- 1Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 2Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 3Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 4Department of Anorectal Surgery, Shenzhen Longhua District Central Hospital, Shenzhen, Guangdong, China
- 5Department of Geriatrics, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
Purpose: Colorectal cancer (CRC) patients may experience inadequate preoperative colonoscopy due to bowel obstruction or inadequate bowel preparation, leading to potential oversight of other polyps. We aimed to identify risk factors for CRC complicated with synchronous high-risk polyps.
Methods: A retrospective analysis of 6,674 CRC patients from December 2014 to September 2018 was conducted. High-risk polyps were defined as adenomas or serrated polyps that were ≥10 mm, or with tubulovillous/villous components or high-grade dysplasia. All other polyps were defined as low-risk polyps. Patients with complete pathological and clinical information were categorized into three groups: the no polyp group, the low-risk polyp group, and the high-risk polyp group. Univariate and multivariate logistic regression analyses were performed to calculate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for all potential risk factors.
Results: Among the 4,659 eligible patients, 848 (18.2%) were found to have low-risk polyps, while 675 (14.5%) were diagnosed with high-risk polyps. In a multivariate logistic regression model, compared to patients without polyps, those with synchronous high-risk polyps were more likely to be male (OR = 2.07), aged 50 or older (OR = 2.77), have early-stage tumors (OR = 1.46), colon tumors (OR = 1.53), NRAS mutant tumors (OR = 1.66), and BRAF wild-type tumors (OR = 2.43).
Conclusion: Our study has identified several risk factors associated with the presence of synchronous high-risk polyps in CRC patients. Based on these findings, we recommend that patients who exhibit these high-risk factors undergo early follow-up of colonoscopy to detect synchronous polyps early.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death worldwide (1). The majority of CRC cases originate from premalignant polyps that accumulate sufficient mutations to progress towards high-grade dysplasia and subsequently CRC (2). Premalignant polyps encompass both conventional adenomas and serrated polyps. Conventional adenomas are further classified into tubular adenomas, tubulovillous adenomas, and villous adenomas, while serrated polyps are classified as traditional serrated adenomas, sessile serrated polyps, and hyperplastic polyps according to the WHO classification (3, 4). The removal of premalignant polyps represents the most effective approach for CRC prevention and may hold greater significance compared to subsequent surveillance measures (5).
The quality of colonoscopy significantly influences the detection of colorectal lesions. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) Guidelines recommend high-quality perioperative colonoscopy to identify and remove synchronous lesions before CRC surgery (6, 7). However, in cases where CRC patients experience bowel obstruction, the endoscope may be unable to pass through the tumor to visualize the proximal bowel, potentially leading to missed lesions such as synchronous polyps. Meanwhile, poor bowel preparation can also lead to incomplete colonoscopy. Incomplete preoperative colonoscopy is associated with an elevated risk of postoperative CRC, particularly metachronous CRC (mCRC). mCRC is defined as a second primary CRC diagnosed at least 6 months after the initial CRC diagnosis, rather than a recurrence of the primary CRC (8). Despite guidelines recommending postoperative colonoscopy for CRC, the incidence of mCRC has not decreased over the past decade (5–7), with the cumulative incidence ranging from 1% to 4% (7). The etiology of mCRC remains undetermined, but missed lesions resulting from incomplete colonoscopy are a major contributing factor (6, 8, 9). For CRC patients who undergo incomplete preoperative colonoscopy, a prompt re-examination via colonoscopy following surgery can facilitate the timely detection and removal of polyps, thereby preventing the occurrence of mCRC.
The aim of this study was to explore the risk factors associated with CRC complicated by synchronous premalignant polyps, particularly high-risk polyps.
Patients and methods
Study population
This retrospective case-control study involved CRC patients who were treated at the Six Affiliated Hospital, Sun Yat-sen University (Guangzhou, China) from December 2014 to September 2018. Approval was obtained from the ethics committee of the Sixth Affiliated Hospital, Sun Yat-sen University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Patients with CRC were identified through inpatient and outpatient discharge diagnoses, colonoscopy reports, or pathology reports from the hospital. All eligible patients had undergone at least one colonoscopy at the Six Affiliated Hospital, Sun Yat-sen University, which included an initial colonoscopy either preoperatively or within six months after surgery. Patients with Peutz-Jeghers syndrome (PJS) or familial adenomatous polyposis (FAP) were excluded from the data collection process.
Case and control identification
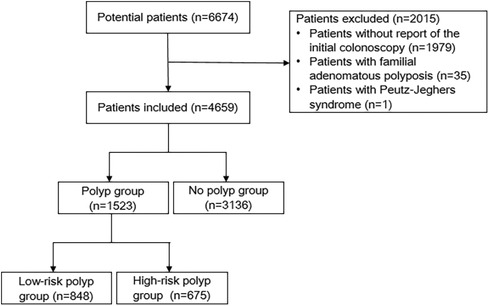
We collected the clinical records, colonoscopy reports, and pathology reports of 6,674 CRC patients from the Six Affiliated Hospital, Sun Yat-sen University. Potential participants were excluded for the following reasons: no recorded preoperative or postoperative colonoscopy within six months (n = 1,979), FAP (n = 35), or PJS (n = 1). Ultimately, a total of 4,659 eligible patients were included in the study. Based on the endoscopy and pathology reports, the study participants were classified into three groups. Participants with at least one synchronous polyp measuring ≥10 mm or exhibiting a villous/tubulovillous component or high-grade dysplasia were categorized as high-risk polyp cases. Participants with synchronous polyps that did not meet the criteria for high-risk polyps were classified as low-risk polyp cases. Controls were participants who had no synchronous polyps detected during the colonoscopy.
Definition of variables
We documented patient, tumor, and polyp information on a standardized form utilizing pre-defined definitions of variables: (1) Patient information encompassed admission number (AD), gender, age, admission date, and body mass index (BMI) (Chinese standard). (2) Tumor characteristics comprised anatomical location, pathologic stage, DNA mismatch repair (MMR), PIK3CA, NRAS, BRAF, and KRAS gene mutation status. (3) Polyp characteristics included number, size, pathological diagnosis results, and anatomical location.
Age was categorized as <50 and ≥50 years old. BMI was categorized as <18.5, 18.5–23.9, and >23.9 kg/m2 in accordance with Chinese classification standards. Tumor anatomical locations were categorized as colon or rectum. Pathologic stage was determined based on the American Joint Committee on Cancer (AJCC) TNM Staging Classification for Carcinoma of the Colon and Rectum (Eighth Edition) (10). In our statistical analysis, patients with stage I and II were consolidated as early-stage patients, while those with stage III and IV were classified as advanced patients. MMR was categorized as deficient MMR (dMMR) and proficient MMR (pMMR). Tumor gene mutation status was classified as mutant or wild-type.
Statistical analysis
The demographic and disease characteristics of the patients were described using descriptive statistics. Proportions were used to present all dichotomous variables. The association between categorical variables based on baseline characteristics was tested using Pearson's χ2 test. Univariate analysis and multivariate logistic regression were utilized to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for all potential risk factors. A significance level of P < 0.05 was considered statistically significant for all analyses, and two-sided tests were conducted. All statistical analyses were performed using SPSS software version 25.0 for Windows.
Results
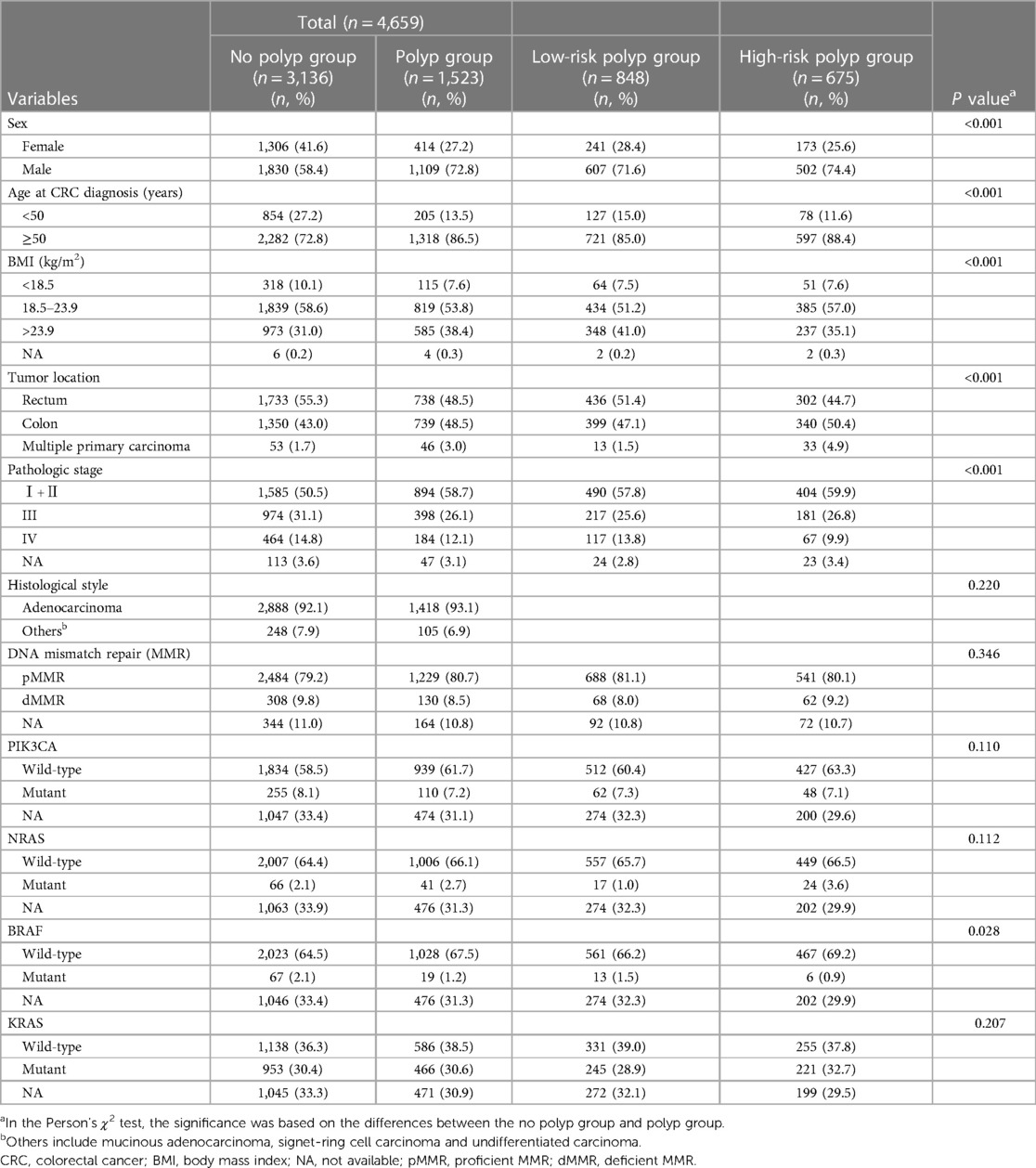
A total of 6,674 CRC patients were initially considered as potential participants for our study. The flow diagram outlining the study process is presented in Figure 1. Ultimately, based on the inclusion and exclusion criteria, a total of 4,659 patients were included in our analysis. Table 1 illustrates the characteristics of CRC patients with or without synchronous polyps. 1,523 patients had polyps, among which 1,295 were found with polyp in preoperative colonoscopy. The median number of polyp found was 2. 675 had high-risk polyps, while 848 had low-risk polyps. Patients with polyps were found to be significantly older, predominantly male, and had higher BMI compared to those without polyps. Notably, a higher proportion of patients with rectal tumors were observed in the no polyp group. Additionally, the polyp group had a higher proportion of patients with early-stage tumors. Furthermore, BRAF mutant tumors were more prevalent in the no polyp group. No significant differences were observed in terms of MMR, KRAS mutation status, NRAS mutation status, and PIK3CA mutation status.
Risk factors for synchronous high-risk polyps
Univariate analysis and multivariate logistic regression analyses were conducted to identify the risk factors associated with synchronous high-risk polyps, as presented in Table 2. In the univariate analysis, male sex, age ≥50, colon tumors, early-stage tumors, NRAS mutant tumors, and BRAF wild-type tumors were significantly associated with an increased risk of high-risk polyps. However, BMI, MMR, PIK3CA mutation status, and KRAS mutation status showed no association with high-risk polyps. All variables that showed statistical significance in the univariate analysis (P < 0.05) were included in the multivariate logistic regression analysis. After adjusting for confounding factors, male sex (OR, 2.07; 95% CI, 1.71–2.50), age ≥50 (OR, 2.77; 95% CI, 2.15–3.56), colon tumors (OR, 1.53; 95% CI, 1.28–1.83), early-stage tumors (OR, 1.46; 95% CI, 1.23–1.75), NRAS mutant tumors (OR, 1.66; 95% CI, 1.01–2.72), and BRAF wild-type tumors (OR, 2.43; 95% CI, 1.03–5.70) remained significantly associated with a higher risk of high-risk polyps. Consequently, we identified male sex, age ≥50, colon tumors, early-stage tumors, NRAS mutant tumors, and BRAF wild-type tumors as independent risk factors for synchronous high-risk polyps.
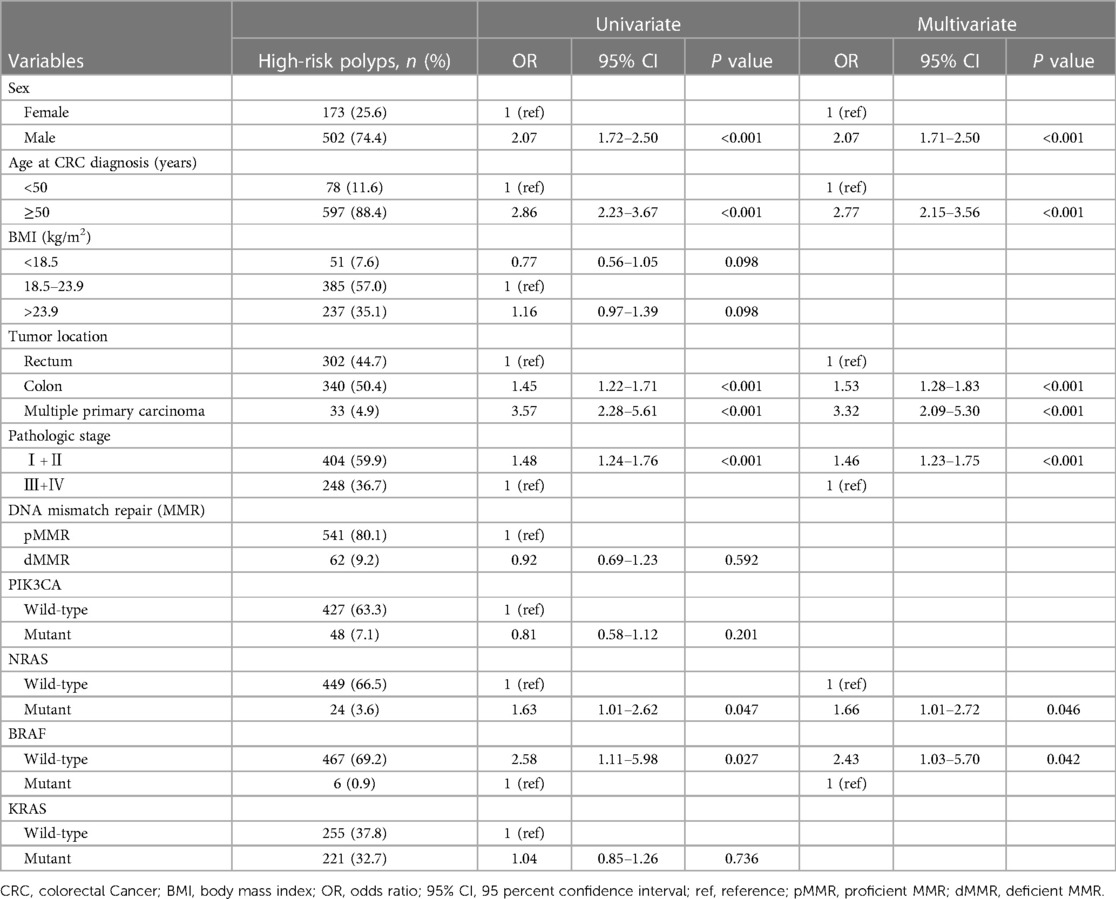
Table 2 Univariate and multivariate logistic regression analysis on risk factors for colorectal cancer complicated with high-risk polyps.
Risk factors for synchronous low-risk polyps
We employed the same methodology to analyze the risk factors associated with synchronous low-risk polyps, and the results are presented in Table 3. In the univariate analysis, male sex, age ≥ 50, BMI > 23.9, colon tumors, and early-stage tumors were significantly associated with an increased risk of low-risk polyps. However, MMR, KRAS mutation status, NRAS mutation status, BRAF mutation status, and PIK3CA mutation status showed no association with low-risk polyps. After adjusting for confounding variables using multivariate logistic regression analysis, male sex (OR, 1.79; 95% CI, 1.52–2.12), age ≥ 50 (OR, 2.07; 95% CI, 1.69–2.55), BMI > 23.9 (OR, 1.52; 95% CI, 1.29–1.79), colon tumors (OR, 1.21; 95% CI, 1.03–1.41), and early-stage tumors (OR, 1.31; 95% CI, 1.12–1.54) remained significantly associated with a higher risk for synchronous low-risk polyps.
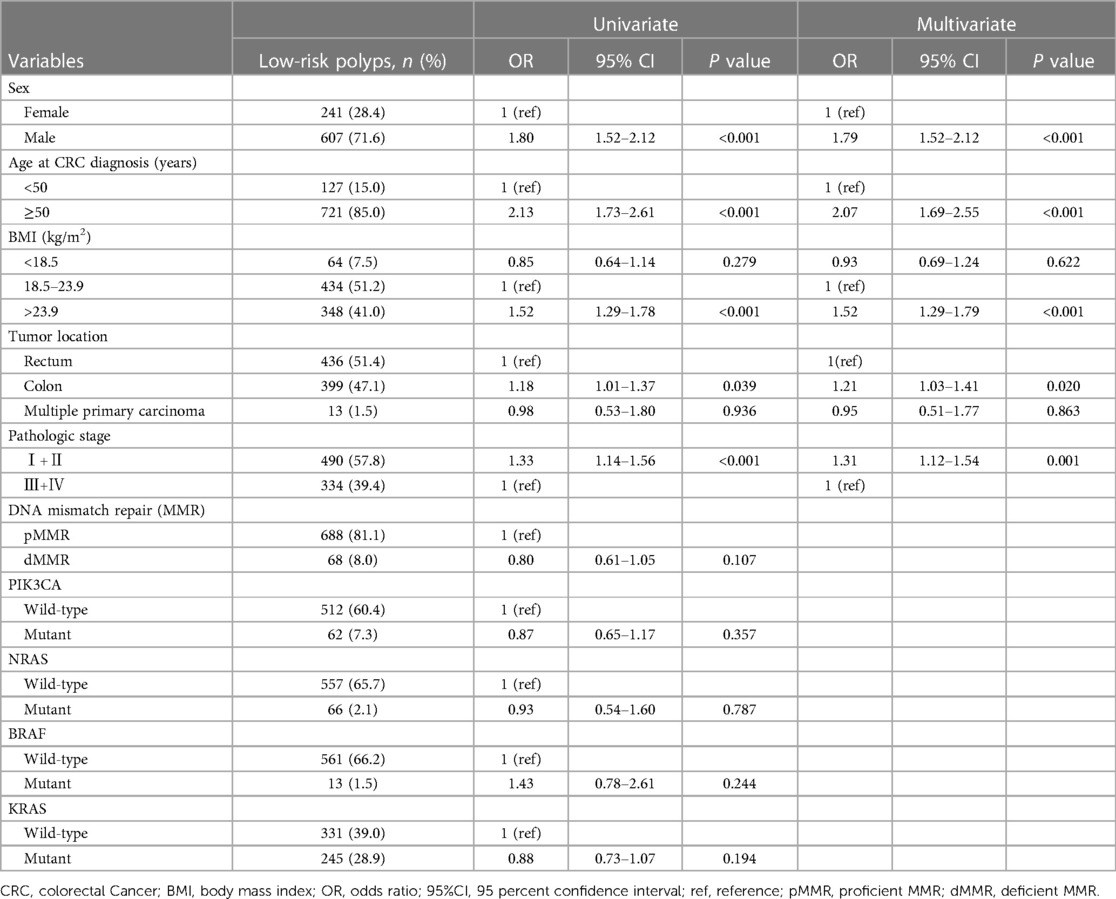
Table 3 Univariate and multivariate logistic regression analysis on risk factors for colorectal cancer complicated with low-risk polyps.
Discussion
Preoperatively missed synchronous premalignant polyps are a significant contributor to the development of mCRC in CRC patients. To identify potential strategies for preventing mCRC, we investigated several risk factors associated with CRC complicated by premalignant polyps. In a multivariate analysis, we identified male sex, age ≥50, colon tumors, early-stage tumors, NRAS mutant tumors, and BRAF wild-type tumors as risk factors for CRC patients with high-risk polyps. Additionally, we analyzed the risk factors of CRC complicated by low-risk polyps and found that the risk was increased in patients with male sex, age ≥ 50, BMI > 23.9, colon tumors, and early-stage tumors.
Following surgery and adjuvant treatment, such as radiation therapy and chemotherapy, many patients with non-metastatic CRC are cured. However, the morbidity and mortality rates among CRC patients have not declined in recent years, potentially due to the occurrence of mCRC. Although many studies have attempted to identify the causes of mCRC, few have been reported. Research has shown that part of the metachronous cancers may be caused by missed lesions (8). Some studies have reported that the miss rate of premalignant polyps found during colonoscopy ranges from 12% to 47% (11–15). Therefore, our study aimed to identify the risk factors associated with CRC complicated by premalignant polyps to identify patients who are more likely to have missed lesions.
Our study confirmed that male and advanced age are risk factors for CRC complicated with synchronous high-risk polyps, which is consistent with the findings of Kazushige Kawai et al. (16). It is well-known that men have a higher incidence of colorectal cancer compared to women. Additionally, the average age of male patients is higher than that of female patients, and advanced age has been established as a strong risk factor for mCRC (17–19). However, current postoperative surveillance practices for CRC patients do not take gender or age into consideration (4–6, 9). Considering our findings, it may be worth considering whether older men should undergo more frequent postoperative surveillance. This could potentially help in reducing the development of mCRC.
In our study, we found that patients with early-stage tumors had a higher risk of synchronous polyps than those with advanced tumors. Interestingly, patients with advanced tumors had a higher likelihood of inadequate colonoscopy due to bowel obstruction, which may result in an underestimation of the number of polyps present in these patients. Our results also showed that tumors located in the colon had a greater risk of synchronous polyps compared to tumors located in the rectum. Previous research has demonstrated that colon tumors are more frequently associated with microsatellite instability, overexpression of epiregulin, chromosomal instability, and epidermal growth factor receptor amplification compared to rectal tumors (20–24). These pathological features may contribute to the increased risk of synchronous polyps in patients with colon tumors.
Currently, several biomarkers play a crucial role in guiding clinicians towards making optimal treatment decisions for CRC patients. These biomarkers include PIK3CA, NRAS, BRAF, KRAS, and DNA mismatch repair genes. They help in the selection of individualized treatment plans based on their respective functions. In our study, we observed that NRAS mutant tumors and BRAF wild-type tumors were identified as risk factors for synchronous high-risk polyps in CRC patients. Traditionally, these biomarkers have been primarily used to guide the treatment of CRC patients. However, our findings indicate an additional role for them. By assessing the mutation status of NRAS and BRAF, we can potentially predict patients at higher risk of synchronous polyps and subsequently intensify postoperative surveillance for these individuals.
The prevention of postoperative CRC through the detection and removal of high-risk polyps is considered a crucial benefit of postoperative colonoscopy. Therefore, various expert guidelines recommend colonoscopy after radical surgery for CRC (4–6, 9). However, several studies have shown that intensive postoperative surveillance, including annual colonoscopies, does not provide a survival benefit compared to standard follow-ups (25–27). Additionally, colonoscopy is an invasive procedure that carries risks, including severe complications such as bleeding and perforation, which can even lead to death. Thus, it is essential to strike a balance between the risk of mCRC and complications when determining the optimal surveillance strategy after curative surgery, in order to avoid unnecessary colonoscopies. However, data on the risk factors for mCRC are limited and often conflicting, and current guidelines do not recommend risk stratification for postoperative endoscopic surveillance (9). In our study, we aimed to identify risk factors through multivariate analysis and stratify patients based on their risk of developing mCRC after surgery. The current findings suggest that male gender, age ≥50, colon tumors, early-stage tumors, NRAS mutant tumors, and BRAF wild-type tumors are risk factors for CRC with high-risk polyps. We recommend that CRC patients with these risk factors, especially those with multiple risk factors, undergo their first postoperative colonoscopy 6 months after curative surgery. Patients who do not have these risk factors can undergo postoperative colonoscopy within one year after surgery, following the standard follow-up protocol (25–27). This approach ensures that patients receive appropriate surveillance while avoiding unnecessary invasive procedures. A well-planned surveillance strategy can optimize the utilization of endoscopy resources, reduce the burden on the medical and health service systems, and promote efficiency. It is important to note that a high-quality index colonoscopy is crucial. If the bowel preparation is inadequate, the patient should undergo a repeat colonoscopy as soon as possible to detect any missed lesions. In cases where the preoperative colonoscopy was incomplete due to intestinal obstruction, a postoperative colonoscopy should also be performed within 6 months after surgery.
Our study possessed several strengths. Firstly, a key advantage was the substantial size of our study population, encompassing nearly 4,659 CRC patients. Secondly, we conducted a comprehensive review of the colonoscopy reports and pathology reports of each patient, categorizing them into the no polyp group, high-risk polyp group, and low-risk polyp group. This stratification was essential due to the differing risk of developing CRC between high-risk and low-risk polyps, with high-risk polyps serving as better predictors of mCRC occurrence. Thirdly, the variables we analyzed, such as sex, age, tumor location, and biomarkers, were readily available, rendering our research results more applicable in clinical practice. Several limitations need to be noted. One significant limitation of this study was its retrospective nature, meaning that we relied on data as recorded in clinical records, colonoscopy reports, and pathology reports. Colonoscopists' subjective estimation of polyp size may have introduced errors, potentially leading to an inflated number of high-risk polyp cases. Secondly, due to the retrospective design, incomplete clinical data were unavoidable. Thirdly, the exclusion of patients without colonoscopy reports may have resulted in selection bias.
Conclusion
Male gender, advanced age, colon tumors, early-stage tumors, NRAS mutant tumors, and BRAF wild-type tumors were identified as characteristics associated with CRC complicated by high-risk polyps. Early follow-up of colonoscopy in patients exhibiting these characteristics is crucial to detect synchronous polyps early.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of the Sixth Affiliated Hospital, Sun Yat-sen University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
DH: Data curation, Methodology, Writing – original draft, Writing – review & editing. JC: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. XJ: Data curation, Formal Analysis, Writing – original draft. HC: Data curation, Formal Analysis, Investigation, Writing – original draft. JH: Conceptualization, Supervision, Validation, Writing – review & editing. ZC: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by National Key Research and Development Program of China, No. 2023YFC2507401, Guangzhou Basic and Applied Basic Research Foundation, No. 202201011160 National Key Clinical Discipline.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. (1988) 319(9):525–32. doi: 10.1056/nejm198809013190901
3. He X, Hang D, Wu K, Nayor J, Drew DA, Giovannucci EL, et al. Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology. (2020) 158(4):852–61.e854. doi: 10.1053/j.gastro.2019.06.039
4. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. (2012) 143(3):844–57. doi: 10.1053/j.gastro.2012.06.001
5. Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. (2020) 69(2):201–23. doi: 10.1136/gutjnl-2019-319858
6. Hassan C, Wysocki PT, Fuccio L, Seufferlein T, Dinis-Ribeiro M, Brandão C, et al. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) guideline. Endoscopy. (2019) 51(3):266–77. doi: 10.1055/a-0831-2522
7. Rex DK. Key quality indicators in colonoscopy. Gastroenterol Rep (Oxf). (2023) 11:goad009. doi: 10.1093/gastro/goad009
8. le Clercq CM, Winkens B, Bakker CM, Keulen ET, Beets GL, Masclee AA, et al. Metachronous colorectal cancers result from missed lesions and non-compliance with surveillance. Gastrointest Endosc. (2015) 82(2):325–33.e322. doi: 10.1016/j.gie.2014.12.052
9. Spada C, Stoker J, Alarcon O, Barbaro F, Bellini D, Bretthauer M, et al. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guideline. Endoscopy. (2014) 46(10):897–915. doi: 10.1055/s-0034-1378092
10. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
11. Bensen S, Mott LA, Dain B, Rothstein R, Baron J. The colonoscopic miss rate and true one-year recurrence of colorectal neoplastic polyps. Polyp prevention study group. Am J Gastroenterol. (1999) 94(1):194–9. doi: 10.1111/j.1572-0241.1999.00796.x
12. Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. (2004) 141(5):352–9. doi: 10.7326/0003-4819-141-5-200409070-00009
13. Patel SG, Ahnen DJ. Prevention of interval colorectal cancers: what every clinician needs to know. Clin Gastroenterol Hepatol. (2014) 12(1):7–15. doi: 10.1016/j.cgh.2013.04.027
14. van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. (2006) 101(2):343–50. doi: 10.1111/j.1572-0241.2006.00390.x
15. Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin. (2006) 56(3):160–7. quiz 185–166. doi: 10.3322/canjclin.56.3.160
16. Kawai K, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Miyata H, et al. Nomogram prediction of metachronous colorectal neoplasms in patients with colorectal cancer. Ann Surg. (2015) 261(5):926–32. doi: 10.1097/sla.0000000000000881
17. Moon CM, Cheon JH, Choi EH, Kim ES, Park JJ, Han SY, et al. Advanced synchronous adenoma but not simple adenoma predicts the future development of metachronous neoplasia in patients with resected colorectal cancer. J Clin Gastroenterol. (2010) 44(7):495–501. doi: 10.1097/MCG.0b013e3181d6bd70
18. Bonithon-Kopp C, Piard F, Fenger C, Cabeza E, O'Morain C, Kronborg O, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum. (2004) 47(3):323–33. doi: 10.1007/s10350-003-0054-1
19. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. (2009) 22(4):191–7. doi: 10.1055/s-0029-1242458
20. Cone MM, Beck DE, Hicks TE, Rea JD, Whitlow CB, Vargas HD, et al. Timing of colonoscopy after resection for colorectal cancer: are we looking too soon? Dis Colon Rectum. (2013) 56(11):1233–6. doi: 10.1097/DCR.0b013e3182a228d1
21. Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. (2012) 61(6):847–54. doi: 10.1136/gutjnl-2011-300865
22. Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol. (2010) 37(3):707–18. doi: 10.3892/ijo_00000720
23. Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. (2014) 25(10):1995–2001. doi: 10.1093/annonc/mdu275
24. Maus MK, Hanna DL, Stephens CL, Astrow SH, Yang D, Grimminger PP, et al. Distinct gene expression profiles of proximal and distal colorectal cancer: implications for cytotoxic and targeted therapy. Pharmacogenomics J. (2015) 15(4):354–62. doi: 10.1038/tpj.2014.73
25. Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. (1998) 114(1):7–14. doi: 10.1016/s0016-5085(98)70626-2
26. Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. (1997) 84(5):666–9.9171758
Keywords: high-risk polyps, colorectal cancer, risk factors, postoperative surveillance, colonoscopy
Citation: He D, Chen J, Jiang X, Chen H, Huang J and Chen Z (2024) Risk factors for synchronous high-risk polyps in patients with colorectal cancer. Front. Surg. 11:1424809. doi: 10.3389/fsurg.2024.1424809
Received: 28 April 2024; Accepted: 11 June 2024;
Published: 24 June 2024.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Giulia Turri, University of Verona, ItalyFrancesk Mulita, General University Hospital of Patras, Greece
Simona Ascanelli, University Hospital of Ferrara, Italy
Giovanni Tomasicchio, Università degli Studi di Bari, Italy
© 2024 He, Chen, Jiang, Chen, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zexian Chen, Y2hlbnp4NTNAbWFpbC5zeXN1LmVkdS5jbg==; Juanni Huang, aHVhbmdqdWFubmkyMDA4MDhAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Degao He
Degao He Junguo Chen
Junguo Chen Xuefei Jiang
Xuefei Jiang Hao Chen1,2,3
Hao Chen1,2,3 Zexian Chen
Zexian Chen