 47(1):84–7. doi: 10.1055/s-0034-1394374
34. Fischer LE, Wolfe BM, Fino N, Elman MR, Flum DR, Mitchell JE, et al. Postbariatric hypoglycemia: symptom patterns and associated risk factors in the longitudinal assessment of bariatric surgery study. Surg Obes Relat Dis. (2021) 17(10):1787–98. doi: 10.1016/j.soard.2021.04.021
35. Kim E, Ershova E, Mazurina N, Komshilova K. A view at postbariatric hypoglycemia by endocrinologist. Obe Metab. (2022) 18(4):471–83. doi: 10.14341/omet12785
36. Hindsø M, Hedbäck N, Svane MS, Møller A, Martinussen C, Jørgensen NB, et al. The importance of endogenously secreted GLP-1 and GIP for postprandial glucose tolerance and β-cell function after Roux-en-Y gastric bypass and sleeve gastrectomy surgery. Diabetes. (2023) 72(3):336–47. doi: 10.2337/db22-0568
37. Herzig D, Schiavon M, Tripyla A, Lehmann V, Meier J, Jainandunsing S, et al. 134-OR: comparison of glucose-insulin homeostasis in people with postprandial hypoglycemia after gastric bypass surgery and nadir plasma glucose <50 mg/dl vs. >50 mg/dl. Diabetes. (2021) 70(Supplement_1):134-OR. doi: 10.2337/db21-134-OR
38. Alsayed Hasan M, Schwartz S, McKenna V, Ing R. An imbalance of pathophysiologic factors in late postprandial hypoglycemia post bariatric surgery: a narrative review. Obes Surg. (2023) 33(9):2927–37. doi: 10.1007/s11695-023-06758-1
39. Idris I. The relative importance of endogenously secreted incretin hormones, GLP-1 and GIP for postprandial glucose tolerance and β-cell function after Roux-en-Y gastric bypass and sleeve gastrectomy surgery. Diabetes Obes Metab. (2023) 1(4):e39. doi: 10.1002/doi2.39
40. Lee CJ, Clark JM, Egan JM, Carlson OD, Schweitzer M, Langan S, et al. Comparison of hormonal response to a mixed-meal challenge in hypoglycemia after sleeve gastrectomy vs gastric bypass. J Clin Endocrinol Metab. (2022) 107(10):e4159–e66. doi: 10.1210/clinem/dgac455
41. D'Hoedt A, Vanuytsel T. Dumping syndrome after bariatric surgery: prevalence, pathophysiology and role in weight reduction - a systematic review. Acta Gastroenterol Belg. (2023) 86(3):417–27. doi: 10.51821/86.3.11476
42. Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. (2009) 6(10):583–90. doi: 10.1038/nrgastro.2009.148
43. Honka H, Salehi M. Postprandial hypoglycemia after gastric bypass surgery: from pathogenesis to diagnosis and treatment. Curr Opin Clin Nutr Metab Care. (2019) 22(4):295–302. doi: 10.1097/MCO.0000000000000574
44. Almby KE, Abrahamsson N, Lundqvist MH, Hammar U, Thombare K, Panagiotou A, et al. Effects of GLP-1 on counter-regulatory responses during hypoglycemia after GBP surgery. Eur J Endocrinol. (2019) 181(2):161–71. doi: 10.1530/EJE-19-0171
45. Craig CM, Lawler HM, Lee CJE, Tan M, Davis DB, Tong J, et al. PREVENT: a randomized, placebo-controlled crossover trial of avexitide for treatment of postbariatric hypoglycemia. J Clin Endocrinol Metab. (2021) 106(8):e3235–e48. doi: 10.1210/clinem/dgab103
46. Zweck E, Hepprich M, Donath MY. Predictors of postprandial hypoglycemia after gastric bypass surgery: a retrospective case-control study. Obes Surg. (2021) 31(6):2497–502. doi: 10.1007/s11695-021-05277-1
47. Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 Analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol. (2013) 169(6):885–9. doi: 10.1530/EJE-13-0504
48. Øhrstrøm CC, Worm D, Kielgast UL, Holst JJ, Hansen DL. Evidence for relationship between early dumping and postprandial hypoglycemia after Roux-en-Y gastric bypass. Obes Surg. (2020) 30(3):1038–45. doi: 10.1007/s11695-020-04387-6
49. Hayashida R, Tsuchiya K, Sekine T, Momose T, Sato F, Sakurada M, et al. A clinical case of insulinoma presenting with postprandial hypoglycemia in a patient with a history of gastric bypass surgery. Intern Med. (2022) 61(8):1189–95. doi: 10.2169/internalmedicine.7428-21
50. Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. (2013) 304(12):G1117–27. doi: 10.1152/ajpgi.00035.2013
51. Ciudin A, Sánchez M, Hernandez I, Cordero E, Fidilio E, Comas M, et al. Canagliflozin: a new therapeutic option in patients that present postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: a pilot study. Obes Facts. (2021) 14(3):291–7. doi: 10.1159/000515598
52. Millstein R, Lawler HM. Hypoglycemia after gastric bypass: an emerging complication. Cleve Clin J Med. (2017) 84(4):319–28. doi: 10.3949/ccjm.84a.16064
53. Xu Q, Zou X, You L, Wu W, Zhu H, Wang L, et al. Surgical treatment for postprandial hypoglycemia after Roux-en-Y gastric bypass: a literature review. Obes Surg. (2021) 31(4):1801–9. doi: 10.1007/s11695-021-05251-x
54. Guarino D, Moriconi D, Mari A, Rebelos E, Colligiani D, Baldi S, et al. Postprandial hypoglycaemia after Roux-en-Y gastric bypass in individuals with type 2 diabetes. Diabetologia. (2019) 62(1):178–86. doi: 10.1007/s00125-018-4737-5
55. Nannipieri M, Belligoli A, Guarino D, Busetto L, Moriconi D, Fabris R, et al. Risk factors for spontaneously self-reported postprandial hypoglycemia after bariatric surgery. J Clin Endocrinol Metab. (2016) 101(10):3600–7. doi: 10.1210/jc.2016-1143
56. Sun W, Zhang Y, Shen Q, Zhang W, Yao Q, Yang Y. Prevalence and risk factors for symptoms suggestive of hypoglycemia and early dumping syndrome after sleeve gastrectomy. Surg Obes Relat Dis. (2019) 15(9):1439–46. doi: 10.1016/j.soard.2019.06.026
57. Lee CJ, Clark JM, Schweitzer M, Magnuson T, Steele K, Koerner O, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring). (2015) 23(5):1079–84. doi: 10.1002/oby.21042
58. Rebelos E, Moriconi D, Scalese M, Denoth F, Molinaro S, Siciliano V, et al. Impact of postprandial hypoglycemia on weight loss after bariatric surgery. Obes Surg. (2020) 30:2266–73. doi: 10.1007/s11695-020-04465-9
59. Douros JD, Tong J, D'Alessio DA. The effects of bariatric surgery on islet function, insulin secretion, and glucose control. Endocr Rev. (2019) 40(5):1394–423. doi: 10.1210/er.2018-00183
60. Gerich J. Pathogenesis and management of postprandial hyperglycemia: role of incretin-based therapies. Int J Gen Med. (2013) 6:877–95. doi: 10.2147/IJGM.S51665
61. Emous M, van den Broek M, Wijma RB, de Heide LJM, van Dijk G, Laskewitz A, et al. Prevalence of hypoglycaemia in a random population after Roux-en-Y gastric bypass after a meal test. Endocr Connect. (2019) 8(7):969–78. doi: 10.1530/EC-19-0268
62. AlTowayan A, Alharbi S, Aldehami M, Albahli R, Alnafessah S, Alharbi AM. Awareness level of hypoglycemia among diabetes Mellitus type 2 patients in al qassim region. Cureus. (2023) 15(2):e35285. doi: 10.7759/cureus.35285
63. Husain KH, Sarhan SF, AlKhalifa HKAA, Buhasan A, Moin ASM, Butler AE. Dementia in diabetes: the role of hypoglycemia. Int J Mol Sci. (2023) 24(12):9846. doi: 10.3390/ijms24129846
64. Yaqub A, Smith EP, Salehi M. Hyperinsulinemic hypoglycemia after gastric bypass surgery: what’s up and what’s down? Int J Obes (Lond). (2018) 42:286–94. doi: 10.1038/ijo.2017.257
65. Haider A, Burks JK, Cheema H, Tejada A. Postprandial hypoglycemia: complication of peptic ulcer surgery. Am J Med. (2017) 130(12):e527–e9. doi: 10.1016/j.amjmed.2017.06.010
66. Schönenberger KA, Cossu L, Prendin F, Cappon G, Wu J, Fuchs KL, et al. Digital solutions to diagnose and manage postbariatric hypoglycemia. Front Nutr. (2022) 9:855223. doi: 10.3389/fnut.2022.855223
67. Yang JC, Zhang GX, Leng C, Chen G, Cheng Z, Du X. Incidence and intensity of early dumping syndrome and its association with health-related quality of life following sleeve gastrectomy. Obes Surg. (2023) 33(11):3510–6. doi: 10.1007/s11695-023-06863-1
68. Van Beek A, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. (2017) 18(1):68–85. doi: 10.1111/obr.12467
69. Masclee GMC, Masclee AAM. Dumping syndrome: pragmatic treatment options and experimental approaches for improving clinical outcomes. Clin Exp Gastroenterol. (2023) 16:197–211. doi: 10.2147/CEG.S392265
70. Yang JY, Lee HJ, Alzahrani F, Choi SJ, Lee WK, Kong SH, et al. Postprandial changes in gastrointestinal hormones and hemodynamics after gastrectomy in terms of early dumping syndrome. J Gastric Cancer. (2020) 20(3):256–66. doi: 10.5230/jgc.2020.20.e24
71. Weiss J, Fewel C, Akinrinade O, Harrington J. Late dumping syndrome preceded by coxsackievirus B4 infection and cholecystectomy. J Surg Case Rep. (2023) 2023(4):rjad205. doi: 10.1093/jscr/rjad205
72. Wong M, Gome JJ, Dreyer R. A delayed presentation of late dumping syndrome after ivor lewis procedure. Cureus. (2023) 15(6):e40877. doi: 10.7759/cureus.40877
73. Van de Velde F, Lapauw B. Late dumping syndrome or postprandial reactive hypoglycaemic syndrome after bariatric surgery. Nat Rev Endocrinol. (2021) 17(5):317. doi: 10.1038/s41574-021-00473-6
74. Shaghouli AA, Ballani R, Mesbah N. Management of late dumping syndrome induced hypoglycemia with GLP-1R agonist. J Endocr Soc. (2021) 5(Supplement_1):A416–A. doi: 10.1210/jendso/bvab048.849
75. Rayas MS, Salehi M. Non-diabetic hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., editors. Endotext. [online source]. South Dartmouth (MA):MDText.com, Inc. (2024).
76. Bjerkan KK, Sandvik J, Nymo S, Johnsen G, Hyldmo ÅA, Kulseng BE, et al. Postbariatric hypoglycemia, abdominal pain and gastrointestinal symptoms after Roux-en-Y gastric bypass explored by continuous glucose monitoring. Obes Res Clin Pract. (2024) 18(1):9–14. doi: 10.1016/j.orcp.2024.02.004
77. Adelmeyer J, Schauer CM, Kann PH. Spontaneous hypoglycemia: should we mind the gap? Long-term follow-up of healthy people who met whipple’s triad criteria. Hormones. (2024) 23(3):447–55. doi: 10.1007/s42000-024-00542-1
78. Malik S, Mitchell JE, Steffen K, Engel S, Wiisanen R, Garcia L, et al. Recognition and management of hyperinsulinemic hypoglycemia after bariatric surgery. Obes Res Clin Pract. (2016) 10(1):1–14. doi: 10.1016/j.orcp.2015.07.003
79. Yang TH, Ziemba R, Shehab N, Geller AI, Talreja K, Campbell KN, et al. Assessment of international classification of diseases, tenth revision, clinical modification (ICD-10-CM) code assignment validity for case finding of medication-related hypoglycemia acute care visits among medicare beneficiaries. Med Care. (2022) 60(3):219–26. doi: 10.1097/MLR.0000000000001682
80. Lazar LO, Sapojnikov S, Pines G, Mavor E, Ostrovsky V, Schiller T, et al. Symptomatic and asymptomatic hypoglycemia post three different bariatric procedures: a common and severe complication. Endocr Pract. (2019). doi: 10.4158/EP-2019-0185
81. Jani S, Bao S. THU276 the use of continuous glucose monitors for management of postbariatric hypoglycemia. J Endocr Soc. (2023) 7(Supplement_1):bvad114.712. doi: 10.1210/jendso/bvad114.712
82. Röhling M, Martin T, Wonnemann M, Kragl M, Klein HH, Heinemann L, et al. Determination of postprandial glycemic responses by continuous glucose monitoring in a real-world setting. Nutrients. (2019) 11(10):2305. doi: 10.3390/nu11102305
83. Yamamoto Y, Togawa T, Sekine O, Ozamoto Y, Fuse J, Azuma C, et al. The oral disposition index calculated from a meal tolerance test is a crucial indicator for evaluating differential normalization of postprandial glucose and triglyceride excursions in morbidly obese patients after laparoscopic sleeve gastrectomy. Endocr J. (2023) 70(12):1141–57. doi: 10.1507/endocrj.EJ23-0241
84. Ramos-Levi AM, Rubio-Herrera MA, Matía-Martín P, Pérez-Ferre N, Marcuello C, Sánchez-Pernaute A, et al. Mixed meal tolerance test versus continuous glucose monitoring for an effective diagnosis of persistent post-bariatric hypoglycemia. J Clin Med. (2023) 12(13):4295. doi: 10.3390/jcm12134295
85. Lobato CB, Pereira SS, Guimarães M, Hartmann B, Wewer Albrechtsen NJ, Hilsted L, et al. A potential role for endogenous glucagon in preventing post-bariatric hypoglycemia. Front Endocrinol (Lausanne). (2020) 11:608248. doi: 10.3389/fendo.2020.608248
86. Maia FF, Araújo LR. Efficacy of continuous glucose monitoring system (CGMS) to detect postprandial hyperglycemia and unrecognized hypoglycemia in type 1 diabetic patients. Diabetes Res Clin Pract. (2007) 75(1):30–4. doi: 10.1016/j.diabres.2006.05.009
87. Alkhaled L, Al-Kurd A, Butsch WS, Kashyap SR, Aminian A. Diagnosis and management of post-bariatric surgery hypoglycemia. Expert Rev Endocrinol Metab. (2023) 18(6):459–68. doi: 10.1080/17446651.2023.2267136
88. Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. (2013) 36(5):1396–405. doi: 10.2337/dc12-1609
89. Vargas EJ, Bazerbachi F, Calderon G, Prokop LJ, Gomez V, Murad MH, et al. Changes in time of gastric emptying after surgical and endoscopic bariatrics and weight loss: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2020) 18(1):57–68.e5. doi: 10.1016/j.cgh.2019.03.047
90. Stier C, Parmar C, Koschker A-C, Bokhari M, Stier R, Chiappetta S. Computed tomography-3D-volumetry: a valuable adjunctive diagnostic tool after bariatric surgery. Mini-invasive Surgery. (2020) 4:18. doi: 10.20517/2574-1225.2019.75
91. Nance ME, Verma R, DeClue C, Reed M, Patel T. Imaging and diagnostic challenges in a patient with refractory hypoglycemia caused by insulinomas related to multiple endocrine neoplasia type 1. Cureus. (2020) 12(5):e8208. doi: 10.7759/cureus.8208
92. Kalik S, Dehghani S, Zaidan J. FRI359 Postprandial hypoglycemia with elevated pro-insulin as A rare presentation of insulinoma. J Endocr Soc. (2023) 7(Supplement_1):bvad114.1292. doi: 10.1210/jendso/bvad114.1292
93. Schoenberger JL, Koh CK, Hor T, Baldwin D, Reddy A, Rondinelli-Hamilton L. Insulin in the medical management of postprandial hypoglycemia in a patient with type 2 diabetes after gastric bypass surgery. Case Rep Endocrinol. (2012) 2012:427565. doi: 10.1155/2012/427565
94. Ab Majida NL, Vanoh D, Md Hashim MN, Zainuddin NS, Ajid M. Nutritional strategies to prevent muscle loss after bariatric surgery. Asian J Med Biomed. (2022) 6(S1):140–2. doi: 10.37231/ajmb.2022.6.S1.564
95. Ternhamar T, Møller A, Martinussen C, Svane MS, Hindsø M, Jørgensen NB, et al. The effects of postprandial exercise and meal glycemic index on plasma glucose and glucoregulatory hormone responses after Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab. (2023) 325(5):E540–e51. doi: 10.1152/ajpendo.00176.2023
96. Cave N, Delaney SJ, Larsen JA. Nutritional management of gastrointestinal diseases. In: Fascetti AJ, Delaney SJ, Larsen JA, Villaverde C, editors. Applied Veterinary Clinical Nutrition. Hoboken, NJ: John Wiley & Sons, Inc. (2023). p. 235–98. doi: 10.1002/9781119375241.ch11
97. Suhl E, Anderson-Haynes SE, Mulla C, Patti ME. Medical nutrition therapy for post-bariatric hypoglycemia: practical insights. Surg Obes Relat Dis. (2017) 13(5):888–96. doi: 10.1016/j.soard.2017.01.025
98. Marques AR, Lobato CB, Pereira SS, Guimarães M, Faria S, Nora M, et al. Insights from the impact of meal composition on glucose profile towards post-bariatric hypoglycemia management. Obes Surg. (2020) 30(1):249–55. doi: 10.1007/s11695-019-04147-1
99. Leutner M, Klimek P, Göbl C, Bozkurt L, Harreiter J, Husslein P, et al. Glucagon-like peptide 1 (GLP-1) drives postprandial hyperinsulinemic hypoglycemia in pregnant women with a history of Roux-en-Y gastric bypass operation. Metab Clin Exp. (2019) 91:10–7. doi: 10.1016/j.metabol.2018.10.006
100. Kandel D, Bojsen-Møller KN, Svane MS, Samkani A, Astrup A, Holst JJ, et al. Mechanisms of action of a carbohydrate-reduced, high-protein diet in reducing the risk of postprandial hypoglycemia after Roux-en-Y gastric bypass surgery. Am J Clin Nutr. (2019) 110(2):296–304. doi: 10.1093/ajcn/nqy310
101. Nor Hanipah Z, Punchai S, Birriel TJ, Lansang MC, Kashyap SR, Brethauer SA, et al. Clinical features of symptomatic hypoglycemia observed after bariatric surgery. Surg Obes Relat Dis. (2018) 14(9):1335–9. doi: 10.1016/j.soard.2018.02.022
102. Keshishian A, Rajtar M, Rosado M. Surgical treatment of clinically significant reactive hypoglycemia nesid-ioblastosis, post-gastric bypass. J Surgical Endocrinol. (2021) 3(1):73–6. doi: 10.36959/608/451
103. Zanley E, Shah ND, Craig C, Lau JN, Rivas H, McLaughlin T. Guidelines for gastrostomy tube placement and enteral nutrition in patients with severe, refractory hypoglycemia after gastric bypass. Surg Obes Relat Dis. (2021) 17(2):456–65. doi: 10.1016/j.soard.2020.09.026
104. van Meijeren J, Timmer I, Brandts H, Janssen I, Boer H. Evaluation of carbohydrate restriction as primary treatment for post-gastric bypass hypoglycemia. Surg Obes Relat Dis. (2017) 13(3):404–10. doi: 10.1016/j.soard.2016.11.004
105. Gertsson J, Hemmingsson JU. Differences in dietary choices in patients who developed postprandial hyperinsulinemic hypoglycemia (dumping syndrome) after Roux-en-Y gastric bypass compared to healthy controls. Clin Nutr Exp. (2018) 22:1–10. doi: 10.1016/j.yclnex.2018.10.003
106. Karimi M, Pirzad S, Shirsalimi N, Ahmadizad S, Hashemi SM, Karami S, et al. Effects of chia seed (Salvia hispanica L.) supplementation on cardiometabolic health in overweight subjects: a systematic review and meta-analysis of RCTs. Nutr Metab (Lond). (2024) 21(1):74. doi: 10.1186/s12986-024-00847-3
107. Younes YR, Cron N, Field BCT, Nayyar V, Clark J, Zachariah S, et al. Proposed treatment strategy for reactive hypoglycaemia. Front Endocrinol (Lausanne. (2024) 15:1332702. doi: 10.3389/fendo.2024.1332702
108. Hepprich M, Wiedemann SJ, Schelker BL, Trinh B, Stärkle A, Geigges M, et al. Postprandial hypoglycemia in patients after gastric bypass surgery is mediated by glucose-induced IL-1β. Cell Metab. (2020) 31(4):699–709.e5. doi: 10.1016/j.cmet.2020.02.013
109. Hepprich M, Schelker B, Wiedemann SJ, Trinh B, Böni-Schnetzler M, Donath MY. 1830-P: empagliflozin as well as anakinra reduces symptomatic postprandial hypoglycemia after gastric bypass. Diabetes. (2019) 68(Supplement_1):1830-P. doi: 10.2337/db19-1830-P
110. Ferreira A, Emara AFA, Herzig D, Melmer A, Vogt AP, Nakas CT, et al. Study protocol for a randomised, double-blind, placebo-controlled crossover trial assessing the impact of the SGLT2 inhibitor empagliflozin on postprandial hypoglycaemia after gastric bypass. BMJ open. (2022) 12(9):e060668. doi: 10.1136/bmjopen-2021-060668
111. Llewellyn DC, Logan Ellis H, Aylwin SJ, Oštarijaš E, Green S, Sheridan W, et al. The efficacy of GLP-1RAs for the management of postprandial hypoglycemia following bariatric surgery: a systematic review. Obesity. (2023) 31(1):20–30. doi: 10.1002/oby.23600
112. Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. (2014) 146(3):669–680.e2. doi: 10.1053/j.gastro.2013.11.044
113. Moreira RO, Moreira RB, Machado NA, Gonçalves TB, Coutinho WF. Post-prandial hypoglycemia after bariatric surgery: pharmacological treatment with verapamil and acarbose. Obes Surg. (2008) 18:1618–21. doi: 10.1007/s11695-008-9569-9
114. Hasebe M, Aizawa-Abe M, Shibue K, Hamasaki A. Successful treatment of postprandial hyperinsulinemic hypoglycemia after billroth-II gastrojejunostomy using octreotide. JCEM Case Rep. (2023) 1(6):luad150. doi: 10.1210/jcemcr/luad150
115. Gonzalez-Gonzalez A, Delgado M, Fraga-Fuentes MD. Use of diazoxide in management of severe postprandial hypoglycemia in patient after Roux-en-Y gastric bypass. Surg Obes Relat Dis. (2013) 9(1):e18–e9. doi: 10.1016/j.soard.2011.05.010
116. Pavan J, Dalla Man C, Herzig D, Bally L, Del Favero S. A PID control algorithm for a post-prandial hypoglycemic clamp study. Annu Int Conf IEEE Eng Med Biol Soc. (2021) 2021:677–80. doi: 10.1109/EMBC46164.2021.9630223
117. Carpentieri GB, Gonçalves S, Casagrande MZ, Mourad WM, Pinto LGC, Zanella MT. SGLT2 Inhibition with empagliflozin as a possible therapeutic option for postprandial hypoglycemia after bariatric surgery. Obes Surg. (2022) 32(8):2664–71. doi: 10.1007/s11695-022-06119-4
118. Vilarrasa N, Bretón I, Ballesteros-Pomar M, Lecube A, Goday A, Pellitero S, et al. Recommendations for the diagnosis and treatment of hypoglycaemia after bariatric surgery. Endocrinol Diabetes Nutr (Engl Ed). (2022) 69(9):723–31. doi: 10.1016/j.endien.2021.09.005
119. Mala T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg Obes Relat Dis. (2014) 10(6):1220–5. doi: 10.1016/j.soard.2014.01.010
120. Hesse UJ, Lenz J, Giulini L, Vladimirov M, Dubecz A, Stein HJ. Minimally invasive conversion of a gastric bypass into sleeve gastrectomy for postprandial hyperinsulinemic hypoglycemia. Obes Surg. (2021) 31:1897–8. doi: 10.1007/s11695-021-05241-z
121. Wang Y, Kassab GS. Efficacy and mechanisms of gastric volume-restriction bariatric devices. Front Physiol. (2021) 12:761481. doi: 10.3389/fphys.2021.761481
122. Nielsen CK, Øhrstrøm CC, Kielgast UL, Hansen DL, Hartmann B, Holst JJ, et al. Dasiglucagon effectively mitigates postbariatric postprandial hypoglycemia: a randomized, double-blind, placebo-controlled, crossover trial. Diabetes Care. (2022) 45(6):1476–81. doi: 10.2337/dc21-2252
123. Sheehan A, Goldfine A, Bajwa M, Wolfs D, Kozuka C, Piper J, et al. Pramlintide for post-bariatric hypoglycaemia. Diabetes. Obe Metab. (2022) 24(6):1021–8. doi: 10.1111/dom.14665
124. Tan M, Lamendola C, Luong R, McLaughlin T, Craig C. Safety, efficacy and pharmacokinetics of repeat subcutaneous dosing of avexitide (exendin 9-39) for treatment of post-bariatric hypoglycaemia. Diabetes Obe Metab. (2020) 22(8):1406–16. doi: 10.1111/dom.14048
125. Øhrstrøm CC, Hansen DL, Kielgast UL, Hartmann B, Holst JJ, Worm D. A low dose of pasireotide prevents hypoglycemia in Roux-en-Y gastric bypass-operated individuals. Obes Surg. (2020) 30(4):1605–10. doi: 10.1007/s11695-019-04248-x
126. Øhrstrøm CC, Worm D, Højager A, Andersen D, Holst JJ, Kielgast UL, et al. Postprandial hypoglycaemia after Roux-en-Y gastric bypass and the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide. Diabetes Obe Metab. (2019) 21(9):2142–51. doi: 10.1111/dom.13796
127. Ritz P, Vaurs C, Bertrand M, Anduze Y, Guillaume E, Hanaire H. Usefulness of acarbose and dietary modifications to limit glycemic variability following Roux-en-Y gastric bypass as assessed by continuous glucose monitoring. Diabetes Technol Ther. (2012) 14(8):736–40. doi: 10.1089/dia.2011.0302
Keywords: hypoglycemia, surgical complication, bariatric surgery, gastrointestinal tract surgery, review
Citation: Karimi M and Kohandel Gargari O (2024) Postprandial hypoglycemia as a complication of bariatric and metabolic surgery: a comprehensive review of literature. Front. Surg. 11:1449012. doi: 10.3389/fsurg.2024.1449012
Received: 14 June 2024; Accepted: 16 October 2024;
Published: 1 November 2024.
Edited by:
Dragomir Dardanov, Lozenetz Hospital, BulgariaReviewed by:
Ioannis I. Lazaridis, University Hospital of Basel, SwitzerlandAsif Mehraj, Apollo Hospital, India
Copyright: © 2024 Karimi and Kohandel Gargari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Karimi, S2FyaW1pOTAxMEBnbWFpbC5jb20=
 Mehdi Karimi
Mehdi Karimi Omid Kohandel Gargari2
Omid Kohandel Gargari2