- Department of Breast Surgery, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Locally advanced breast cancer (LABC) remains a significant clinical challenge, particularly in developing countries. While neoadjuvant systemic therapy (NST) has improved the pathological complete response (pCR) rates, particularly in HER2-positive and triple-negative breast cancer patients, surgical management post-NST continues to evolve. The feasibility of omitting surgery and the increasing consideration of breast-conserving surgery, immediate reconstruction in LABC patients are important areas of exploration. Accurate assessment of tumor response to NST through advanced imaging and minimally invasive biopsies remains pivotal, though challenges persist in reliably predicting pCR. Additionally, axillary lymph node management continues to evolve, with emerging strategies aiming to minimize the extent of surgery in patients who achieve nodal downstaging post-NST. Minimizing axillary lymph node dissection in favor of less invasive approaches is gaining attention, though further evidence is needed to establish its oncological safety. The potential for personalized treatment approaches, reducing surgical morbidity, and improving quality of life are key goals in managing LABC, while maintaining the priority of achieving favorable long-term outcomes.
Introduction
LABC is commonly referred to inoperable cancers which surgical resection is impossible without systemic therapy and absence of distant metastases. In general, clinically stage III breast cancer was included (1, 2).
The implementation of a comprehensive breast cancer screening program has resulted in a comparatively low prevalence of LABC in developed nations (3). It still remains a big challenge in developing countries. For instance, in India, 47% of breast cancer cases are diagnosed at stage III (4). Despite the elevated risk of recurrence and metastasis, LABC can still be curable if local control is attained. Due to the use of dual human epidermal growth factor receptor 2 (HER2) blockade and platinum-based neoadjuvant chemotherapy, the rate of pCR rate in HER2 positive (HER2+) or triple-negative breast cancer (TNBC) patients has increased to more than 30% (5). Mastectomy and axillary lymph node dissection (ALND) are commonly employed as the standard surgical procedures for patients diagnosed with LABC. Surgery is performed with the objective of completely excising the primary tumor, as well as any adjacent skin or muscular involved. As the treatment approach for breast cancer transitions from “maximum tolerable” to “minimum effective” treatment, it is important to consider if there are additional surgical options available for patients with LABC, while considering the pretreatment stage and response to NST. This review seeks to investigate the potential for omitting breast surgery and the viability of breast-conserving surgery (BCS) or immediate reconstruction (IR) without compromising oncological safety for LABC. Additionally, as well as to identify targeted patients for ALND exemption, thus promoting individualized surgical options for LABC patients.
Ways to evaluate the effectiveness of NST and possibility of omitting surgery
Assessing the response of breast cancer patients to NST before surgery is essential for tailoring personalized surgical plans and treatment strategies. In cases where patients achieve a clinical complete response (cCR), meaning no detectable cancer is found through physical examination and imaging, it may even be possible to consider omitting surgery (6). Magnetic resonance imaging (MRI) is more accurate in predicting pCR and residual disease compared to clinical examination, ultrasound and mammography (7, 8). However, MRI can either overestimate or underestimate residual disease. Overestimation may occur due to fibrosis, necrotic tumors, or residual benign masses, while underestimation can be caused by no mass lesions, invasive lobular carcinoma, hormone receptor-positive (HR+) tumors, nonconcentric shrinkage patterns, antiangiogenic therapy, or late-enhancing foci (9). Therefore, the accuracy of MRI is still falling short of clinical expectations. Moreover, the accuracy varies significantly across different molecular subtypes, with the highest sensitivity observed in TNBC and the lowest in HR+/HER2-subtypes (10). Therefore, relying solely on imaging results is insufficient. In recent years, multiple trials have explored the predictive value of image-guided minimally invasive biopsy (MIB) techniques, such as core needle biopsy (CNB), vacuum-assisted biopsy (VAB), and fine-needle aspiration (FNA), for determining breast pCR following NST. For example, the study by Sutton et al. (11) found that MRI-guided VAB can increase the accuracy of predicting pCR to 95%. However, Hemert et al. (12) found that small residual lesions (4–7 mm) are often tended to be missed in biopsy procedures. A meta-analysis (13) of nine trials involving 1,030 breast cancer patients found that, while the pooled sensitivity and specificity of MIB were 0.72 [95% confidence interval (CI): 0.61–0.81] and 0.99 [95% CI: 0.89–1.00], respectively, current image-guided MIB methods are still not accurate enough for reliably predicting breast pCR after NST.
The question of whether patients achieving cCR or pCR through MIB can be exempted from breast surgery has been addressed in previous retrospective studies by Ring (14) and Clouth (15), which included patients with stage III breast cancer. Their findings indicate that omitting breast surgery does not affect survival outcomes in the long run. However, there are no studies specifically examining the exemption of surgery in LABC. A multicenter phase II clinical trial (NCT02945579) (16) led by MD Anderson Cancer Center is exploring the possibility of omitting surgery after NST, but it has excluded LABC patients. Despite the pooled analysis published in Lancet (17) showing that patients who achieve pCR exhibit improved long-term survival rates, a recent meta-analysis (18) of 54 clinical studies found only a weak association between pCR and both disease-free survival (DFS) and overall survival (OS). The meta-analysis concluded that pCR should not be considered the primary endpoint in trials of NST for breast cancer. Currently, there are no effective methods available for accurately assessing pCR. Moreover, pCR cannot be considered a primary endpoint in research, as patients with LABC who achieve pCR are not yet exempt from surgery.
Breast surgery
Feasibility and safety of BCS in LABC patients
Is BCS considered a safe treatment option for LABC patients who exhibit positive response to NST? This paragraph explores the influence of tumor shrinkage patterns on the feasibility of BCS, evaluates the criteria for selecting patients for BCS, and discusses the implications of different margin definitions on clinical outcomes. The crucial aspect of BCS is to achieve a negative pathological margin, so it's important to understand the pattern of tumor regression. The tumor shrinkage patterns following NST predominantly exhibit concentric and non-concentric characteristics. Wang et al. (19) classified residual tumor morphology into three categories: isolated residual tumors (61%), multifocal and patchlike (33%), and main residues with satellite lesions (6%). Most tumors exhibited isolated concentric shrinkage, while the other two types demonstrated non-concentric shrinkage. The primary tumor's size directly influenced its concentric shrinkage pattern, with larger tumors more often showing non-concentric shrinkage, which complicates the attainment of negative margins. The application of BCS after NST is theoretically limited to tumors exhibiting concentric shrink patterns. For multiple lesions in the same quadrant, BCS can be attempted. The primary tumor in LABC is typically large and prone to be non-concentric. As a result, it is necessary to conduct imaging comparisons before and after NST in order to comprehensively assess the patterns of tumor shrinkage. Bi et al. (20) conducted a study on 3D MRI reconstruction of residual tumors, suggesting that a 50% reduction in the longest diameter and a size of ≤2 cm post-NST could qualify patients for BCS. This criterion could potentially expand the BCS-eligible patient population. After a median follow-up of 77 months, the rate of recurrence or metastasis was 7.1%. The National Comprehensive Cancer Network and St. Gallen consensus (21, 22) define negative margins for BCS after NST as “no ink on tumor,” consistent with criteria for BCS without NST. However, a 2022 meta-analysis in the British Medical Journal (23) challenged this standard, finding that close margins (defined as no tumor on ink but <2 mm) were linked to a higher risk of local recurrence and metastasis compared to negative margins (≥2 mm), even when accounting for adjuvant chemotherapy and radiotherapy (P < 0.001). This raises concerns about the adequacy of the “no tumor on ink” criterion for BCS post-NST. The safety of applying non-NST margin criteria to NST cases remains inconclusive due to insufficient high-level evidence.
The rate of BCS after NST in LABC patients ranged from 12.5% to 43.4% in several retrospective studies (24–27). BCS was found to be oncologically safe for LABC patients who responded well to NST. Younger patients, those with smaller tumors, and those achieving pCR were more frequently selected for BCS. Additionally, patients in the NST-BCS group were more commonly found to have HER2+/HR- or TNBC (24, 27), as well as non-invasive lobular carcinoma, compared to the mastectomy group (25, 26).
Sun et al. (28) conducted a meta-analysis of 16 studies, finding no significant difference in local recurrence-free survival (LRFS) between the BCS and mastectomy groups (P = 0.26). However, DFS and OS were higher in the BCS group (P < 0.01). This may be attributed to the higher pCR rates in the BCS group (29), which is associated with improved DFS and OS. While these results imply that BCS maybe safe for LABC patients who have a favorable response to NST, it should be noted that the studies referenced are all retrospective. Therefore, high-quality medical evidence is still needed to confirm these conclusions.
Feasibility and safety of IR after NST
Breast reconstruction offers patients who cannot undergo BCS an opportunity for a more aesthetically pleasing breast shape and can help mitigate some of the negative effects of total mastectomy. Considerations include the benefits of immediate reconstruction (IR) versus delayed reconstruction (DR), the oncological safety of different reconstruction techniques, and the effects of combining these procedures with NST and radiation therapy.IR is associated with higher physical and psychological satisfaction compared to DR, and patients desiring reconstruction may opt for IR without compromising safety (30). Procedures such as nipple-sparing, skin-sparing, or skin-reducing mastectomies allow for IR, with nipple-sparing mastectomies requiring a negative margin at the posterior of the nipple-areola complex (31). There is a lack of high-quality evidence confirming the oncological safety of nipple-sparing mastectomy combined with reconstruction. However, several retrospective studies indicate that IR after NST does not increase the risk of local recurrence or negatively impact long-term survival. For instance, Meli et al. (32) found that there is no significant difference in local recurrence or survival between patients who undergo nipple-sparing mastectomy with or without NST, suggesting that IR is a viable and safe option. Wu et al. (33) found no significant differences in long-term outcomes including 5-year LRFS, DFS, or OS between patients who had IR after NST and those who had NR. This indicates that opting for IR does not compromise long-term survival, thus reinforcing the safety and desirability of IR. However, some caution is advised. A study by Song et al. (34) highlighted those patients with tumors exceeding 3 cm who received IR had a lower 5-year DFS compared to those who had no reconstruction, suggesting that IR may be more appropriate for smaller tumors (≤3 cm). For stage T4 breast cancer, particularly inflammatory breast cancer, Pawloski et al. (35) found that IR significantly increased the likelihood of postoperative complications and delayed the start of radiotherapy, often by more than 8 weeks. Due to these complications and the observation that the average time until the first recurrence was 18 months within a median follow-up of 4.2 years, the study recommended postponing reconstruction for at least 18 months after surgery. Wu et al. (36) reported no significant differences in LRFS, DFS, or OS between patients with poor responses to NST who underwent nipple-sparing or skin-sparing IR and those who had mastectomy alone. This suggests that the response to NST should not solely determine the choice of IR. A meta-analysis (37) of 17 studies involving 3,249 patients examined the effect of NST on postoperative complications associated IR. The analysis found that neoadjuvant NST did not significantly raise the overall risk of postoperative complications (P = 0.34). The analysis did show a statistically significant rise in the rate of implant or expander loss (P = 0.03). This suggests that while NST does not broadly elevate the risk of complications, it may specifically heighten the risk of implant-related issues.
There is widespread agreement that postmastectomy radiation therapy can lead to skin discoloration and reduction in size of the nipple-areola complex (38). In the meantime, the 2022 recommendations from the Oncoplastic Breast Consortium (39) generally agree that post-mastectomy radiation therapy (PMRT) raises the risk of complications in all forms of implant-based breast reconstruction. Most experts in the field concur that PMRT carries a lower overall long-term risk of complications after immediate autologous reconstruction compared to implant-based reconstruction. In order to avoid delaying PMRT after IR, a reverse sequence (RS) of NST, preoperative radiotherapy, mastectomy and IR has been proposed. Paillocher et al. (40) included 111 patients with RS, with a median follow-up of 31.6 months. The 5-year DFS and OS were 93.2% and 98.3%, respectively, and patient satisfaction was high (17/20). In this study, radiotherapy was feasible 4 weeks after the end of NST in the RS group, while immediate autologous latissimus dorsi breast reconstruction surgery was feasible 6–8 weeks following the conclusion of radiotherapy in the standard sequence (SS) group, and RS could shorten the treatment time. Maire et al. (41) compared the RS and SS approaches using the autologous latissimus dorsi flap with or without an implant. With a median follow-up of 61.7 months, there was no significant difference between the groups in OS (P = 0.44) or RFS (P = 0.30). Postoperative morbidity also did not differ significantly between the two groups (P = 0.51). In the RS group, the average time from the end of radiotherapy to surgery was 5.9 weeks, compared to 8.4 weeks in the SS group from surgery to the start of radiotherapy, indicating that RS could significantly shorten treatment time (P < 0.001). To further explore the optimization of treatment timelines, the ongoing single-arm clinical trial NCT05412225 (42) is investigating the feasibility of preoperative radiotherapy followed by total mastectomy and autologous IR in LABC patients. This approach aims to avoid delays in radiotherapy after IR.
For LABC patients, doctors should guide them to fully understand the process, risks and benefits of reconstructive surgery, and be clear about the expected results of surgery. Patients with original large tumor, IR should be performed with great cautiousness. T4 stage, especially inflammatory breast cancer, IR is not recommended. Patients who are willing to reconstruct and need radiation therapy can receive radiation therapy before surgery after NST to shorten the treatment time and at the same time maintain the aesthetics of the breast after reconstruction.
Axillary lymph node management after NST
The use of NST has significantly changed the approach to axillary lymph node management in breast cancer. Traditionally, ALND was performed for patients with clinically positive nodes (cN+), but recent efforts have explored less invasive alternatives. The primary goal is to strike a balance between reducing surgical morbidity and maintaining oncological safety for these patients. A meta-analysis (43) including 33 studies revealed that axillary lymph node pCR rates by breast cancer subtypes in patients with cN+ were 60%, 45%, 48%, and 18% for HR-/HER2+, HR+/HER2+, HR-/HER2-, and HR+/HER2-, respectively. This suggests that patients with HER2+ and TNBC may be eligible for less extensive axillary surgery. Data from the Netherlands Cancer Registry (44) revealed that between 2006 and 2016, there was a notable increase in the rate of patients with initially negative axillary lymph nodes with non-invasive diagnostic methods (cN0) who underwent sentinel lymph node biopsy (SLNB) after NST, rising from 33% to 62%. Additionally, the rate of patients with cN+ who underwent ALND decreased from 99% to 63% (P < 0.01). There is ongoing debate about the conditions under which ALND can be safely omitted after NST. This discussion is particularly relevant when lymph nodes initially assessed as positive before treatment (cN+) are found to be negative upon pathological examination after treatment, as determined through SLNB (ypN0). The European Breast Cancer Research Association of Surgical Trialists (EUBREAST) conducted a global survey (45) in 2020, highlighting differing expert opinions on axillary management post-NST. Key points of contention include whether ALND can be omitted for patients whose positive nodes become negative (cN+→ ypN0) and the appropriate treatment for patients with sentinel lymph nodes (SLNs) showing isolated tumor cells (ypN0[i+]) or micrometastases (ypN1[mi]).
Data from large clinical trials (46–48) have found that NST potentially increase the FNR of SLNB due to its effects on axillary lymphatic reflux patterns, disruption of lymphatic structures, and induction of fibrosis. Several meta-analyses have also confirmed that the use of dual-tracer sampling and removing a minimum of three SLNs are effective in reducing FNR (49, 50). In addition, a strategy that involves marking nodes with biopsy-confirmed metastases prior to initiating NST and subsequently performing SLNB with targeted axillary dissection (TAD) has been shown to effectively reduce FNR. Anderson Cancer Center (51) revealed FNR of 10.1% and 1.4% for SLNB and SLNB in combination with TAD, respectively (P = 0.03).
Safety of cN+→ ypN0 exemption from ALND
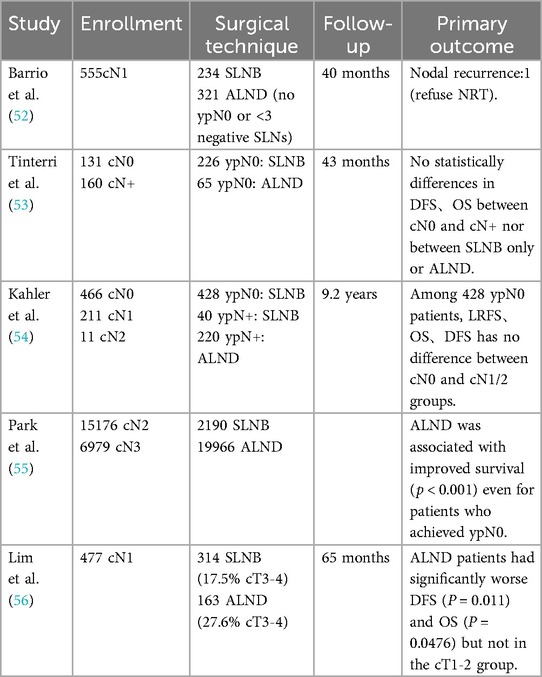
There is some controversy in the guidelines as to whether cN+→ypN0 patients should be spared from ALND. Barrio et al. (52) conducted an analysis on a cohort of 610 patients diagnosed with cN1. Among those patients, 91% who were ypN0 underwent SLNB alone. It was found that 42% of these patients had three or more SLNs removed, and 70% of them received regional nodal irradiation (RNI). At a median follow-up of 40 months, recurrence in the axillary nodes was noted in just one patient, who did not undergo RNI. This study suggested that cN1→ ypN0 patients, and who had three or more SLNs identified through SLNB, may not require ALND. Tinterri et al. (53) studied 291 patients who were ypN0 after SLNB, including 131 who were cN0 and 160 who were cN+ before treatment. After a median follow-up of 43 months, the local recurrence rates in the axillary nodes were 2.3% for patients with cN0 and 1.3% for those with cN+. There were no significant differences in DFS and OS between the cN0 and cN+ groups or between those who had SLNB and those who had ALND. Similarly, Kahler et al. (54) analyzed 688 ypN0 patients after SLNB, with a median follow-up of 9.2 years. They observed local axillary recurrence rates of 1.8% for cN0 patients and 1.5% for cN1-2 patients, with no significant difference in DFS and OS between the groups. These retrospective studies consistently show that cN+ → ypN0 patients do not necessarily need ALND. However, some limitations exist, such as Tinterri et al.'s lack of detailed information about cN+ patients and Kahler et al.'s inclusion of only 12 cases of cN2 patients. In contrast, Park et al. (55) analyzed data from 22,156 cN2-3 patients in the National Cancer Database. Of these, 2,190 (9.9%) underwent SLNB. After adjusting for relevant factors, the study found that ALND was linked to a reduced risk of mortality compared to SLNB, even in patients who achieved pCR. In a study conducted by Lim et al. (56), 477 patients with cN1→ ypN0 were analyzed. At a median follow-up of 65 months, patients who underwent ALND had worse DFS (P = 0.011) and OS (P = 0.0476) compared to those who had only SLNB. They noted that the ALND group had a higher number of patients with larger tumors (T3-4). However, in the subgroup of patients with smaller tumors (cT1–2), there was no significant difference in DFS and OS between the two groups. The details of the corresponding retrospective studies are provided in Table 1.
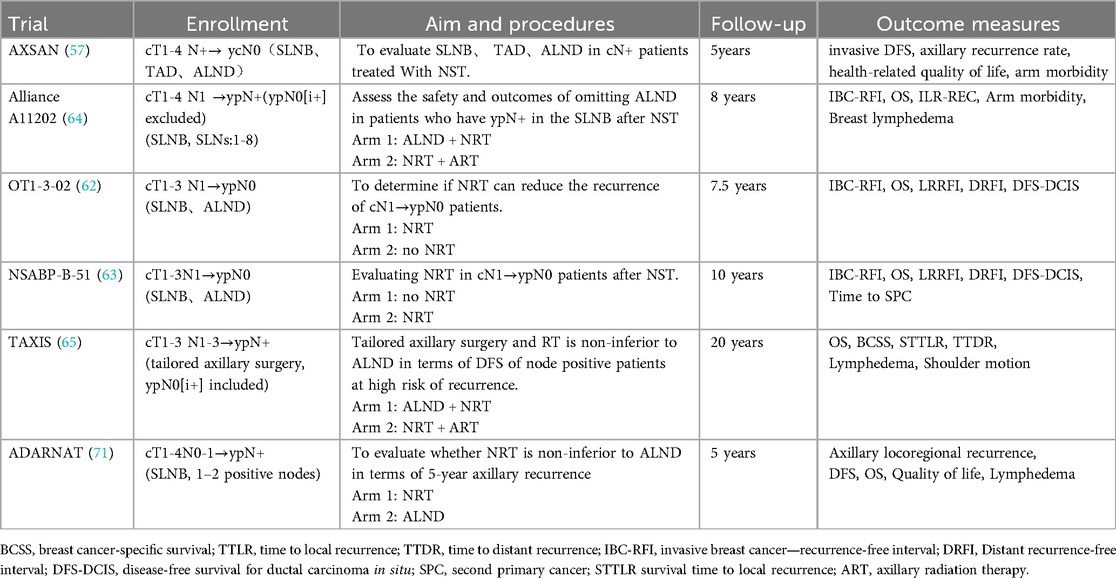
The AXSANA trial (57), a multi-center prospective study, aims to recruit a total of 3,000 patients by the year 2030. The aim of this study is to evaluate the feasibility and safety of various surgical techniques, including ALND, SLNB, and TAD, in patients with positive lymph nodes. In summary, for LABC patients, particularly those achieving ypN0 status, it may be appropriate to consider less invasive surgical options like SLNB, especially when dealing with smaller tumors and fewer affected lymph nodes. However, for patients with more extensive disease, ALND may still be warranted until the final results of the AXSANA trial are available.
Axillary lymph node management for ypN0(i+) and ypN1(mi)
The management of ypN0(i+) or ypN1(mi) remains a topic of debate. Wong et al. (58) showed that the 5-year DFS of ypN0(i+) and ypN1(mi), ypN0 patients in the Dana-Farber/Brigham and Women's Cancer Center (DFBWCC) was 73.5%, 74.7%, and 88.4% (P < 0.001); the 5-year OS in the NCDB database was 82. 8%, 79.5%, and 88.9% (P < 0.001). Subgroup analyses indicated that ypN0(i+) and ypN1(mi) exhibited a poorer prognosis compared to ypN0, particularly in cases of HER2+ and TNBC. Pending results from large clinical trials, this study suggested that patients with ypN0(i+) and ypN1(mi) should undergo ALND. In contrast, a retrospective study (59) conducted in the Netherlands revealed that there was no difference in the 5-year OS (P = 0.889) or DFS (P = 0.613) rates between patients with ypN0(i+) and ypN1(mi) compared to those with ypN0. The potential explanation for this disparity, aside from population variation, could be attributed to the fact that 70.4% of ypN0(i+) and 80.3% of ypN1(mi) cases included in the DFBWCC study ultimately underwent ALND, whereas in the Netherlands study, all patients underwent ALND. Kantor et al. (60) analyzed 4,496 patients with HR+/HER2-, finding no statistically difference between ypN0 or ypN+ in LRFS, OS, and DFS between those who underwent ALND and patients who did not. Based on these findings, the study suggested that ypN0(i+) and ypN1(mi) patients may not need ALND after neoadjuvant endocrine therapy. The international multicenter retrospective OPBC-05/ICARO study (61) examined 583 patients with ypN0(i+). Among them, 182 patients received ALND, while 401 did not. Among those who underwent ALND, 30% were found to have additional positive nodes. There was no significant difference in the 5-year rate of any oncologic outcomes. Consequently, the study suggests that routine ALND may not be necessary for this patient population. Existing studies present conflicting findings regarding the potential exemption of ypN0(i+) and ypN1(mi) from ALND. In the EUBREAST survey (45), it was found that 32.3% of the experts recommended no additional treatment for ypN0(i+) patients, while 33.1% suggested RNI. A total of 34.8% of the experts recommended ALND for ypN1(mi) patients, while 30.4% expressed a preference for RNI. The prevailing viewpoint among experts at the 2021 St. Gallen Conference (22) was in favor of RNI as opposed to ALND for patients with ypN0(i+), ypN1(mi). However, definitive guidelines are pending the results of ongoing clinical trials. The OT1-3-02 (62) and NSABP B-51/RTOG 1,304 (63) clinical trials were conducted to enroll patients with ypN0(i+) in order to examine the potential benefits of RNI. The Alliance A11202 (64) and TAXIS (65) phase III trials are evaluating the safety of omitting ALND in patients with ypN1(mi). The details of the corresponding retrospective studies are provided in Table 2.
Possibility of ALND omission in ypN+ patients
ALND is commonly used as a standard treatment for breast cancer patients with positive SLNs, but it is associated with significant morbidity. Recently, there has been interest in finding less invasive alternatives that maintain oncological safety. Efforts have been made to investigate the potential of SLNB and RNI as a safe alternative to ALND in certain cases. The ACOSOG Z0011 study (66) included 892 female patients with T1 or T2 breast cancer who underwent BCS and had metastases in one or two SLNs without palpable axillary lymphadenopathy, were followed for a median of 9.3 years. The results indicated that SLN alone was not inferior to that of patients treated with ALND. The AMAROS Trial (67) included 1,425 patients with cT1-2, node-negative breast cancer and a positive sentinel node biopsy, who were randomly assigned to either ALND or NRT. The 10-year analysis shows that both treatments resulted in a low axillary recurrence rate, with no significant differences in OS, DFS, or locoregional control. Though these two clinical trials did not include patients who received NST, they still provide a potential option for patients with LABC. Efforts have been made to find less invasive procedures for ypN+ patients.
The retrospective study conducted by Almahariq et al. (68) included patients with cT1-3N1 who were converted to ypN1 after NST from the NCDB. Out of the total sample, 1,313 patients underwent ALND and 304 patients underwent SLNB. All patients received RNI. The study found a statistically significant difference in 5-year OS between the two groups, with a higher survival rate in the ALND group compared to the SLNB (P = 0.01). Furthermore, the multivariate analysis revealed that SLNB was linked to lower survival rates (P < 0.001). A different conclusion was reached by Park et al. (69), who conducted a study incorporating data from 14 medical centers in South Korea. The study included 1,103 cases of ALND and 170 cases of SLNB, with all patients receiving RNI. With a median follow-up period of 75.3 months, the study found no statistically significant disparity in DFS (P = 0.406) or OS (P = 0.083) between two groups, and multivariate analysis indicated SLNB did not compromise oncological outcomes, suggesting that exemption from ALND could be a feasible option for ypN+ patients receiving RNI. The study conducted by Almahariq et al. (68) encountered limitations in data extraction from the NCDB, resulting in the specification of SLNs ranging from 1 to 4. On the other hand, the study conducted by Park et al. (69) did not impose any restrictions on the number of positive lymph nodes either prior to or following NST, but the median number of SLNs in this study was 6. A study conducted by Moo et al. (70) involving 273 patients with positive SLNs undergoing ALND revealed a high incidence of ALND positivity across all molecular subtypes, with no significant difference observed between micrometastases and macrometastases. Therefore, they recommend performing ALND for patients with positive SLNs, regardless of molecular subtype.
Several ongoing trials are exploring these alternatives. Alliance A11202 (64) is an ongoing phase III clinical trial to enroll 2012 cN1 patients with positive SLNs after NST, with one group receiving ALND followed by RNI and the other group receiving RNI alone. TAXIS (65) is a multinational, multicenter phase III clinical trial aimed at assessing the viability and effectiveness of exempting cN1-2 patients with positive SLNs from ALND, which includes both patients who receive NST or not. ADARNAT (71) is a multicenter, randomized, open-label phase 3 trial involving 1,660 patients with 1-2positive SLNs post-NST, across 50 Spanish centers. Patients will be assigned at random to either a group receiving NRT without ALND or a group undergoing ALND. The primary outcome is 5-year axillary recurrence. Table 3 provides detailed information on all the ongoing trials mentioned in this section.
Discussion
In conclusion, the management of LABC remains complex, requiring tailored to individual patient profiles. Challenges still persist in predicting complete responses to NST and omitting surgery seems inappropriate for LABC patients at this time. Existing retrospective studies have demonstrated the safety of BCS in patients who respond well to NST. However, the standard for negative margins after NST needs to be further validated through large-scale randomized controlled trials. For LABC patients with a desire for reconstruction, IR does not compromise tumor safety and does not increase complications. Patients who have large initial tumors should be cautious, and IR at T4 is not recommended. Patients with a desire for IR who still need radiotherapy after surgery, a reverse treatment sequence of NST, preoperative radiotherapy, mastectomy, and IR is feasible. Regarding axillary lymph node management, guidelines emphasize the importance of dual tracer imaging and the identification of three or more SLNs due to the increased FNR following NST. Some of the available retrospective studies had SLNs less than 3, which could potentially contribute to the significant variability in the results. Therefore, it is imperative to include SLN ≥3 as a crucial criterion in the design of clinical trials that explore exemptions from ALND. Based on the findings of retrospective studies, patients who have cN0-1 →ypN0 can potentially be excluded from undergoing ALND. However, it takes extreme caution when treating patients with cN2-3 →ypN0, and ALND is strongly recommended in such cases. As to whether the presence of micrometastases or macrometastases in the SLNs can exempt patients from ALND, the results of the available studies are inconsistent and definitive conclusions will have to await the long-term survival data from the various ongoing clinical trials. We have observed that few clinical studies on axillary lymph node management have considered the impact of different molecular subtypes. As noted by Swarnkar (72), persistent positive lymph nodes after treatment may suggest a more aggressive tumor in HER2-positive and TNBC patients, as these subtypes are generally more responsive to NST compared to luminal subtypes. This observation implies that such cases might necessitate more aggressive surgical approaches, such as ALND. Additional research is required to confirm this hypothesis and to further refine treatment strategies. Overall, the surgical approach following NST for LABC should be tailored based on pre-treatment clinical characteristics, NST efficacy, and the patient's overall condition. A balanced consideration of the benefits and risks, aligned with the patient's preferences, should guide a collaborative decision between the patient and the surgeon.
Author contributions
ZS: Writing – original draft, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. KL: Supervision, Visualization, Writing – original draft. YG: Supervision, Validation, Writing – original draft. NJ: Supervision, Validation, Writing – original draft. MY: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Training Program for Innovative Key Talents of Traditional Chinese Medicine (19-Z-1-13).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tryfonidis K, Senkus E, Cardoso MJ, Cardoso F. Management of locally advanced breast cancer-perspectives and future directions. Nat Rev Clin Oncol. (2015) 12(3):147–62. doi: 10.1038/nrclinonc.2015.74
2. Aebi S, Karlsson P, Wapnir IL. Locally advanced breast cancer. Breast. (2022) 62:S58–62. doi: 10.1016/j.breast.2021.12.011
3. Kadys A, Gremke N, Schnetter L, Kostev K, Kalder M. Intercontinental comparison of women with breast cancer treated by oncologists in Europe, Asia, and Latin America: a retrospective study of 99,571 patients. J Cancer Res Clin Oncol. (2023) 149(10):7319–26. doi: 10.1007/s00432-023-04681-7
4. Dhanushkodi M, Sridevi V, Shanta V, Rama R, Swaminathan R, Selvaluxmy G, et al. Locally advanced breast cancer (LABC): real-world outcome of patients from cancer institute, Chennai. JCO Glob Oncol. (2021) 7:767–81. doi: 10.1200/GO.21.00001
5. Al-Tweigeri T, Elshenawy M, Badran A, Omar A, Suleman K, Al Malik O, et al. Impact of pathologic complete response following neoadjuvant chemotherapy ± trastuzumab in locally advanced breast cancer. J Oncol. (2021) 2021:6639763. doi: 10.1155/2021/6639763
6. Tasoulis MK, Muktar S, Smith I, Roche N, MacNeill F. Omission of breast surgery in selected breast cancer patients with excellent response to neoadjuvant systemic therapy. Eur J Surg Oncol. (2024) 50(6):108277. doi: 10.1016/j.ejso.2024.108277
7. Palshof FK, Lanng C, Kroman N, Benian C, Vejborg I, Bak A, et al. Prediction of pathologic complete response in breast cancer patients comparing magnetic resonance imaging with ultrasound in neoadjuvant setting. Ann Surg Oncol. (2021) 28(12):7421–9. doi: 10.1245/s10434-021-10117-8
8. Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. (2013) 105(5):321–33. doi: 10.1093/jnci/djs528
9. Reig B, Lewin AA, Du L, Heacock L, Toth HK, Heller SL, et al. Breast MRI for evaluation of response to neoadjuvant therapy. Radiographics. (2021) 41(3):665–79. doi: 10.1148/rg.2021200134
10. Janssen LM, den Dekker BM, Gilhuijs KGA, van Diest PJ, van der Wall E, Elias SG. MRI To assess response after neoadjuvant chemotherapy in breast cancer subtypes: a systematic review and meta-analysis. NPJ Breast Cancer. (2022) 8(1):107. doi: 10.1038/s41523-022-00475-1
11. Sutton EJ, Braunstein LZ, El-Tamer MB, Brogi E, Hughes M, Bryce Y. Accuracy of magnetic resonance imaging-guided biopsy to verify breast cancer pathologic complete response after neoadjuvant chemotherapy: a nonrandomized controlled trial. JAMA Netw Open. (2021) 4(1):e2034045. doi: 10.1001/jamanetworkopen.2020.34045
12. van Hemert AKE, van Duijnhoven FH, van Loevezijn AA, Loo CE, Wiersma T, Groen EJ, et al. Biopsy-Guided pathological response assessment in breast cancer is insufficient: additional pathology findings of the MICRA trial. Ann Surg Oncol. (2023) 30(8):4682–9. doi: 10.1245/s10434-023-13476-6
13. Li Y, Zhou Y, Mao F, Lin Y, Zhang X, Shen S, et al. The diagnostic performance of minimally invasive biopsy in predicting breast pathological complete response after neoadjuvant systemic therapy in breast cancer: a meta-analysis. Front Oncol. (2020) 10:933. doi: 10.3389/fonc.2020.00933
14. Ring A, Webb A, Ashley S, Allum WH, Ebbs S, Gui G, et al. Is surgery necessary after complete clinical remission following neoadjuvant chemotherapy for early breast cancer?. J Clin Oncol. (2003) 21(24):4540–5. doi: 10.1200/JCO.2003.05.208
15. Clouth B, Chandrasekharan S, Inwang R, Smith S, Davidson N, Sauven P. The surgical management of patients who achieve a complete pathological response after primary chemotherapy for locally advanced breast cancer. Eur J Surg Oncol. (2007) 33(8):961–6. doi: 10.1016/j.ejso.2006.12.006
16. Kuerer HM, Smith BD, Krishnamurthy S, Yang WT, Valero V, Shen Y, et al. Eliminating breast surgery for invasive breast cancer in exceptional responders to neoadjuvant systemic therapy: a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2022) 23(12):1517–24. doi: 10.1016/S1470-2045(22)00613-1
17. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. (2014) 384(9938):164–72. doi: 10.1016/S0140-6736(18)32772-7
18. Conforti F, Pala L, Sala I, Oriecuia C, De Pas T, Specchia C, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. Br Med J. (2021) 375:e066381. doi: 10.1136/bmj-2021-066381
19. Wang S, Zhang Y, Yang X, Fan L, Qi X, Chen Q, et al. Shrink pattern of breast cancer after neoadjuvant chemotherapy and its correlation with clinical pathological factors. World J Surg Oncol. (2013) 11(1):166. doi: 10.1186/1477-7819-11-166
20. Bi Z, Qiu PF, Yang T, Chen P, Song XR, Zhao T, et al. The modified shrinkage classification modes could help to guide breast conserving surgery after neoadjuvant therapy in breast cancer. Front Oncol. (2022) 12:982011. doi: 10.3389/fonc.2022.982011
21. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network. (2022) 20(6):691–722. doi: 10.6004/jnccn.2022.0030
22. Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the st. Gallen international consensus guidelines for treatment of early breast cancer 2021. Ann Oncol. (2021) 32(10):1216–35. doi: 10.1016/j.annonc.2021.06.023
23. Bundred JR, Michael S, Stuart B, Cutress RI, Beckmann K, Holleczek B, et al. Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. Br Med J. (2022) 378:e070346. doi: 10.1136/bmj-2022-070346
24. Sang Y, Zhou X, Chi W, Chen J, Yang B, Hao S, et al. Surgical options of the breast and clinical outcomes of breast cancer patients after neoadjuvant chemotherapy: a single-center retrospective study. Front Oncol. (2022) 12:984587. doi: 10.3389/fonc.2022.984587
25. Mazor AM, Mateo AM, Demora L, Sigurdson ER, Handorf E, Daly JM, et al. Breast conservation versus mastectomy in patients with T3 breast cancers (> 5 cm): an analysis of 37,268 patients from the national cancer database. Breast Cancer Res Treat. (2019) 173(2):301–11. doi: 10.1007/s10549-018-5007-4
26. Shin HC, Han W, Moon HG, Im SA, Moon WK, Park IA, et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann Surg Oncol. (2013) 20(8):2582–9. doi: 10.1245/s10434-013-2909-6
27. Cho JH, Park JM, Park HS, Park S, Kim SI, Park BW. Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. J Surg Oncol. (2013) 108(8):531–6. doi: 10.1002/jso.23439
28. Sun Y, Liao M, He L, Zhu C. Comparison of breast-conserving surgery with mastectomy in locally advanced breast cancer after good response to neoadjuvant chemotherapy: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). (2017) 96(43):e8367. doi: 10.1097/MD.0000000000008367
29. Pawloski KR, Barrio AV. Breast surgery after neoadjuvant systemic therapy. Transl Breast Cancer Res. (2024) 5:13. doi: 10.21037/tbcr-23-50
30. Zhong T, Hu J, Bagher S, Vo A, O'Neill AC, Butler K, et al. A comparison of psychological response, body image, sexuality, and quality of life between immediate and delayed autologous tissue breast reconstruction: a prospective long-term outcome study. Plast Reconstr Surg. (2016) 138(4):772–80. doi: 10.1097/PRS.0000000000002536
31. Colwell AS, Taylor EM. Recent advances in implant-based breast reconstruction. Plast Reconstr Surg. (2020) 145(2):421e–32e. doi: 10.1097/PRS.0000000000006510
32. Meli EZ, De Santis A, Cortese G, Manna E, Mastropietro T, La Pinta M, et al. Nipple-Sparing mastectomy after neoadjuvant chemotherapy: definitive results with a long-term follow-up evaluation. Ann Surg Oncol. (2023) 30(4):2163–72. doi: 10.1245/s10434-022-13035-5
33. Wu ZY, Kim HJ, Lee JW, Chung IY, Kim JS, Lee SB, et al. Long-term oncologic outcomes of immediate breast reconstruction vs conventional mastectomy alone for breast cancer in the setting of neoadjuvant chemotherapy. JAMA Surg. (2020) 155(12):1142–50. doi: 10.1001/jamasurg.2020.4132
34. Song Y, Sun S, Li D, Han J, Niu M, Luo S, et al. Long-term oncologic safety of immediate reconstructive surgery in patients with invasive breast cancer: a retrospective matched-cohort study. World J Surg Oncol. (2021) 19(1):348. doi: 10.1186/s12957-021-02450-9
35. Pawloski KR, Barrio AV, Gemignani ML, Sevilimedu V, Le T, Dayan J, et al. Reconstruction in women with T4 breast cancer after neoadjuvant chemotherapy: when is it safe? J Am Coll Surg. (2021) 233(2):285–93. doi: 10.1016/j.jamcollsurg.2021.04.016
36. Wu ZY, Kim HJ, Lee J, Chung IY, Kim J, Lee SB, et al. Oncologic outcomes of immediate breast reconstruction in young women with breast cancer receiving neoadjuvant chemotherapy. Breast Cancer Res Treat. (2022) 191(2):345–54. doi: 10.1007/s10549-021-06428-9
37. Varghese J, Gohari SS, Rizki H, Faheem M, Langridge B, Kümmel S, et al. A systematic review and meta-analysis on the effect of neoadjuvant chemotherapy on complications following immediate breast reconstruction. Breast. (2021) 55:55–62. doi: 10.1016/j.breast.2020.11.023
38. Jagsi R, Momoh AO, Qi J, Hamill JB, Billig J, Kim HM, et al. Impact of radiotherapy on complications and patient-reported outcomes after breast reconstruction. J Natl Cancer Inst. (2018) 110(2):157–65. doi: 10.1093/jnci/djx148
39. Weber WP, Shaw J, Pusic A, Wyld L, Morrow M, King T, et al. Oncoplastic breast consortium recommendations for mastectomy and whole breast reconstruction in the setting of post-mastectomy radiation therapy. Breast. (2022) 63:123–39. doi: 10.1016/j.breast.2022.03.008
40. Paillocher N, Florczak AS, Richard M, Classe JM, Oger AS, Raro P, et al. Evaluation of mastectomy with immediate autologous latissimus dorsi breast reconstruction following neoadjuvant chemotherapy and radiation therapy: a single institution study of 111 cases of invasive breast carcinoma. Eur J Surg Oncol. (2016) 42(7):949–55. doi: 10.1016/j.ejso.2016.03.024
41. Maire M, Debled M, Petit A, Fournier M, Macgrogan G, Quenel-Thueux N, et al. Neoadjuvant chemotherapy and radiotherapy for locally advanced breast cancer: safety and efficacy of reverse sequence compared to standard technique? Eur J Surg Oncol. (2022) 48(8):1699–705. doi: 10.1016/j.ejso.2022.04.022
42. ClinicalTrials.gov A Study of an Alternative Treatment Approach (Preoperative Radiotherapy, Then Mastectomy, Then Immediate Reconstruction Surgery) in People with T4 Breast Cancer. (2022). Available online at: https://clinicaltrials.gov/ct2/show/NCT05412225 (Accessed August 13, 2024).
43. Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML, et al. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg. (2021) 156(6):e210891. doi: 10.1001/jamasurg.2021.0891
44. Simons JM, Koppert LB, Luiten EJT, van der Pol CC, Samiei S, de Wilt JHW, et al. De-escalation of axillary surgery in breast cancer patients treated in the neoadjuvant setting: a Dutch population-based study. Breast Cancer Res Treat. (2020) 180(3):725–33. doi: 10.1007/s10549-020-05589-3
45. Gasparri ML, de Boniface J, Poortmans P, Gentilini OD, Kaidar-Person O, Banys-Paluchowski M, et al. Axillary surgery after neoadjuvant therapy in initially node-positive breast cancer: international EUBREAST survey. Br J Surg. (2022) 109(9):857–63. doi: 10.1093/bjs/znac217
46. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA. (2013) 310(14):1455–61. doi: 10.1001/jama.2013.278932
47. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. (2013) 14(7):609–18. doi: 10.1016/S1470-2045(13)70166-9
48. Classe JM, Loaec C, Gimbergues P, Alran S, de Lara CT, Dupre PF, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. (2019) 173(2):343–52. doi: 10.1007/s10549-018-5004-7
49. Simons JM, van Nijnatten TJA, van der Pol CC, Luiten EJT, Koppert LB, Smidt ML. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. (2019) 269(3):432–42. doi: 10.1097/SLA.0000000000003075
50. Tee SR, Devane LA, Evoy D, Rothwell J, Geraghty J, Prichard RS, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. (2018) 105(12):1541–52. doi: 10.1002/bjs.10986
51. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. (2016) 34(10):1072–8. doi: 10.1200/JCO.2015.64.0094
52. Barrio AV, Montagna G, Mamtani A, Sevilimedu V, Edelweiss M, Capko D, et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy-A rare event. JAMA Oncol. (2021) 7(12):1851–5. doi: 10.1001/jamaoncol.2021.4394
53. Tinterri C, Sagona A, Barbieri E, Di Maria Grimaldi S, Caraceni G, Ambrogi G, et al. Sentinel lymph node biopsy in breast cancer patients undergoing neo-adjuvant chemotherapy: clinical experience with node-negative and node-positive disease prior to systemic therapy. Cancers (Basel). (2023) 15(6):1719. doi: 10.3390/cancers15061719
54. Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, Vicini E, Morigi C, Corso G, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. (2021) 47(4):804–12. doi: 10.1016/j.ejso.2020.10.014
55. Park TS, Thomas SM, Rosenberger LH, Fayanju OM, Plichta JK, Blitzblau RC, et al. The association of extent of axillary surgery and survival in women with N2-3 invasive breast cancer. Ann Surg Oncol. (2018) 25(10):3019–29. doi: 10.1245/s10434-018-6587-2
56. Lim SZ, Yoo TK, Lee SB, Kim J, Chung IY, Ko BS, et al. Long-term outcome in patients with nodal-positive breast cancer treated with sentinel lymph node biopsy alone after neoadjuvant chemotherapy. Breast Cancer Res Treat. (2024) 203(1):95–102. doi: 10.1007/s10549-023-07104-w
57. ClinicalTrials.gov AXillary Surgery After NeoAdjuvant Treatment (AXSANA). (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04373655 (Accessed August 13, 2024).
58. Wong SM, Almana N, Choi J, Hu J, Gagnon H, Natsuhara K, et al. Prognostic significance of residual axillary nodal micrometastases and isolated tumor cells after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. (2019) 26(11):3502–9. doi: 10.1245/s10434-019-07517-2
59. van Nijnatten TJ, Simons JM, Moossdorff M, de Munck L, Lobbes MB, van der Pol CC, et al. Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: isolated tumor cells and micrometastases carry a better prognosis than macrometastases. Breast Cancer Res Treat. (2017) 163(1):159–66. doi: 10.1007/s10549-017-4157-0
60. Kantor O, Wong S, Weiss A, Metzger O, Mittendorf EA, King TA. Prognostic significance of residual nodal disease after neoadjuvant endocrine therapy for hormone receptor-positive breast cancer. NPJ Breast Cancer. (2020) 6:35. doi: 10.1038/s41523-020-00177-6
61. Montagna G, Laws A, Ferrucci M, Mrdutt M, Polidorio N, Sevilimedu V, et al. Abstract GS02-02: are nodal ITCs after neoadjuvant chemotherapy an indication for axillary dissection? The OPBC05/EUBREAST-14R/ICARO study. Cancer Res. (2024) 84(9_Supplement):GS02-02. doi: 10.1158/1538-7445.sabcs23-gs02-02
62. Mamounas EP, Bandos H, White JR, Julian TB, Khan AJ, Shaitelman SF, et al. Abstract OT1-3-02: will chest wall and regional nodal radiotherapy post mastectomy or the addition of regional nodal radiotherapy to breast radiotherapy post lumpectomy reduce the rate of invasive cancer events in patients with positive axillary nodes who convert to ypN0 af. Cancer Res. (2015) 75(9_Supplement):OT1–3–02. doi: 10.1158/1538-7445.sabcs14-ot1-3-02
63. Mamounas EP, White JR, Bandos H, Julian TB, Kahn AJ, Shaitelman SF, et al. NSABP B-51/RTOG1304: randomized phase III clinical trial evaluating the role of postmastectomy chest wall and regional nodal XRT (CWRNRT) and post-lumpectomy RNRT in patients (pts) with documented positive axillary (ax) nodes before neoadjuvant chemotherapy (NC) who convert to pathologically negative ax nodes after NC. J Clin Oncol. (2014) 32(15_suppl):TPS1141. doi: 10.1200/jco.2014.32.15_suppl.tps1141
64. ClinicalTrials.gov Alliance A11202 trial (2013). Available online at: https://clinicaltrials.gov/ct2/show/NCT01901094 (Accessed August 13, 2024).
65. Henke G, Knauer M, Ribi K, Hayoz S, Gérard MA, Ruhstaller T, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. (2018) 19(1):667. doi: 10.1186/s13063-018-3021-9
66. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs No axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. (2017) 318(10):918–26. doi: 10.1001/jama.2017.11470
67. Bartels SAL, Donker M, Poncet C, Sauvé N, Straver ME, van de Velde CJH, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial. J Clin Oncol. (2023) 41(12):2159–65. doi: 10.1200/JCO.22.01565
68. Almahariq MF, Levitin R, Quinn TJ, Chen PY, Dekhne N, Kiran S, et al. Omission of axillary lymph node dissection is associated with Inferior survival in breast cancer patients with residual N1 nodal disease following neoadjuvant chemotherapy. Ann Surg Oncol. (2021) 28(2):930–40. doi: 10.1245/s10434-020-08928-2
69. Park Y, Shin YS, Kim K, Shin KH, Chang JH, Kim SS, et al. Omission of axillary lymph node dissection in patients with ypN+ breast cancer after neoadjuvant chemotherapy: a retrospective multicenter study (KROG 21-06). Eur J Surg Oncol. (2023) 49(3):589–96. doi: 10.1016/j.ejso.2022.11.099
70. Moo TA, Pawloski KR, Flynn J, Edelweiss M, Le T, Tadros A, et al. Is residual nodal disease at axillary dissection associated with tumor subtype in patients with low volume sentinel node metastasis after neoadjuvant chemotherapy? Ann Surg Oncol. (2021) 28(11):6044–50. doi: 10.1245/s10434-021-09910-2
71. Garcia-Tejedor A, Ortega-Exposito C, Salinas S, Luzardo-González A, Falo C, Martinez-Pérez E, et al. Axillary lymph node dissection versus radiotherapy in breast cancer with positive sentinel nodes after neoadjuvant therapy (ADARNAT trial). Front Oncol. (2023) 13:1184021. doi: 10.3389/fonc.2023.1184021
Keywords: locally advanced breast cancer, individualized treatment, neoadjuvant systemic therapy, surgery, pathological complete response
Citation: Sun Z, Liu K, Guo Y, Jiang N and Ye M (2024) Surgery paradigm for locally advanced breast cancer following neoadjuvant systemic therapy. Front. Surg. 11:1410127. doi: 10.3389/fsurg.2024.1410127
Received: 31 March 2024; Accepted: 27 August 2024;
Published: 6 September 2024.
Edited by:
Ugo Marone, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Min-Ying Lydia Su, University of California, Irvine, United StatesKuo Chen, First Affiliated Hospital of Zhengzhou University, China
Copyright: © 2024 Sun, Liu, Guo, Jiang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meina Ye, eWVtZWluYTIwMDJAMTI2LmNvbQ==
 Ziyue Sun
Ziyue Sun Kexin Liu
Kexin Liu