- 1Department of Cardiovascular Medicine, Beijing Shijitan Hospital Affiliated to Capital University of Medical Sciences, Beijing, China
- 2Department of Diagnostic Ultrasound, Beijing Anzhen Hospital Affiliated to Capital University of Medical Sciences, Beijing, China
Background: An intra-aortic balloon pump (IABP) is a mechanical circulatory device frequently used in patients undergoing coronary artery bypass grafting (CABG). As a treatment for perioperative haemodynamic instability, IABP insertion often implicates an adverse outcome. This study aimed to investigate the age- and sex-related disparity in risk factors for perioperative IABP insertion in CABG patients.
Methods: A total of 2,460 CABG patients were included and divided into subgroups by age (elderly subgroup, ≥65 years; young subgroup, <65 years) and sex. Basic characteristics were compared between IABP and non-IABP patients in the overall patient group and the subgroups. Multivariate logistic analysis was used to investigate the significant risk factors for perioperative IABP application, and interaction effects among the potential risk factors were analysed. Combined receiver operating characteristic analysis was used to evaluate the prediction value of combined risk factors.
Results: The overall patient group had a mean age of 61.5 years. The application rate of perioperative IABP was 8.0%. A left ventricular ejection fraction (LVEF) <50% significantly correlated with perioperative IABP application in the overall patient group and the subgroups. Traditional factors such as myocardial infarction history, atrial fibrillation history, and intraoperative estimated blood loss were significant risk factors in certain subgroups. Small dense low-density lipoprotein levels were significantly associated with IABP insertion in the male subgroup and young subgroup. The area under the curve of combined risk factors was significantly higher than that of LVEF <50% alone in the overall patient group and subgroups.
Conclusion: Age- and sex-related differences were present in the risk factor distribution for perioperative IABP insertion in CABG patients.
Background
Coronary artery bypass grafting (CABG) is one of the most important treatments for severe coronary atherosclerotic heart disease (1). An intra-aortic balloon pump (IABP) is a frequently used mechanical circulatory device in patients undergoing CABG (2). As an adjunctive treatment for haemodynamic instability, intra- or postoperative IABP insertion often implicates an adverse outcome of CABG (3). For developing counties, the use of an IABP brings an extra economic burden to patients.
Impaired left ventricle systolic function is a widely recognised risk factor in IABP insertion and it can be revealed by preoperative routine echocardiography (4). Other potential risk factors, such as comorbidities and specific biomarkers that have emerged recently as prognosis predictors of CABG, may also provide additive value (5). There are no well-defined criteria for perioperative IABP application, and thus it is important to identify potential controllable risk factors and improve preoperative management (6).
Recently, scholars highlighted the importance of age- and sex-related disparities in the risk factor distribution and outcomes in CABG patients (7). It is believed that patients of an advanced age may require more mechanical support during cardiac surgery, and females often had worse outcomes (8, 9). As far as we know, few studies have been conducted on age- and sex-related differences in the risk factor distribution for perioperative IABP application. This study aimed to investigate the risk factors for perioperative IABP insertion in different subgroups of CABG patients.
Patients and methods
Patients
We retrospectively reviewed 3,507 patients (>18 years of age) undergoing CABG in the Department of Cardiac Surgery at Anzhen Hospital between 1 January 2017 and 31 December 2018. Patients undergoing emergency surgery (N = 151) were excluded due to incomplete preoperative data. Patients who received prophylactic preoperative IABP support were also excluded (N = 51). A total of 845 patients were excluded for missing blood biochemical or ultrasonographic results. Overall, 2,460 patients were included for analysis (shown in Figure 1). The overall patient group was divided into subgroups by age (elderly, ≥65 years, N = 988; young <65 years, N = 1,472) and sex (male, N = 1,877; female, N = 583). Each subgroup was subsequently divided into non-IABP and IABP groups.

Figure 1. Flow chart of the study. CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump.
Routine preoperative examinations were performed, including medical history collection, a physical examination, blood biochemical tests, an electrocardiogram, a carotid ultrasound, and echocardiography. Carotid artery stenosis (CAS) was defined as ≥50% diameter stenosis of the internal or common carotid artery according to the Society of Radiologists through ultrasound consensus (10). A left ventricular ejection fraction (LVEF) <50% was defined as a lower than normal left ventricular systolic function (11). All images were acquired by certified experienced clinicians using Philips (Bothell, WA, USA), GE (Waukesha, WI, USA), or Hitachi (Tokyo, Japan) ultrasound imaging systems. Intraoperative estimated blood loss (EBL) ≥1,000 ml (75th percentile of the overall patient group) was defined as a large amount of EBL. Patients with IABP insertion received IABP support intra or postoperatively under the following circumstances of haemodynamic instability: (1) sudden ventricular fibrillation during an operation or in an intensive care unit (ICU) that could not be corrected using medications; (2) other kinds of arrhythmia that occurred repeatedly and could not be corrected by medications; (3) a sudden decrease in blood pressure during surgery that could not be maintained using vasopressors; and (4) other signs of decreased cardiac function, including a decrease in urine output or cold feet. In-hospital all-cause death was defined as death from any cause during the period of hospitalisation.
Statistical analysis
Continuous variables were described as mean ± standard deviation or median with interquartile range. Categorical variables were described as numbers and percentages. Univariate comparisons between groups were performed using the chi-square test for categorical variables and Student's t-test or a Mann–Whitney rank-sum test for continuous variables, as appropriate. Multivariate logistic regression analysis was applied to explore significant risk factors for perioperative IABP application in the overall patient group and subgroups. Potential covariates were included in adjusted models according to univariate logistic regression analysis results. A combined receiver operating characteristic (ROC) analysis was performed to analyse the association between combined risk factors and IABP application. Analyses were performed using SPSS 26.0. (SPSS, Chicago, IL, USA), and p < 0.05 was considered statistically significant.
Results
Baseline characteristics
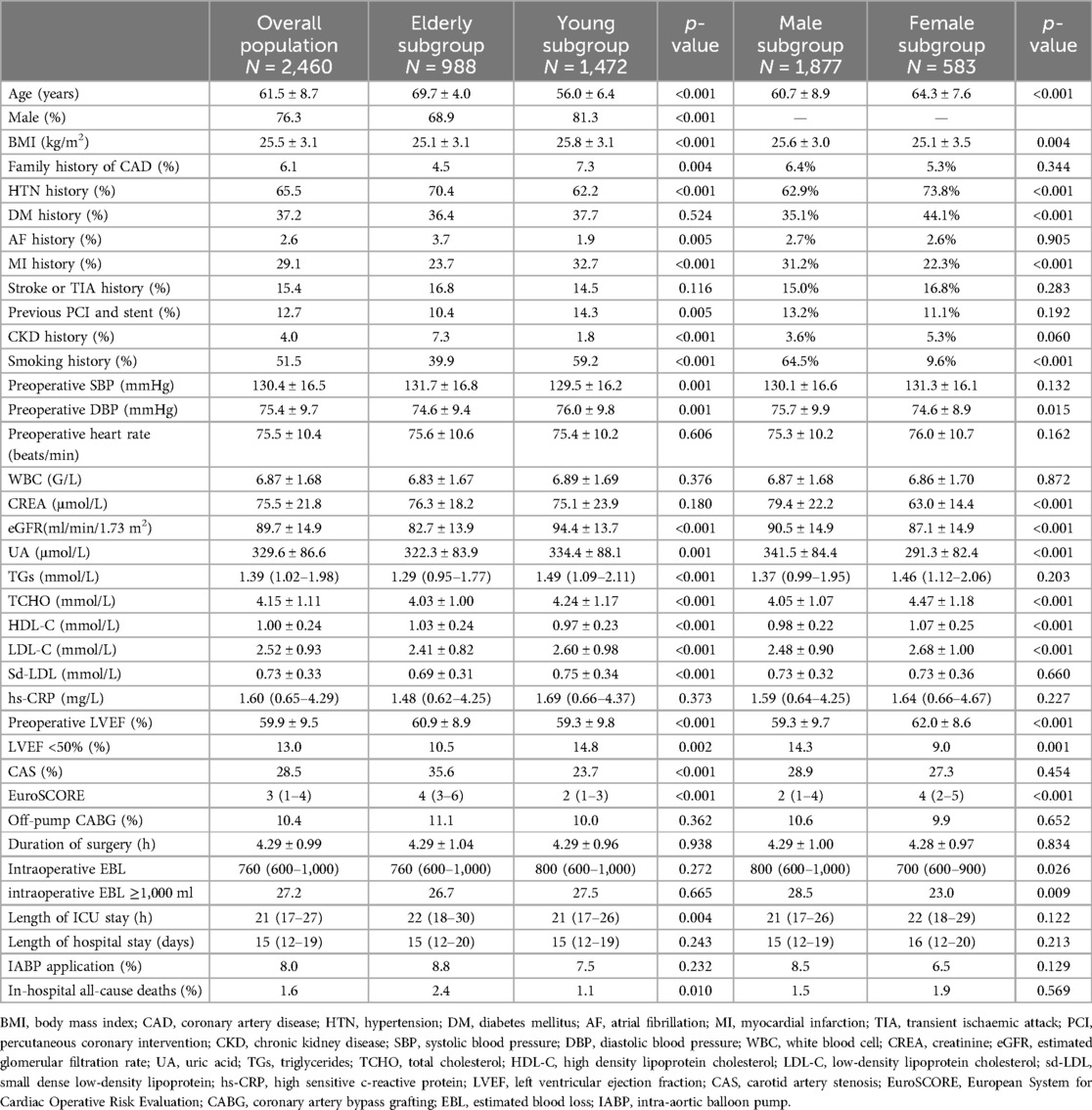
The study included 2,460 patients (mean age of 61.5 years), the majority of which were male (76.3%). The elderly subgroup (age ≥65 years) had higher prevalences of atrial fibrillation (AF), hypertension (HTN), chronic kidney disease (CKD), and CAS. The young subgroup (age <65 years) had a higher body mass index (BMI), higher prevalence of smoking and myocardial infarction (MI) history, and higher blood lipid levels. The male subgroup had a higher prevalence of MI history and a high BMI, whereas the female subgroup had higher prevalences of diabetes mellitus (DM), HTN, and CKD and higher blood lipid levels. IABP application rates were comparable between the male and female subgroups (8.5% vs. 6.5%, p = 0.129) and between the elderly and young subgroups (8.8% vs. 7.5%, p = 0.232). The basic characteristics are summarised in Table 1.
Comparison between IABP and non-IABP patients
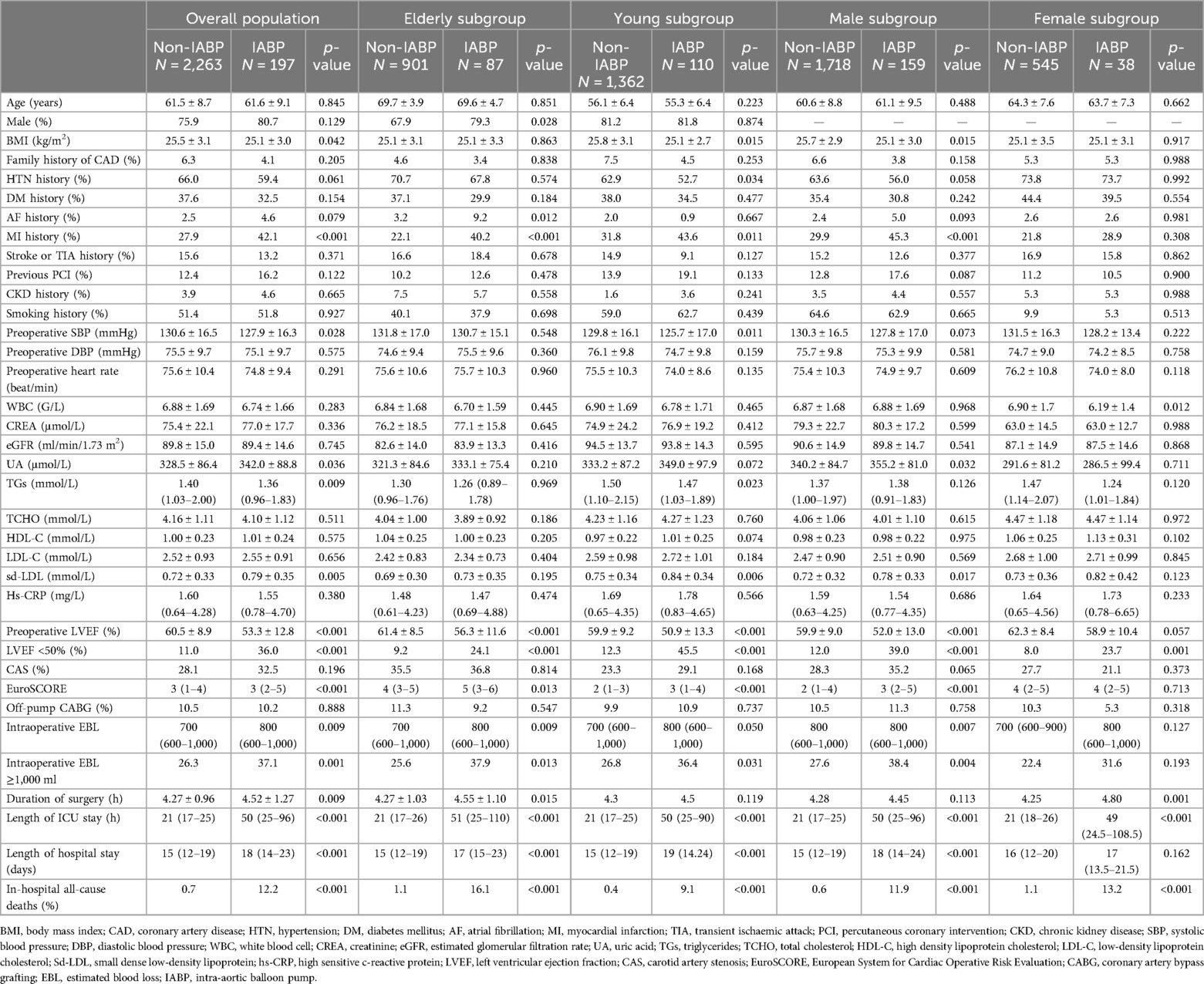
In the overall patient group, IABP patients had higher prevalences of HTN, AF, and MI history, higher baseline uric acid (UA) and small dense low-density lipoprotein (sd-LDL) levels, and lower baseline systolic blood pressure (SBP), BMI, and LVEF. In the elderly subgroup, there were a higher proportion of males in the IABP patients and higher prevalences of AF and MI history. In the young subgroup, IABP patients had a higher prevalence of MI history, higher baseline UA and sd-LDL levels, and lower BMI. In the male subgroup, IABP patients had higher prevalences of AF and MI history, and higher baseline UA and sd-LDL levels. In the female subgroup, IABP patients had age, comorbidities, and blood lipid levels that were comparable with non-IABP patients, although they had a lower baseline LVEF.
In addition, in the overall patient group and subgroups, IABP patients generally had a lower baseline LVEF, a larger amount of intraoperative EBL, longer hospital and ICU stays, and a higher incidence of in-hospital all-cause death. A comparison of the baseline characteristics between IABP and non-IABP patients is summarised in Table 2.
Risk factors for perioperative IABP application in the overall patient group
In the overall patient group, univariate logistic analysis showed that BMI, HTN history, MI history, AF history, preoperative SBP, UA, sd-LDL, triglycerides (TGs), LVEF <50%, EBL ≥1,000 ml, and European System for Cardiac Operative Risk Evaluation (EuroSCORE) significantly correlated with the application of a perioperative IABP. Multivariate logistic analysis showed that LVEF <50% (OR, 3.44; p < 0.001), sd-LDL (OR, 2.08; p = 0.001), and EBL ≥1,000 ml (OR, 1.61; p = 0.003) were significant risk factors. LVEF <50% and sd-LDL had a synergistic effect (OR, 5.71; p < 0.001). Results are shown in Supplementary Tables S1, S2 and Figure 2.

Figure 2. Forest plot of a multivariate logistic regression analysis of risk factors associated with perioperative IABP insertion in the overall patient group. LVEF <50% (OR, 3.44; p < 0.001), sd-LDL levels (OR, 2.08; p = 0.001), and EBL ≥1,000 ml (OR, 1.61; p = 0.003) were significant risk factors.
Differences in risk factors for perioperative IABP application in subgroups
In the elderly subgroup, MI history (OR, 1.91; p = 0.010), AF history (OR, 2.90; p = 0.013), LVEF <50% (OR, 2.44; p = 0.003), and EBL ≥1,000 ml (OR, 1.80; p = 0.014) were significant risk factors for perioperative IABP application. LVEF <50% had a synergistic effect with AF history (OR, 6.99, p = 0.034) and MI history (OR, 4.36, p < 0.001). In the young subgroup, sd-LDL (OR, 2.59; p < 0.001) emerged as a significant risk factor along with LVEF <50% (OR, 5.54; p < 0.001). LVEF <50% and sd-LDL had a synergistic effect (OR, 7.66; p < 0.001). In the male subgroup, AF history (OR, 2.44; p = 0.032), sd-LDL levels (OR, 1.97; p = 0.007), LVEF <50% (OR, 3.59; p < 0.001), and EBL ≥1,000 ml (OR, 1.66; p = 0.005) were significant risk factors. LVEF <50% and sd-LDL had a synergistic effect (OR, 5.57; p < 0.001). In the female subgroup, white blood cell (WBC) (OR, 0.760; p = 0.015) and LVEF <50% (OR, 3.61; p = 0.002) were significant risk factors. Results are shown in Supplementary Tables S1, S2 and Figure 3.
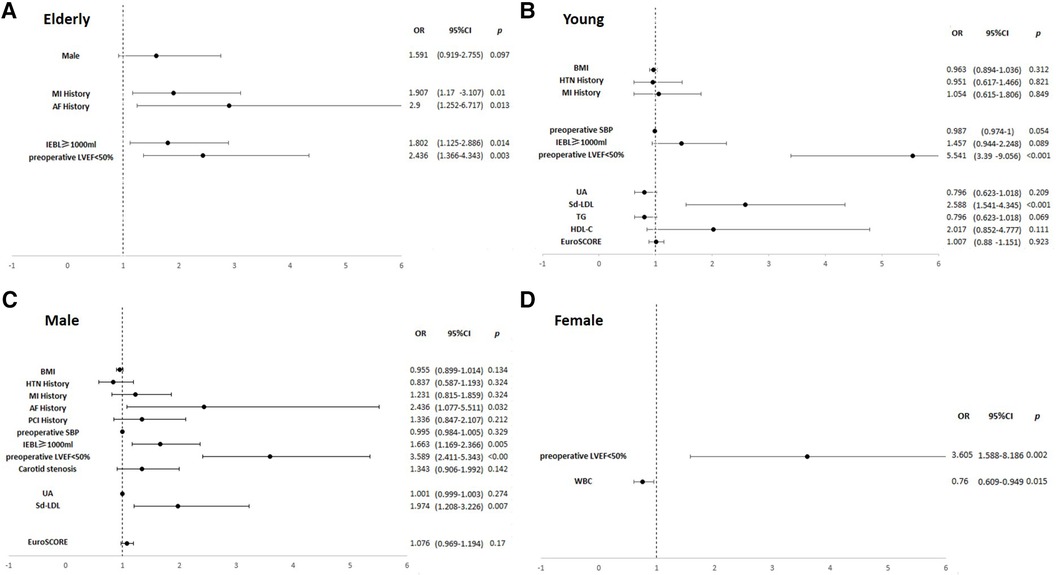
Figure 3. Forest plots of multivariate logistic regression analyses of risk factors associated with perioperative IABP insertion in the subgroups. (A) The elderly subgroup. (B) The young subgroup. (C) The male subgroup. (D) The female subgroup. In the elderly subgroup, MI history (OR, 1.91; p = 0.010), AF history (OR, 2.90; p = 0.013), LVEF <50% (OR, 2.44; p = 0.003), and EBL ≥1,000 ml (OR, 1.80; p = 0.014) were significant risk factors. In the young subgroup, sd-LDL (OR, 2.59; p < 0.001) and LVEF <50% (OR, 5.54; p < 0.001) were significant risk factors. In the male subgroup, AF history (OR, 2.44; p = 0.032), sd-LDL levels (OR, 1.97; p = 0.007), LVEF <50% (OR, 3.59; p < 0.001), and EBL ≥1,000 ml (OR, 1.66; p = 0.005) were significant risk factors. In the female subgroup, WBC (OR, 0.760; p = 0.015) and LVEF <50% (OR, 3.61; p = 0.002) were significant risk factors.
ROC analyses
In the overall patient group, the area under the curve (AUC) of LVEF <50% for IABP application was 0.625 [95% confidence interval (CI) (0.591–0.659); p < 0.001] and the combined AUC of LVEF <50%, EBL ≥1,000 ml, and sd-LDL was 0.669 [95% CI (0.626–0.712); p < 0.001]. In the elderly subgroup, the AUC of LVEF <50% was 0.575 [95% CI (0.529–0.621); p = 0.001] and the combined AUC of AF history, MI history, LVEF <50%, and EBL ≥1,000 ml was 0.653 [95% CI (0.591–0.715); p < 0.001]. In the young subgroup, the AUC of LVEF <50% was 0.666 [95% CI (0.618–0.714); p < 0.001] and the combined AUC of LVEF <50% and sd-LDL was 0.724 [95% CI (0.671–0.778); p < 0.001]. In the male subgroup, the AUC of LVEF <50% was 0.635 [95% CI (0.596–0.674); p = 0.020] and the combined AUC of AF history, LVEF <50%, sd-LDL, and EBL ≥1,000 ml was 0.693 [95% CI (0.646–0.739); p = 0.024]. In the female subgroup, the AUC of LVEF <50% was 0.579 [95% CI (0.509–0.648); p = 0.027] and the combined AUC of LVEF <50% and WBC was 0.0.675 [95% CI (0.588–0.762); p < 0.001]. The AUC of the combined risk factors was significantly higher than that of LVEF alone in each subgroup. Results of the ROC analysis are shown in Table 3 and Figures 4, 5.
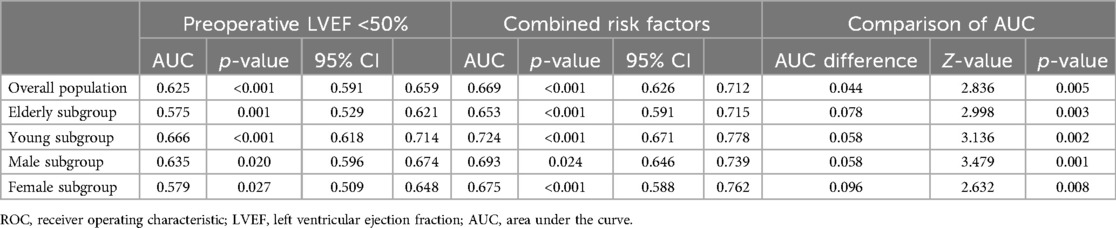
Table 3. ROC analyses of risk factors for perioperative IABP application in the overall population and subgroups.
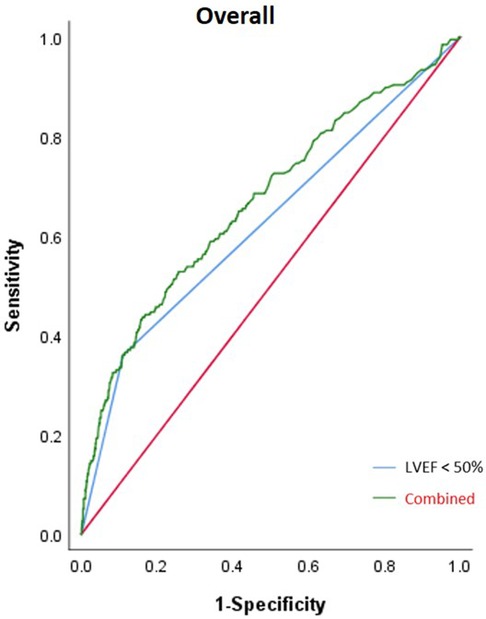
Figure 4. Result from the ROC analysis of the overall patient group. The AUC of LVEF <50% for IABP application was 0.625 (p < 0.001) and the combined AUC of LVEF <50%, intraoperative EBL ≥1,000 ml, and sd-LDL was 0.669 (p < 0.001).

Figure 5. Results from ROC analyses of the subgroups. (A) The elderly subgroup. (B) The young subgroup. (C) The male subgroup. (D) The female subgroup. In the elderly subgroup, the AUC of LVEF <50% was 0.575 (p = 0.001) and the combined AUC of AF history, MI history, LVEF <50%, and intraoperative EBL ≥1,000 ml was 0.653 (p < 0.001). In the young subgroup, the AUC of LVEF <50% was 0.666 (p < 0.001) and the combined AUC of LVEF <50% and sd-LDL was 0.724 (p < 0.001). In the male subgroup, the AUC of LVEF <50% was 0.635 (p = 0.020) and the combined AUC of AF history, LVEF <50%, sd-LDL, and intraoperative EBL ≥1,000 ml was 0.693 (p = 0.024). In the female subgroup, the AUC of LVEF <50% was 0.579 (p = 0.027) and the combined AUC of LVEF <50% and WBC was 0.675 (p < 0.001). The AUC of the combined risk factors was significantly higher than that of LVEF alone in each subgroup (p < 0.01).
Discussion
The most important findings of our study include the following: (1) in different subgroups, different factors provided complementary values to a lower baseline LVEF, a generally accepted risk factor for perioperative IABP insertion; (2) the AUC of combined risk factors in each subgroup was significantly higher than that of LVEF alone; and (3) besides traditional risk factors, such as MI history or AF history, baseline sd-LDL level emerged as a risk factor in certain subgroups.
There were no well-defined criteria for perioperative IABP insertion and multiple factors should be taken into consideration (9). As an important haemodynamic parameter, LVEF was recognised as a predictor of a poor prognosis for CABG patients. Fallahzadeh et al. indicated that patients with a severely reduced baseline LVEF were at a higher risk of mortality after CABG (12). Recently, Kumar et al. proposed that preoperative three-dimensional LVEF could predict intra and postoperative IABP insertion in CABG patients (4). Our result showed that a lower than normal baseline LVEF (<50%) was significantly associated with perioperative IABP application in the overall patient group and subgroups, which was consistent with previous studies.
In addition to traditional risk factors, our data revealed that baseline sd-LDL significantly correlated with IABP insertion in the young subgroup and male subgroup. Krychtiuk et al. proposed that among coronary artery disease (CAD) patients with high sd-LDL levels, monocyte subset distribution is skewed to a more “pro-inflammatory” profile (13). These cells then respond upon activation with a higher production of inflammatory cytokines (14, 15). During major surgeries such as CABG, the cardiovascular system develops specific reactions (activation of the inflammatory cascade, which involves numerous cytokines and chemokines) against the stress (16, 17). The inflammatory responses induced by surgery might contribute to unstable haemodynamics when IABP is needed (18, 19). Therefore, an elevated level of sd-LDL may exert its effects through the modulation of the monocyte subset distribution to a rather pro-inflammatory profile. In addition, Norata et al. proposed that sd-LDL can induce inflammatory responses in endothelial cells, and subsequent endothelial dysfunction makes it difficult for the cardiovascular system to adapt to the haemodynamic changes after major surgeries (20).
The timing and intensity of the response mentioned above vary among individuals. For instance, patients of different ages may have different oxidative stress statuses (different cytokine and chemokine levels), which may lead to different responses to the stress (21). In addition, studies have proposed that the drivers of coronary microvascular dysfunction may differ by sex. Inflammation predominates in males, whereas ventricular remodelling and fibrosis play a major role in females (22). In our study, the role of sd-LDL was more prominent in the young subgroup and male subgroup. Our result indicated that physicians may pay more attention to the inflammatory background before cardiac surgery in certain subgroups.
Our data failed to reveal the significant correlation of sd-LDL with perioperative IABP insertion in females and the elderly subgroup. We speculate that one of the possible explanations might be that the distributions of sd-LDL were also influenced by age, sex, and menopausal status. In males, sd-LDL levels showed an increasing phase followed by a decreasing phase and the summit was reached at approximately 60 years of age. In females, the increasing phase was followed by a plateaued phase, which may have occurred due to the impact of the postmenopausal status (23). Therefore, we speculate that the decrease in sd-LDL after middle age and the impact of the menopausal status may have concealed the role of sd-LDL in elderly and female patients. In addition, female CABG patients had more comprehensive comorbidities, and the drugs they routinely took may have had a certain impact on sd-LDL levels (24).
Previous studies have reported that sd-LDL is correlated with CAD severity and a higher incidence of adverse events. One of the possible explanations might be that sd-LDL promotes atherosclerosis plaque progression through its strong pro-atherosclerotic effect (24–26). Therefore, we assume that the association of sd-LDL with IABP application may be attributed the fact that patients with a higher sd-LDL level may have more severe CAD, which leads to a subsequent IABP insertion. However, CAD severity is often estimated by angiography. In our data, all patients (CABG patients) had established severe CAD; we assume it may be not easy to stratify the severity using only imaging results. Sd-LDL level may serve as a complementary factor to help stratify the risk of CABG patients.
Finally, we found synergistic effects between certain potential risk factors. LVEF <50% and sd-LDL could promote each other's effect in the overall patient group, the male subgroup, and the younger subgroup. We assume the possible explanation may be that abnormal lipid metabolism influences cardiac function through various mechanisms. Previous studies have reported that lipotoxicity can promote stiffening and inflammation of the cardiac tissue (27). Although pathological evidence is lacking, our result revealed that patients with higher sd-LDL levels and a lower LVEF were more likely to experience haemodynamic instability. However, we have to admit that LVEF could be influenced by several factors, and the synergistic effect between LVEF and sd-LDL has to be confirmed in future studies. These two factors were not significantly associated with IABP use in the elderly subgroup, perhaps due to both being influenced by an abnormal lipid metabolism; young patients may have more obvious consequences as the cardiac function of the elderly has already been impaired by other factors, such as ageing. Therefore, physicians should pay some attention to lipid metabolism in young and male patients in addition to their cardiac function and comorbidity. In the elderly group, we found MI history and AF history both had a synergistic effect with lower LVEF. This result was consistent with clinical understanding. However, our study only included in-hospital mortality, and as for long-term mortality, further study is needed. For instance, in this CABG population, there were patients with preserved LVEF, such as those with heart failure with a preserved ejection fraction (HFpEF), who may have even worse long-term outcomes (28).
The study had some limitations. First, our study was a single-centre study that indicated the situation in one hospital. Therefore, we only provided a preliminary result of IABP risk prediction in CABG patients, which needs to be validated in a larger population. Second, we excluded patients with preoperative IABP insertion. As there is no well-defined guideline, prophylactic IABP may involve the physician’s personal judgement. The third limitation was that we used EuroSCORE instead of EuroSCORE II due to incomplete information. In our study, we aimed to reveal the preoperative risk of the patients and we assume EuroSCORE may be sufficient. The next limitation was that the influence of drugs was not taken into consideration. A large proportion of patients took certain drugs, such as antidiabetic or antihyperlipidemic drugs, which may affect their baseline levels of biochemical markers. However, the effect was not easy to evaluate.
Conclusion
Our study confirmed the significant correlation of traditional risk factors (LVEF, MI history, AF history, and intraoperative EBL) with perioperative IABP insertion, and baseline sd-LDL level emerged as a biochemical risk factor in certain subgroups. In addition, we found a disparity in risk factors; different factors provided complementary values to a lower baseline LVEF in different subgroups. Our result provided complementary knowledge to this field, and it is crucial to be aware of the difference in risk factor distribution in subgroups during the preoperative evaluation of CABG patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the committee waived the need for informed consent from the patients due to the retrospective nature of this study.
Author contributions
JG: Conceptualization, Formal Analysis, Methodology, Writing – original draft. QZ: Data curation, Writing – original draft. YC: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1395518/full#supplementary-material
Abbreviations
CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; sd-LDL, small dense low-density lipoprotein; EBL, estimated blood loss.
References
1. Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur J Cardiothorac Surg. (2019) 55(1):4–90. doi: 10.1093/ejcts/ezy289
2. Notarianni A, Tickoo M, Bardia A. Mechanical cardiac circulatory support: an overview of the challenges for the anesthetist. Curr Anesthesiol Rep. (2021) 11(4):421–8. doi: 10.1007/s40140-021-00486-x
3. Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, van Domburg RT, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. (2009) 30(17):2102–8. doi: 10.1093/eurheartj/ehp292
4. Kumar S, Malik V, Chauhan S, Das D, Hote MP, Devagourou V. Comparison of left ventricular global longitudinal strain with ejection fraction as a predictor for peri-operative IABP insertion in patients undergoing off-pump coronary artery bypass grafting: a pilot study. Ann Card Anaesth. (2023) 26(3):295–302. doi: 10.4103/aca.aca_144_22
5. Plicner D, Stoliński J, Wąsowicz M, Gawęda B, Hymczak H, Kapelak B, et al. Preoperative values of inflammatory markers predict clinical outcomes in patients after CABG, regardless of the use of cardiopulmonary bypass. Indian Heart J. (2016) 68(Suppl 3):S10–5. doi: 10.1016/j.ihj.2016.10.002
6. Grant SW, Ouzounian M. Closing the sex gap in cardiac surgery outcomes: more work to be done. Eur J Cardiothorac Surg. (2022) 61(3):703–4. doi: 10.1093/ejcts/ezab429
7. Hosseini K, Yavari N, Pashang M, Jalali A, Nalini M, Majdi Nassab F, et al. Sex difference in the risk factor distributions and outcomes after coronary artery bypass graft surgery in the young population. Eur J Cardiothorac Surg. (2022) 62(1):ezab475. doi: 10.1093/ejcts/ezab475
8. Chen FT, Chou AH, Chan YH, Wu VC, Lin CP, Hung KC, et al. Sex-related differences on the risks of in-hospital and late outcomes after acute aortic dissection: a nationwide population-based cohort study. PLoS One. (2022) 17(2):e0263717. doi: 10.1371/journal.pone.0263717
9. Lorusso R, Heuts S, Jiritano F, Scrofani R, Antona C, Actis Dato G, et al. Contemporary outcomes of cardiac surgery patients supported by the intra-aortic balloon pump. Interact Cardiovasc Thorac Surg. (2022) 35(1):ivac091. doi: 10.1093/icvts/ivac091
10. Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis—society of radiologists in ultrasound consensus conference. Radiology. (2003) 229(2):340–6. doi: 10.1148/radiol.2292030516
11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
12. Fallahzadeh A, Sheikhy A, Ajam A, Sadeghian S, Pashang M, Shirzad M, et al. Significance of preoperative left ventricular ejection fraction in 5-year outcome after isolated CABG. J Cardiothorac Surg. (2021) 16(1):353. doi: 10.1186/s13019-021-01732-3
13. Krychtiuk KA, Kastl SP, Pfaffenberger S, Lenz M, Hofbauer SL, Wonnerth A, et al. Association of small dense LDL serum levels and circulating monocyte subsets in stable coronary artery disease. PLoS One. (2015) 10(4):e0123367. doi: 10.1371/journal.pone.0123367
14. Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. (2002) 168(7):3536–42. doi: 10.4049/jimmunol.168.7.3536
15. Westerberg M, Bengtsson A, Ricksten A, Jeppsson A. Tumor necrosis factor gene polymorphisms and inflammatory response in coronary artery bypass grafting patients. Scand Cardiovasc J. (2004) 38(3):172–7. doi: 10.1080/14017430410031795
16. Perros AJ, Esguerra-Lallen A, Rooks K, Chong F, Engkilde-Pedersen S, Faddy HM, et al. Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. J Cell Mol Med. (2020) 24(8):4791–803. doi: 10.1111/jcmm.15154
17. Preeshagul I, Gharbaran R, Jeong KH, Abdel-Razek A, Lee LY, Elman E, et al. Potential biomarkers for predicting outcomes in CABG cardiothoracic surgeries. J Cardiothorac Surg. (2013) 8:176. doi: 10.1186/1749-8090-8-176
18. Reina-Couto M, Silva-Pereira C, Pereira-Terra P, Quelhas-Santos J, Bessa J, Serrão P, et al. Endothelitis profile in acute heart failure and cardiogenic shock patients: Endocan as a potential novel biomarker and putative therapeutic target. Front Physiol. (2022) 13:965611. doi: 10.3389/fphys.2022.965611
19. Sjauw KD, Engström AE, Henriques JP. Percutaneous mechanical cardiac assist in myocardial infarction. Where are we now, where are we going? Acute Card Care. (2007) 9(4):222–30. doi: 10.1080/17482940701534818
20. Norata GD, Raselli S, Grigore L, Garlaschelli K, Vianello D, Bertocco S, et al. Small dense LDL and VLDL predict common carotid artery IMT and elicit an inflammatory response in peripheral blood mononuclear and endothelial cells. Atherosclerosis. (2009) 206(2):556–62. doi: 10.1016/j.atherosclerosis.2009.03.017
21. Zhang RJ, Yu XY, Wang J, Lv J, Yu MH, Wang L, et al. Comparison of in-hospital outcomes after coronary artery bypass graft surgery in elders and younger patients: a multicenter retrospective study. J Cardiothorac Surg. (2023) 18(1):53. doi: 10.1186/s13019-023-02163-y
22. Chandramoul C, Ting TW, Tromp J, Agarwal A, Svedlund S, Saraste A, et al. Sex differences in proteomic correlates of coronary microvascular dysfunction among patients with heart failure and preserved ejection fraction. Eur J Heart Fail. (2022) 24(4):681–4. doi: 10.1002/ejhf.2435
23. Izumida T, Nakamura Y, Sato Y, Ishikawa S. Association among age, sex, menopausal status and small dense low-density lipoprotein cholesterol: a cross-sectional study. BMJ Open. (2021) 11(2):e041613. doi: 10.1136/bmjopen-2020-041613
24. Lemaire A, Soto C, Salgueiro L, Ikegami H, Russo MJ, Lee LY. The impact of age on outcomes of coronary artery bypass grafting. J Cardiothorac Surg. (2020) 15(1):158. doi: 10.1186/s13019-020-01201-3
25. Sekimoto T, Koba S, Mori H, Sakai R, Arai T, Yokota Y, et al. Small dense low-density lipoprotein cholesterol: a residual risk for rapid progression of non-culprit coronary lesion in patients with acute coronary syndrome. J Atheroscler Thromb. (2021) 28(11):1161–74. doi: 10.5551/jat.60152
26. Jin X, Yang S, Lu J, Wu M. Small, dense low-density lipoprotein-cholesterol and atherosclerosis: relationship and therapeutic strategies. Front Cardiovasc Med. (2022) 8:804214. doi: 10.3389/fcvm.2021.804214
27. Cinato M, Andersson L, Miljanovic A, Laudette M, Kunduzova O, Borén J, et al. Role of perilipins in oxidative stress-implications for cardiovascular disease. Antioxidants (Basel). (2024) 13(2):209. doi: 10.3390/antiox13020209
28. Kapłon-Cieślicka A, Benson L, Chioncel O, Crespo-Leiro MG, Coats AJS, Anker SD, et al. A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction—insights from the ESC-HFA EORP heart failure long-term registry [published correction appears in Eur J Heart Fail. 2023 Mar;25(3):443 and Eur J Heart Fail. 2024 Jan;26(1):193]. Eur J Heart Fail. (2022) 24(2):335–50. doi: 10.1002/ejhf.2408
Keywords: coronary artery bypass grafting (CABG), intra-aortic balloon pump (IABP), left ventricular ejection fraction (LVEF), age, sex
Citation: Gao J, Zhao Q and Cheng Y (2024) Age- and sex-related differences in risk factors for perioperative intra-aortic balloon pump application in patients undergoing coronary artery bypass grafting. Front. Surg. 11:1395518. doi: 10.3389/fsurg.2024.1395518
Received: 4 March 2024; Accepted: 9 August 2024;
Published: 3 September 2024.
Edited by:
Giuseppe Gatti, Azienda Sanitaria Universitaria Giuliano Isontina, ItalyReviewed by:
Antonino S. Rubino, Kore University of Enna, ItalyPhilemon Gukop, St George's University Hospitals NHS Foundation Trust, United Kingdom
Copyright: © 2024 Gao, Zhao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Cheng, Y2hlbmd5aTgzMDRAMTYzLmNvbQ==
 Junyi Gao1
Junyi Gao1 Yi Cheng
Yi Cheng