- 1General Surgery Unit, Ospedale Santa Maria Delle Croci - AUSL Romagna, Ravenna, Italy
- 2General Surgery Unit, Ospedale per gli Infermi - AUSL Romagna, Faenza, Italy
- 3Medical Oncology Unit, Ospedale per gli Infermi - AUSL Romagna, Faenza, Italy
- 4Medical Oncology Unit, Ospedale Santa Maria Delle Croci - AUSL Romagna, Ravenna, Italy
- 5Department of Radiation Oncology, Maria Cecilia Hospital, Cotignola, Italy
- 6Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
Background: Rectal cancer (RC) commonly affects older patients. Total Neoadjuvant Therapy (TNT) has been introduced to improve local and systemic control of RC. The aim was to present real-world data of older patients receiving TNT followed by surgery after a frailty assessment and verify feasibility and safety of this approach.
Methods: This was a single-center retrospective study which enrolled all patients ≥70 years of age with RC who underwent TNT followed by surgery between November 2017 and April 2022. Data regarding cancer characteristics, neoadjuvant chemoradiotherapy (CRT), and toxicity were recorded. All patients underwent surgery 12–16 weeks after the end of therapy. Intra- and postoperative outcomes were recorded. Pre- and postoperative functional evaluation was carried out.
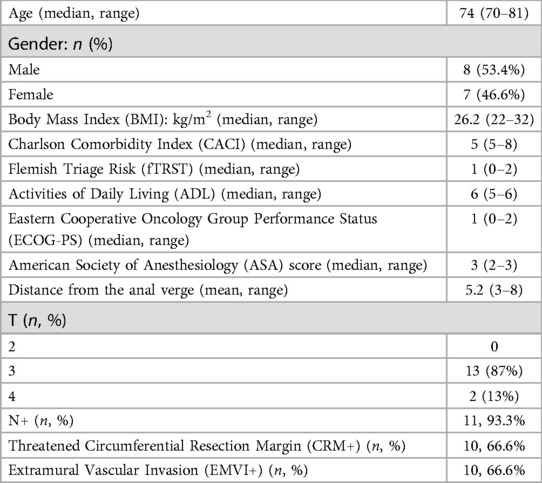
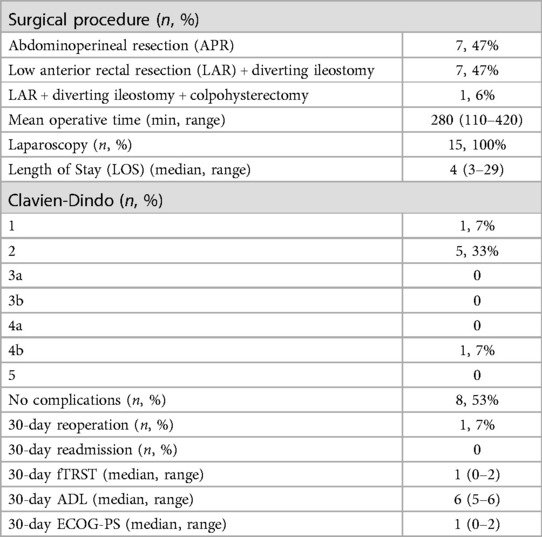
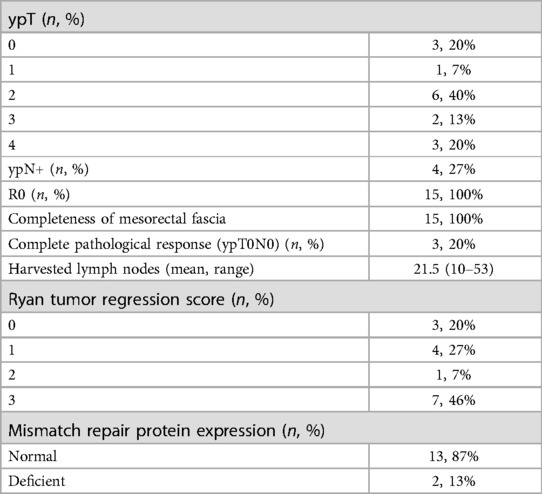
Results: Fifteen patients were enrolled. Mean age was 74 (70–81) years. Mean distance of the tumor from the anal verge was 5.2 cm. Fourteen patients had positive nodes (93.3%), 11 (73.3%) showed involvement of the circumferential margin (CRM+) and 10 (66.6%) had extramural vascular invasion (EMVI+). Ten patients (66.6%) received mFOLFOX-6 and 5 CAPOX (33.3%) followed by CRT. After CRT, positive nodes were reported in 4 cases (26.6%), CRM+ in 4 (26.6%), and EMVI+ in 1 (6.6%). Transanal total mesorectal excision (taTME) was performed in all cases. Median operative time was 280 min (110–420). Median length of stay was 4 days (3–29). One Clavien-Dindo grade 4 complication, no readmissions, and no variations in pre- and postoperative functional status within 30 days from surgery were reported. No positive distal or CRMs were detected. Three pathologic complete responses were reported (20%).
Conclusions: TNT followed by TME is feasible and safe in older patients, with good clinical and oncologic outcomes. Patient evaluation is crucial for maximizing cancer care in fit older patients.
Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer in the world and the second most deadly, with approximately 2 million new cases and 1 million deaths reported in 2018 (1, 2). Rectal cancer (RC) represents a third of all CRC cases and commonly affects older people. The median age at the time of diagnosis is 70 years, and relative incidence increases with age, having a peak incidence at 80–84 years (3). Due to the progressive aging of the population, the overall number of older patients with RC is expected to increase even more. Unfortunately, older patients are often undertreated or overtreated based on their chronological age alone (4, 5), generating a significant disparity as compared with younger patients (6). Chronological age remains one of the most used variables to assess outcomes of several medical and surgical treatments. Due to the increasing of life expectancy over time, older age reference points of 70 and 75 years have been used (4, 7, 8). Older adults are a heterogeneous population that covers a spectrum from frail to fit patients; however, treatment continues to be insufficiently tailored to each patient's specific needs. There is a paucity of trials addressing the risks and benefits of all aspects of RC care (9).
The standard treatment of RC is challenging for older patients as it often involves neoadjuvant chemoradiation therapy (as in the case of locally advanced RC), extended surgery (often involving a temporary or permanent stoma creation) and then more adjuvant care. In addition, new regimens based on extended preoperative chemotherapy treatment have been introduced into guidelines under the term Total Neoadjuvant Therapy (TNT) (10, 11). The thinking behind more intense preoperative management is to improve local and systemic control in high-risk patients showing threatened circumferential resection margins (CRM+s), the presence of extramural vascular invasion (EMVI+) and metastatic mesorectal and extramesorectal lymph nodes (12–14). The results of this new regimen seem to be extremely encouraging, and many centers around the world have incorporated TNT into their multidisciplinary treatment strategy (15–17). Unfortunately, to date, none of the randomized control trials testing TNT have been designed for older patients who have again been left out of the innovation process, only then to often be the recipients of these treatments in the real world. At the same time, if TNT is going to show sustained oncologic benefits, as it currently seems, prejudice and misjudgment towards older patients may prevent many people from receiving optimal care, only based on chronological age or perceived, non-assessed vulnerability.
The aim of this study was to present real-world data regarding older patients receiving TNT followed by minimally invasive Total Mesorectal Excision (TME) after a multidimensional frailty assessment. The primary goal is to verify whether this approach would be feasible and safe, and the secondary goal of this study is to show possible short-term oncologic outcomes improvements.
Methods
Patient characteristics
This was a single-center retrospective study based on a prospectively maintained database that enrolled all patients ≥70 years of age with biopsy-proven locally advanced RC (LARC) who underwent TNT followed by surgery between November 2017 and April 2022. Preoperative staging included colonoscopy, magnetic resonance imaging (MRI) and computed tomography (CT) according to the 8th edition of the American Joint Commission on Cancer (AJCC) staging system (18). All patients received initial frailty screening including the Charlson Comorbidity Index (CCI), Flemish Triage Risk (fTRST), Activities of Daily Living (ADL), Eastern Cooperative Oncology Group Performance Status (ECOG-PS) and nutritional screening. All the cases were discussed at diagnosis, after completion of the staging, and after treatment and re-staging by a multidisciplinary team (MDT).
The local MDT included colorectal surgeons, oncologists, radiotherapists, radiologists, pathologists, gastroenterologists, and geriatricians. The study was conducted in accordance with the latest revision of the Declaration of Helsinki and approved by the local Institutional Review Board (Comitato Etico della Romagna, C.E.ROM.). Informed consent was obtained from all subjects and/or their legal guardian(s).
Data regarding the tumor-node-metastasis (TNM) stage, the distance of the tumor from the anal verge, the total number of neoadjuvant chemo-radiotherapy (nCRT) cycles received, any toxicity during treatment and the need for dose reduction were prospectively recorded. All the patients underwent surgery 12–16 weeks after the end of radiation therapy. No watch-and-wait strategy was offered to patients undergoing TNT based on the insufficient knowledge of the long-term results of these patients by the time the protocol was adopted (November 2017). It was always made clear that the decision to undergo a TNT vs. standard chemoradiation was based on the goal of possibly obtaining improved long-term oncological outcomes rather than achieving a complete clinical complete response or pursuing sphincter-saving surgery.
Surgical options were collegially discussed first, during MDT, and with the patients and families then. The main criteria to offer a restorative vs. a non-restorative procedure (permanent colostomy) were based on oncologic criteria (invasion or threatened sphincter complex); patients' pre-existing clinical conditions (fecal incontinence, lack of independence in the self-care); patients' and family preferences. The contact of a patients' advocate/witness (mainly former patients in the same age group who underwent similar pathways) was offered to patients and families in order to give the possibility of a fellow patient giving him/her personal opinion about the current quality of life after the surgery.
All the surgeries were performed using a minimally invasive approach. The TME was performed using a synchronous abdominal and transanal approach (taTME). Intra- and postoperative outcome data were recorded, including operative time, conversion rates, length of stay (LOS), 30-day complications according to the Clavien-Dindo scale (19) and the need for re-operation or re-admission. The postoperative data consisted of a full pathological report with tumor regression grade including the according to Ryan and mismatch repair protein expression (20). Postoperative functional evaluation was carried out using fTRST, ADL and ECOG-PS.
TNT protocols
TNT was offered to patients in case high-risk features were reported during the staging including at least one of the following: threatened circumferential resection margins (CRM+s), the presence of extramural vascular invasion (EMVI+), metastatic mesorectal and extramesorectal lymph nodes.
The TNT was defined as neoadjuvant chemotherapy (nCT) followed by short-course (5 × 5 Gy) or standard long-course radiotherapy (28 daily fractions of 1.8 Gy up to 50.4 Gy or 25 fractions of 2 Gy up to 50 Gy) with concurrent capecitabine (twice-daily oral capecitabine 825 mg/m2). Optional field reduction was recommended after 45 Gy (1.8 Gy schedule) or 46 Gy (2 Gy schedule), with the last fractions delivered to the tumor bed. The clinical target for radiotherapy (RT) included the entire mesorectum with the primary tumor and relevant regional lymph nodes. All the patients underwent RT starting 3 weeks from the end of the nCT.
The nCT regimen consisted of 4 cycles of CAPOX (capecitabine 1,000 mg/m2 orally twice daily on days 1–14, oxaliplatin 130 mg/m2 intravenously on day 1, and a chemotherapy-free interval between days 15–21) or 8 cycles of modified FOLFOX-6 (mFOLFOX-6) which consisted of oxaliplatin (85 mg/m2) given as a 2-h intravenous infusion, followed by leucovorin (400 mg/m2) given as a 2-h intravenous infusion, followed by fluorouracil (400 mg/m2) given as an intravenous bolus and then as continuous intravenous infusion (2,400 mg/m2) over 46 h every 14 days. The choice of CAPOX or mFOLFOX-6 was determined by the treating physician and according to hospital policy. In the case of toxicity, there was a dose reduction of 25% or more.
Multimodality treatment feasibility was assessed based on patient postoperative functional status, and evaluation of treatment completion and toxicity in addition to the need for a dose reduction. The chemotherapy dosing for the first cycle of treatment was categorized as standard or dose reduced as per the National Comprehensive Cancer Network (NCCN) guidelines (21). The patients were followed from the beginning until the end of the chemotherapy course. Toxicity was measured at each clinical encounter using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 5.0 (22).
Statistical analysis
The continuous variables were measured using median, mean and range. The categorical variables were described by count and relative frequency. The data were analyzed using STATA 16.1 (StataCorp, College Station, Texas). The primary aim was to evaluate surgical and oncologic outcomes in older patients who underwent TNT for LARC. The secondary aim was to explore the feasibility of this emerging approach in this subset of patients.
Results
From November 2017 to April 2022, a total of 15 patients were found to be eligible for this analysis. Baseline demographics, frailty screening and rectal cancer clinicopathological characteristics are summarized in Table 1. The mean age of patients was 74 (range 70–81) years; 8 were male (8/15, 53.3%). The median BMI was 26.2 (range 22–32) kg/m2. The mean distance of the tumor from the anal verge was 5.2 cm. During pre-treatment staging, 14 patients had positive nodes (14/15, 93.3%), 11 patients (11/15, 73.3%) showed involvement of the circumferential resection margin (CRM+) and EMVI+ was reported in 10 cases (10/15, 66.6%).
A frailty assessment was carried out at baseline (see Table 1). Ten patients (10/15, 66.6%) received mFOLFOX-6 and 5 CAPOX (5/15, 33.3%). Minimal toxicity was reported in both groups, necessitating a dose reduction during treatment. All the patients completed their neoadjuvant chemotherapy plan. Details regarding the treatment regimen, toxicity, and pre- and post-treatment staging are summarized in Table 2. Fourteen patients (14/15, 93.3%) underwent long-course RT with concurrent capecitabine whereas one patient underwent short-course treatment. The degree of toxicity was also acceptable during the radiation therapy with the exception of one patient (1/15, 6.6%) who could not complete the treatment due to diarrhea, dehydration, and acute kidney failure.
All the patients showed radiological partial response (PR) after TNT, and no interval disease progression was recorded. At restaging after treatment, positive nodes were reported in 4 cases (4/15, 26.6%), the CRM+ was still described by the MRI in 4 cases (4/15, 26.6%), and EMVI+ in 1 patient (1/15, 6.6%).
Details regarding the surgical procedures are summarized in Table 3. All procedures were performed using a laparoscopic approach; no conversion to open surgery was recorded. The median operating time was 280 min (range 110–420). An abdominoperineal resection (APR) was performed in 7 cases (7/15, 47%), a TaTME with coloanal anastomosis and diverting loop ileostomy was performed in 7 patients (7/15, 47%). One patient (1/15, 6%) required an APR associated with a colpohysterectomy due to a locally advanced tumor. All the patients were treated according to the standard Enhanced Recovery After Surgery (ERAS) protocol. Median LOS was 4 days (range 3–29). Postoperative complications were minimal; however, 1 patient had a Clavien-Dindo grade 4 complication (anastomotic leak and acute cholecystitis which required reintervention and intensive care unit management). No readmission within 30 days from surgery was reported. No patient died within 90 days from surgery. A pathologic complete response (pCR) to TNT was reported in 3 cases (3/15, 20%). All the patients had R0 resections, regardless of their preoperative staging, with negative circumferential and distal resection margins at final pathology (see Table 4). No patients underwent adjuvant therapy, regardless of the tumor stage.
When functional assessment was repeated, 30 days after surgical treatment, no differences were reported between before and after treatment (see Table 3).
Discussion
The present study showed that TNT followed by TME was feasible and safe in fit older patients, having good short-term oncologic outcomes. The present results show optimal compliance to TNT, which was completed with acceptable toxicity in all cases. Regarding postoperative complications, only one patient had a Clavien-Dindo grade 4 complication, and neither reintervention nor deaths within 30 days after surgery were reported.
Age should not be the main determinant in the treatment decision-making process; individual patient frailty should be defined to promote optimal care tailored to each patient's specific needs (8). Older adults are a heterogeneous population covering a spectrum from fit to vulnerable to frail; therefore, individual evaluation of patient performance is the correct way of defining the feasible pathways for each patient. In the present series, patient evaluations were always presented during the MDT by a geriatrician or a frailty expert; these evaluations impacted the decision-making process, helping to define which patients should undergo full TNT.
The time spent assessing frailty was well spent as it was shown that older patients, selected based on their fitness level, could complete a multi-treatment maximally impactful pathway, such as TNT, for RC management. All the patients enrolled in the present study showed no differences in ADL, fTRST, and ECOG-PS score before and after completing treatment (medical and surgical) for LARC with limited toxicity and a low postoperative complication rate. Optimal cancer care, even when highly impactful such as in the case of TNT, should no longer be denied to older patients based on age alone.
The idea of TNT for LARC has been included as a pre-operative treatment option by the Clinical Practice Guidelines in Oncology (National Comprehensive Cancer Network, NCCN) since 2018, although only recently have the results of the RAPIDO and PRODIGE23 trials started to change clinical practice (16, 17). These two randomized clinical trials have addressed the effects of TNT for LARC over the standard neoadjuvant chemoradiotherapy (nCRT), adopting short-course radiotherapy (SC-RT) and induction nCT, respectively. In both studies, patients enrolled in the TNT arm showed a better pathologic complete response (pCR) rate and disease control, increased disease-free survival (DFS), and decreased disease-related treatment failure with better tolerance as compared to adjuvant therapy. Therefore, the TNT approach has gained interest as a possible means of improving long-term oncologic outcomes in patients with LARC.
The RAPIDO study, which is based on short-course radiation and systemic chemotherapy, experimental arm included 189 patients older than 65 (39% of the population). Unfortunately, not only 65 years seems to be a suboptimal cutoff to identify the older population, and a subgroup analysis is not provided in the publication. What has been shown instead, following the RAPIDO regimen, is that only 78% vs. 85% (standard arm) of patients underwent an optimal TME with intact mesorectum and that 1/10 patients had an R1 resection (16). Our much smaller experience shows in contrast that best surgical care can be achieved in a higher percentage of patients.
To date, no study specifically reporting on TNT in older patients has been published; however, many centers are also clearly incorporating this option in their armamentarium for treating this group. Moreover, this is happening without any specific oncologic trial being run on this group or the patient fitness level being assessed (in many cases) (23). Older patients are excluded based only on age alone or included without knowledge regarding their frailty (4). This has once again been shown by Aparicio et al. who analyzed 110 patients over 75 years of age who were managed for CRC and found that older patients received sub-standard treatment in 52% of cases. The majority underwent surgery whereas only a few underwent chemo- or radiotherapy (24).
Accurate diagnosis and pre-operative assessment are crucial for choosing the appropriate treatment, especially for older patients. Artificial intelligence (AI) is the new promising tool for achieving this goal. Machine learning (ML) is a field of AI that can develop mathematical algorithms capable of automatically learning different tasks with minimal human involvement. ML includes convolutional neural networks (CNNs), neural networks, and deep learning (DL). Both ML and DL have been massively adopted over these years to increase precision in diagnosis. Radiomics analyses quantitative data from diagnostic images that can be combined with ML to find other features beyond those obtained by radiologists. DL has been proven efficient in extracting information about polyp detection, cellular characteristics of RC, tumor environment and ratio, and estimating patient survival (25, 26). Regarding CT colonoscopy, further research is required to establish the role of DL in optimizing RC diagnosis, but DL could have a promising involvement in data extraction from CT scans which can increase the rate of diagnosing extraperitoneal CRC with a sensitivity of about 95% (27). However, MRI has been established as the most valuable imaging modality for primary staging and restaging after neoadjuvant treatment for RC and AI could improve accuracy in diagnosis and prognosis. Decision support tools based on MRI radiomics and ML have been developed to assist radiologists in differentiating parietal layers involved in the tumor and establish the T stage. AI has been proposed also as a tool to optimize the evaluation of lymph nodes (LNs) status. Recently, Ma et al. have been demonstrated that ML models could differentiate N0 from N1/N2 stages with a sensitivity and a specificity of 79% and 72%, respectively (28). Some authors also explored the possibility of predicting pCR through radiomics nomograms with promising results, even if it is not possible to draw a reliable conclusion (29, 30). An early and accurate diagnosis and prediction of neoadjuvant therapy response could significantly improve the management of patients with RC, mostly older patients who primarily benefit from the development of a tailored treatment.
In the present study, all patients were able to complete the entire neoadjuvant treatment pathway, although dose reduction was needed in the majority of cases. This was in contrast to what had previously been published by Margalit et al. who analyzed the rate of treatment deviation in 36 patients 75 years of age and older having rectal cancer treated with combined modality therapy. They reported an 83% rate of early treatment termination, interruption, or dose reduction. The rate of treatment deviation did not differ between patients who had preoperative or postoperative chemoradiotherapy (19% vs. 17%, p = 1.00); no significant differences in the deviation between patients with no to mild vs. moderate to severe comorbidities (p = 0.66) or no to moderate vs. severe comorbidities (p = 1.00) were reported (31).
The role of minimally invasive surgery (MIS), such as conventional or robotic-assisted, in reducing surgical trauma and improving postoperative recovery has been broadly discussed and analyzed for colorectal surgery; however, limited evidence is available regarding the effectiveness of the MIS approach in older patients with rectal cancer (32, 33). Several studies have demonstrated that age should not be considered the only significant predictor of postoperative morbidity and laparoscopic rectal cancer surgery can be safely performed, even in older patients (34, 35).
In the present study, all surgeries were performed using a conventional laparoscopic approach (namely taTME), and no conversion to an open approach was reported. The advantages of MIS become more important in geriatric patients who have limited resources for overcoming surgical stress when compared to younger counterparts. In the past, some authors argued that longer operating times and the need for the pneumoperitoneum and Trendelenburg position, which reduce venous return and potentially compromise ventilation, might significantly reduce the safety of the MIS approach in these patients (36); however, the above was not experiences in our series.
The majority of patients in our study underwent non-restorative procedures. In rectal cancer surgery, the choice between low anterior resection (LAR) with diverting loop ileostomy (DLI) and APR depends on the distance of the tumor from the anal verge/involvement of the sphincter complex, the technical feasibility of an anastomosis, the patient's clinical conditions and preferences, in light of the non-insignificant rate of suboptimal functional results after a colo-anal anastomosis (37).
In fact, besides clinical and surgical considerations, health-related quality of life (HRQoL) should be considered an important parameter of treatment efficacy. Data from the Dutch Colorectal Surgical Audit group about older patients who underwent low anterior resection (LAR) with diverting loop ileostomy (DLI) have shown that ileostomy closure was performed only in 68% of cases in the age range of 71–80, whereas only 60% of patients >81 years old had their stoma reversed. Moreover, DLI is a risk factor for readmission also after ileostomy closure (38). The recently published GOSAFE study also showed that QoL is not improved, specifically in this group of patients, by a restorative procedure while complication rate (certainly higher after a colo-anal anastomosis as compared to an inter-sphincteric APR) clearly affects short and long-term QoL (39). Indeed, several studies showed that older patients with permanent stoma have comparable HRQoL to older patients without a stoma or to normal population (40–42).
Balancing pros and cons of a coloanal anastomosis with the potential harm derived from postoperative complications might be difficult in older patients due to their limited physiologic reserves, therefore in these cases and inter-sphincteric APR seemed a viable option, after a thorough shared decision-making process. Appropriate counseling about patients' expectations and the potential side effects of a definitive DLI is advised in order to identify patients' real goals and understand which patients will be able to complete surgical treatment successfully (9).
In the present study, the median LOS was 4 days, and no readmission were reported. The present results may be biased due to a population of relatively fit patients with low frailty scores and an acceptable number of comorbidities. However, these findings point out the importance of careful patient selection based on performance scores, frailty assessment, and tumor characteristics. Moreover, the reduction in LOS due to MIS combined with enhanced recovery pathways improved postoperative functional recovery as reported in the present study group.
The vast majority of the patients underwent long-course RT (LCRT) as the treatment of choice at our center. Recent data about LR after TNT with short-course radiation therapy from the RAPIDO trial has shown an association with an increased risk of LRR and a higher rate of suboptimal TME surgery (16, 43, 44). None of this evidence was generated specifically on a study population focusing on older patients, which is the essence of the current work. In this series, the selection of a SCRT protocol was limited to 2 patients who had severe logistic limitations and couldn't achieve consistency with the 25-day long LCRT.
Early oncologic outcomes were also noted, with all the patients being able to have an R0 resection. Three patients (3/15; 20%) experienced a pCR while clear distal and circumferential margins were reported in all cases regardless of the initial staging of the cancer. Despite not being able to report on the long-term effects of TNT on disease-free and overall survival due to the limited duration of the follow up, this study showed that the rate of pCR and CRM positivity was similar/better to what has been previously reported on younger patients (45, 46). This was a promising early result for older patients affected by rectal cancer.
Conclusions
Despite the relevant incidence of rectal cancer in the older population there are few studies focusing specifically on this group. The Authors' experience regarding maximizing cancer care for fit older patients showed that extended treatment was feasible, even in the geriatric population when properly screened for frailty with good perioperative, functional, and oncologic outcomes.
The hope is that, starting from limited observational experiences such as this, more space will be given to geriatric patients in larger, prospective, oncologic trials in order to identify proper care tailored to each patient's specific needs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitato Etico della Romagna (C.E.ROM.). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. FD: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. GT: Data curation, Investigation, Validation, Writing – review & editing. ST: Conceptualization, Methodology, Validation, Writing – review & editing. EG: Investigation, Validation, Writing – review & editing. JC: Conceptualization, Data curation, Investigation, Validation, Writing – review & editing. FM: Writing – review & editing. ER: Writing – review & editing. AG: Writing – review & editing. GU: Conceptualization, Investigation, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
IM is faculty and invited speaker at Olympus SE Rectal cancer and minimally invasive surgery courses.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. Erratum in: CA Cancer J Clin. 2020 70(4):313. doi: 10.3322/caac.21492
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Rutten HJ, den Dulk M, Lemmens VE, van de Velde CJ, Marijnen CA. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol. (2008) 9(5):494–501. doi: 10.1016/S1470-2045(08)70129-3
4. Birch RJ, Taylor JC, Downing A, Spencer K, Finan PJ, Audisio RA, et al. Rectal cancer in old age -is it appropriately managed? Evidence from population-based analysis of routine data across the English National Health Service. Eur J Surg Oncol. (2019) 45(7):1196–204. doi: 10.1016/j.ejso.2019.01.005
5. De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol. (2014) 15(1):23–34. doi: 10.1016/S1470-2045(13)70546-1
6. Babaei M, Jansen L, Balavarca Y, Sjövall A, Bos A, van de Velde T, et al. Neoadjuvant therapy in rectal cancer patients with clinical stage II to III across European countries: variations and outcomes. Clin Colorectal Cancer. (2018) 17(1):e129–42. doi: 10.1016/j.clcc.2017.09.002
7. Vironen JH, Sainio P, Husa AI, Kellokumpu IH. Complications and survival after surgery for rectal cancer in patients younger than and aged 75 years or older. Dis Colon Rectum. (2004) 47(7):1225–31. doi: 10.1007/s10350-004-0557-4
8. Saur NM, Davis BR, Montroni I, Shahrokni A, Rostoft S, Russell MM, et al. The American society of colon and rectal surgeons clinical practice guidelines for the perioperative evaluation and management of frailty among older adults undergoing colorectal surgery. Dis Colon Rectum. (2022) 65(4):473–88. doi: 10.1097/DCR.0000000000002410
9. Montroni I, Ugolini G, Saur NM, Spinelli A, Rostoft S, Millan M, et al. Personalized management of elderly patients with rectal cancer: expert recommendations of the European society of surgical oncology, European society of coloproctology, international society of geriatric oncology, and American college of surgeons commission on cancer. Eur J Surg Oncol. (2018) 44(11):1685–702. doi: 10.1016/j.ejso.2018.08.003
10. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: rectal cancer, version 6.2020. J Natl Compr Canc Netw. (2020) 18(7):806–15. doi: 10.6004/jnccn.2020.0032
11. Commission on Cancer National Accreditation Program for Rectal cancer 2020 Standards and Resources. Available online at: https://www.facs.org/quality-progr ams/cancer/naprc/standards/2020 (accessed July 16, 2021)
12. Horesh N, Freund MR, Garoufalia Z, Gefen R, Nagarajan A, Suarez E, et al. Total neoadjuvant therapy is a predictor for complete pathological response in patients undergoing surgery for rectal cancer. J Gastrointest Surg. (2022) 26(12):2579–84. doi: 10.1007/s11605-022-05463-1
13. Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. (2020) 3(12):e2030097. doi: 10.1001/jamanetworkopen.2020.30097
14. Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. (2020) 271(3):440–8. doi: 10.1097/SLA.0000000000003471
15. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. (2014) 15(2):184–90. doi: 10.1016/S1470-2045(13)70599-0
16. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22(1):29–42. Erratum in: Lancet Oncol. 2021 22(2):e42. doi: 10.1016/S1470-2045(20)30555-6
17. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22(5):702–15. doi: 10.1016/S1470-2045(21)00079-6
18. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer (2017).
19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
20. Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. (2005) 47(2):141–6. doi: 10.1111/j.1365-2559.2005.02176.x
21. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2022) 20(10):1139–67. doi: 10.6004/jnccn.2022.0051
22. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Available online at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf (accessed November 27, 2017).
23. Macmillan Cancer Support. The age old Excuse: The Under Treatment of Older Cancer Patients. Macmillan Cancer Support (2012). Cancer services coming of age: learning from the improving cancer treatment assessment and support for older people project. London, UK: Department of Health, Age UK (2012).
24. Aparicio T, Navazesh A, Boutron I, Bouarioua N, Chosidow D, Mion M, et al. Half of elderly patients routinely treated for colorectal cancer receive a sub-standard treatment. Crit Rev Oncol Hematol. (2009) 71(3):249–57. doi: 10.1016/j.critrevonc.2008.11.006
25. Bousis D, Verras GI, Bouchagier K, Antzoulas A, Panagiotopoulos I, Katinioti A, et al. The role of deep learning in diagnosing colorectal cancer. Prz Gastroenterol. (2023) 18(3):266–73. doi: 10.5114/pg.2023.129494
26. Chlorogiannis DD, Verras GI, Tzelepi V, Chlorogiannis A, Apostolos A, Kotis K, et al. Tissue classification and diagnosis of colorectal cancer histopathology images using deep learning algorithms. Is the time ripe for clinical practice implementation? Prz Gastroenterol. (2023) 18(4):353–67. doi: 10.5114/pg.2023.130337
27. Wang X, Guo C, Zha Y, Xu K, Liu X. Diagnosis of nonperitonealized colorectal cancer with computerized tomography image features under deep learning. Contrast Media Mol Imaging. (2022) 2022:1886406. doi: 10.1155/2022/1886406
28. Ma X, Shen F, Jia Y, Xia Y, Li Q, Lu J. MRI-based radiomics of rectal cancer: preoperative assessment of the pathological features. BMC Med Imaging. (2019) 19(1):86. doi: 10.1186/s12880-019-0392-7
29. Wang J, Liu X, Hu B, Gao Y, Chen J, Li J. Development and validation of an MRI-based radiomic nomogram to distinguish between good and poor responders in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. Abdom Radiol (NY). (2021) 46(5):1805–15. doi: 10.1007/s00261-020-02846-3
30. Liu Z, Zhang XY, Shi YJ, Wang L, Zhu HT, Tang Z, et al. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res. (2017) 23:7253–62. doi: 10.1158/1078-0432.CCR-17-1038
31. Margalit DN, Mamon HJ, Ancukiewicz M, Kobayashi W, Ryan DP, Blaszkowsky LS, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys. (2011) 81(5):e735–41. doi: 10.1016/j.ijrobp.2010.12.056
32. Zheng J, Feng X, Yang Z, Hu W, Luo Y, Li Y. The comprehensive therapeutic effects of rectal surgery are better in laparoscopy: a systematic review and meta-analysis. Oncotarget. (2017) 8(8):12717–29. doi: 10.18632/oncotarget.14215
33. Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. (2009) 96(9):982–9. doi: 10.1002/bjs.6662
34. Manceau G, Hain E, Maggiori L, Mongin C, Prost À la Denise J, Panis Y. Is the benefit of laparoscopy maintained in elderly patients undergoing rectal cancer resection? An analysis of 446 consecutive patients. Surg Endosc. (2017) 31(2):632–42. doi: 10.1007/s00464-016-5009-4
35. Hoshino N, Fukui Y, Hida K, Sakai Y. Short-term outcomes of laparoscopic surgery for colorectal cancer in the elderly versus non-elderly: a systematic review and meta-analysis. Int J Colorectal Dis. (2019) 34(3):377–86. doi: 10.1007/s00384-019-03234-0
36. Harris SN, Ballantyne GH, Luther MA, Perrino AC Jr. Alterations of cardiovascular performance during laparoscopic colectomy: a combined hemodynamic and echocardiographic analysis. Anesth Analg. (1996) 83(3):482–7. doi: 10.1213/00000539-199609000-00007
37. Luna-Pérez P, Rodríguez-Ramírez S, Hernández-Pacheco F, Gutiérrez De La Barrera M, Fernández R, Labastida S. Anal sphincter preservation in locally advanced low rectal adenocarcinoma after preoperative chemoradiation therapy and coloanal anastomosis. J Surg Oncol. (2003) 82(1):3–9. doi: 10.1002/jso.10185
38. Jonker FH, Tanis PJ, Coene PP, Gietelink L, van der Harst E, Dutch Surgical Colorectal Audit Group. Comparison of a low Hartmann’s procedure with low colorectal anastomosis with and without defunctioning ileostomy after radiotherapy for rectal cancer: results from a national registry. Colorectal Dis. (2016) 18(8):785–92. doi: 10.1111/codi.13281
39. Montroni I, Ugolini G, Saur NM, Rostoft S, Spinelli A, Van Leeuwen BL, et al. Predicting functional recovery and quality of life in older patients undergoing colorectal cancer surgery: real-world data from the international GOSAFE study. J Clin Oncol. (2023) 41(34):JCO2202195. doi: 10.1200/JCO.22.02195
40. Pachler J, Wille-Jørgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. (2012) 12(12):CD004323. doi: 10.1002/14651858.CD004323.pub4
41. Cornish JA, Tilney HS, Heriot AG, Lavery IC, Fazio VW, Tekkis PP. A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann Surg Oncol. (2007) 14(7):2056–68. doi: 10.1245/s10434-007-9402-z
42. Orsini RG, Thong MS, van de Poll-Franse LV, Slooter GD, Nieuwenhuijzen GA, Rutten HJ, et al. Quality of life of older rectal cancer patients is not impaired by a permanent stoma. Eur J Surg Oncol. (2013) 39(2):164–70. doi: 10.1016/j.ejso.2012.10.005
43. Dijkstra EA, Nilsson PJ, Hospers GAP, Bahadoer RR, Meershoek-Klein Kranenbarg E, Roodvoets AGH, et al. Locoregional failure during and after short-course radiotherapy followed by chemotherapy and surgery compared to long-course chemoradiotherapy and surgery - a five-year follow-up of the RAPIDO trial. Ann Surg. (2023) 278(4):e766–72. doi: 10.1097/SLA.0000000000005799
44. Giunta EF, Bregni G, Pretta A, Deleporte A, Liberale G, Bali AM, et al. Total neoadjuvant therapy for rectal cancer: making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat Rev. (2021) 96:102177. doi: 10.1016/j.ctrv.2021.102177
45. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. (2015) 16(8):957–66. doi: 10.1016/S1470-2045(15)00004-2
Keywords: rectal cancer, elderly, neoadjuvant therapy, transanal total mesorectal excision, functional recovery
Citation: Montroni I, Di Candido F, Taffurelli G, Tamberi S, Grassi E, Corbelli J, Mauro F, Raggi E, Garutti A and Ugolini G (2024) Total neoadjuvant therapy followed by total mesorectal excision for rectal cancer in older patients real world data and proof of concept. Front. Surg. 11:1448073. doi: 10.3389/fsurg.2024.1448073
Received: 26 June 2024; Accepted: 4 November 2024;
Published: 19 November 2024.
Edited by:
Vito D'Andrea, Sapienza University of Rome, ItalyReviewed by:
Francesk Mulita, General Hospital of Eastern Achaia- Unit of Aigio, GreeceJoão Sousa, University of Brasilia, Brazil
Copyright: © 2024 Montroni, Di Candido, Taffurelli, Tamberi, Grassi, Corbelli, Mauro, Raggi, Garutti and Ugolini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Di Candido, ZnJhbmNlc2NhZGljYW5kaWRvQGdtYWlsLmNvbQ==
†ORCID:
Francesca Di Candido
orcid.org/0000-0002-2284-4357
 Isacco Montroni1
Isacco Montroni1 Francesca Di Candido
Francesca Di Candido