- 1Department of Colorectal Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Key Laboratory of Biological Treatment of Zhejiang Province, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 3Key Laboratory of Integrated Traditional Chinese and Western Medicine Research on Anorectal Diseases of Zhejiang Province, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: Colorectal cancer (CRC) is the third most common cancer worldwide. Patients diagnosed with stage I CRC typically do not require postoperative adjuvant treatment. However, postoperative recurrence is present in at least 40% of patients with CRC and often occurs in those with stage I disease. This study aimed to elucidate the current status of recurrence and clinicopathological characteristics in patients with stage I CRC.
Methods: Data of indicated patients were obtained from 18 registries in Surveillance, Epidemiology, and End Results (SEER). The multivariable Fine–Gray regression model was used to identify the mortality risk of patients. Disparities in survival were analyzed using Kaplan–Meier curves. Logistic regression was employed to identify factors associated with recurrent risk overestimation.
Results: Our study indicated a recurrence rate of 15.04% (1,874/12,452) in stage I CRC cases. Notably, we identified race, age, T stage, and carcinoembryonic antigen (CEA) levels as independent risk factors for tumor recurrence, substantially impacting prognosis. Furthermore, gender, race (Black), age (>65 years), elevated CEA levels, and refusal or unknown status regarding radiotherapy significantly correlated with an adverse prognosis in patients with stage I CRC.
Conclusions: We identified certain key clinicopathological features of patients with stage I CRC and demonstrated the survival benefits of radiotherapy, offering a new perspective on stage I CRC follow-up and treatment recommendations.
Introduction
Colorectal cancer (CRC) ranks as the third most prevalent cancer globally and is the second-largest contributor to cancer-related fatalities (1, 2). Despite the implementation of population-based CRC screening in the 1990s, which led to a more than 35% reduction in CRC incidence across the general population, a concerning trend has emerged: there has been an almost two-fold increase in CRC incidence among younger adults within the same time frame (3–6).
Surgery and chemotherapy are the most common therapeutic approaches for CRC. Surgery is used for stage I CRC and yields a 5-year survival rate of approximately 90% (7). Despite advancements in surgical methodologies and postoperative monitoring, the recurrence of CRC after pathologically confirmed complete resection remains a formidable challenge. Recurrence impacts a minimum of 40% of patients with CRC, often manifesting within the initial 3 years of disease emergence (8). According to the National Comprehensive Cancer Network (NCCN) guidelines, patients diagnosed with stage I CRC typically do not require postoperative adjuvant treatment (9, 10) and are usually advised to undergo follow-up examinations every 6 months postsurgery (9). However, real-world clinical observations often indicate postoperative recurrence in patients with stage I CRC (11). Additionally, patients with large tumors, previously assumed to require chemotherapy, are occasionally diagnosed with stage I CRC (12). Consequently, this study aimed to characterize the current landscape of recurrence patterns and clinicopathological characteristics among patients with stage I CRC.
Methods
Patients
We obtained all data from 18 registries of the Surveillance, Epidemiology, and End Results (SEER) using SEER * Stat 8.4.1 software. Ethical approval was not required since SEER is a publicly available database. Our study focused on patients who were diagnosed with stage I CRC between 2010 and 2015 according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site codes (C18.0–C18.9, C19.9, C20.9) and ICD-O-3 behavior codes (malignant). Extracted variables included patient identification, age at diagnosis, gender, race, tumor grade, tumor size, American Joint Committee on Cancer (seventh edition) T stage, carcinoembryonic antigen (CEA) status, surgery code, treatment (chemotherapy, radiation), vital status, and survival duration in months. We excluded patients with incomplete clinical data, those diagnosed by autopsy, or those solely reported in death certificates.
We established a variable to track local recurrence by forming a patient cohort that met our inclusion criteria. This cohort comprised individuals with an initial tumor at a specific site who later developed the same tumor at an identical location (including tumors in situ). during subsequent follow-up. More precisely, the identification of CRC in situ at the original site, based on ICD site codes, constituted the definition of a local recurrence.
Statistical analysis
We conducted comparisons among patient demographics, tumor characteristics, and treatment details using the χ2 test. To pinpoint the independent predictors of recurrence in patients with CRC, logistic regression analysis was employed. Covariates linked to overall survival (OS) were evaluated via a multivariable Cox proportional hazards model. The proportional hazards assumption was assessed using Kaplan–Meier curves and time-dependent covariates. In instances where this assumption was violated, the time-dependent Cox model was used. The findings were presented as hazard ratios (HRs) accompanied by 95% confidence intervals (CIs). Statistical analyses were carried out using SPSS Version 22.0 (IBM Corp.) and GraphPad Prism Version 8 (GraphPad Software) software.
Results
Clinical characteristics of the enrolled patients
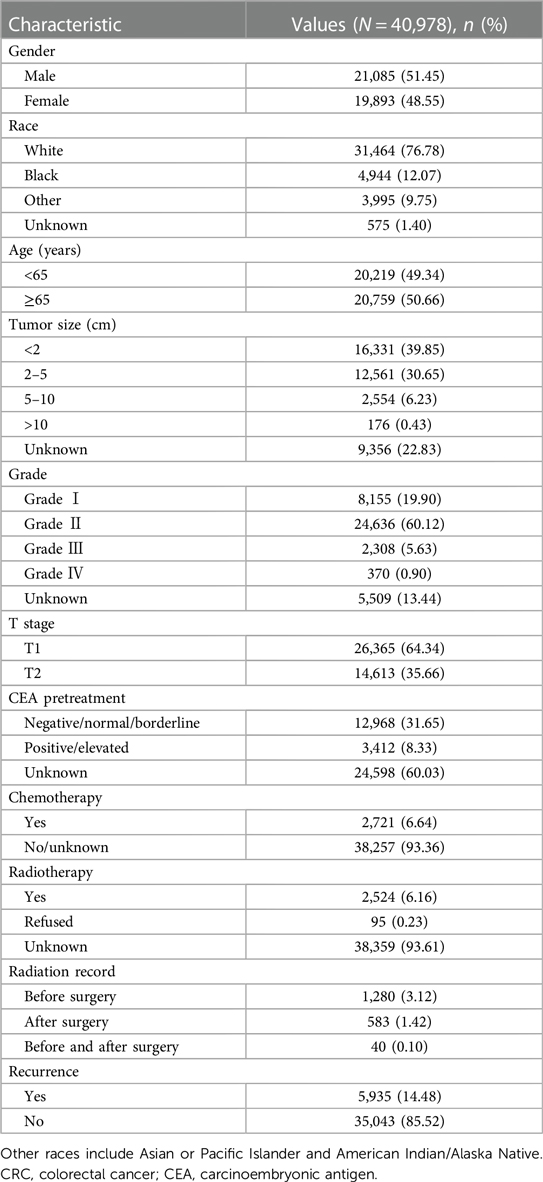
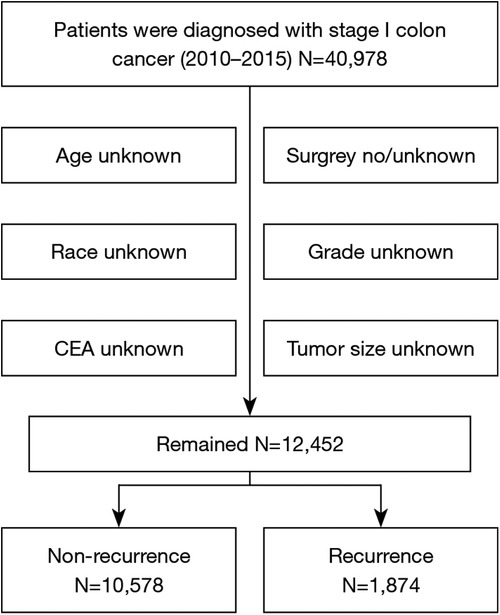
A total of 40,978 patients diagnosed with stage I CRC between 2010 and 2015 were included in the study, and their comprehensive clinicopathological details are presented in Table 1. All patients underwent surgical treatment. Those with incomplete information were excluded, such as in cases where race, CEA status, grade, tumor size, or surgical details were unknown (Figure 1). Upon final analysis, 12,452 patients were eligible for inclusion, comprising 10,578 patients (84.95%) with no recurrence and 1,874 patients (15.04%) with tumor recurrence.
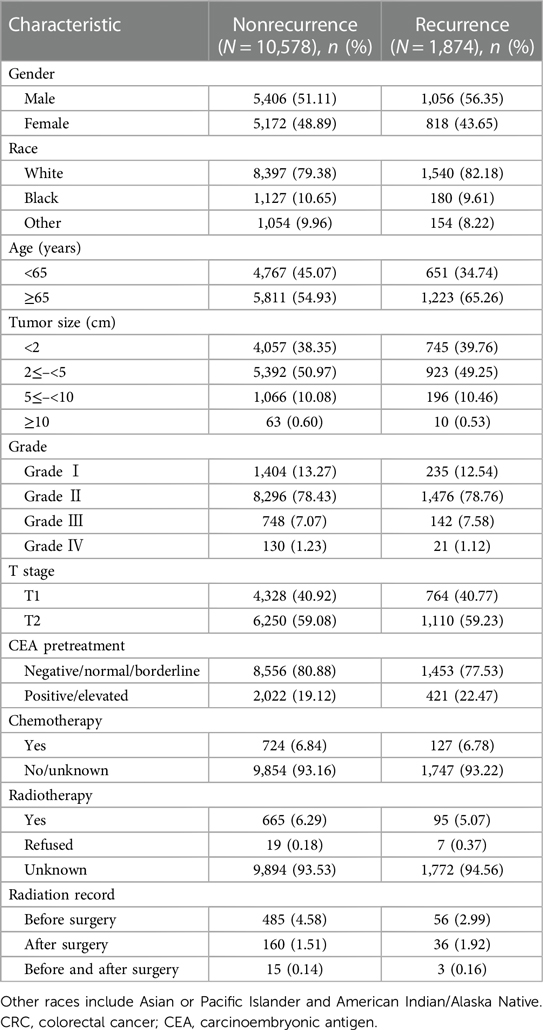
Among the 1,874 patients who experienced recurrence, 1,056 (56.35%) were male, 1,540 (82.18%) were White, 1,223 (65.26%) were aged over 65 years, and the majority were diagnosed with T2 (59.23%) disease. Additionally, 421 (22.47%) were CEA positive, 127 (6.78%) underwent chemotherapy, and 95 (5.07%) received radiotherapy. Furthermore, detailed information on tumor size and grade was collected. A comprehensive baseline analysis of clinical characteristics based on recurrence status is presented in Table 2.
Mortality risk analysis of stage I CRC
To investigate the factors influencing survival in stage I CRC, we conducted both univariate and multivariate Cox regression analyses. Figure 2 illustrates the associations with an overestimation of mortality risk. Male gender exhibited a higher risk of mortality overestimation in comparison to female gender (female vs. male: HR = 0.850; 95% CI: 0.787–0.919; P < 0.01). Moreover, Black individuals demonstrated a greater likelihood of mortality risk overestimation (Black vs. White: HR = 1.334; 95% CI: 1.185–1.503; P < 0.01), and other races were associated with a significant protective effect (other vs. White: HR = 0.708; 95% CI: 0.608–0.826; P < 0.01). Age over 65 years emerged as an independent risk factor (<65 vs. ≥65 years: HR = 0.296; 95% CI: 0.269–0.326; P < 0.01). Additionally, patients classified as T2 exhibited a heightened mortality risk compared to those classified as T1 (T1 vs. T2: HR = 0.804; 95% CI: 0.741–0.872; P < 0.01). Elevated pretreatment CEA levels were also linked to increased mortality risk in contrast to normal CEA levels (negative/normal vs. positive/elevated: HR = 0.586; 95% CI: 0.538–0.638; P < 0.01). Furthermore, individuals who experienced recurrence demonstrated a higher mortality risk compared to those without recurrence (nonrecurrence vs. recurrence: HR = 0.713; 95% CI: 0.651–0.782; P < 0.01).
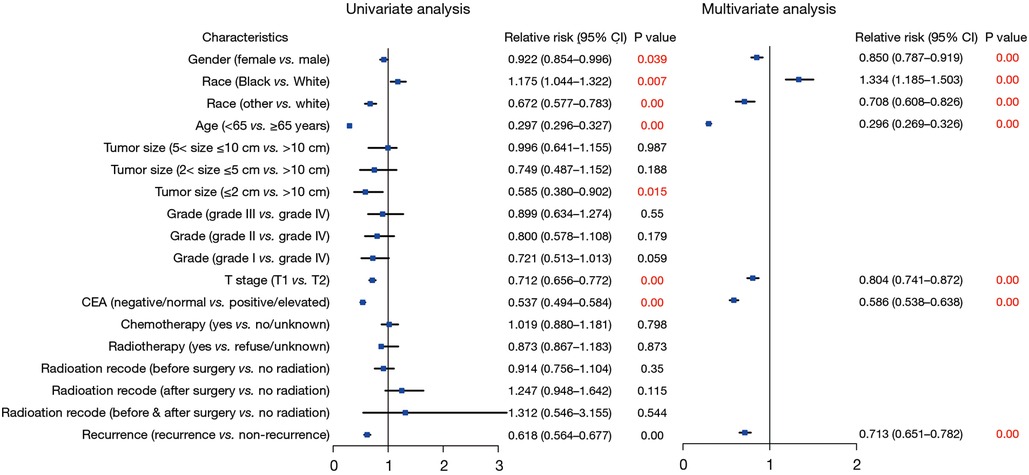
Figure 2. Forest plot of Cox regression analysis for assessing the risks associated with survival in patients with stage I CRC. CRC, colorectal cancer.
Additional analysis with the Kaplan–Meier survival curve highlighted significantly worse survival rates among patients with stage I CRC with recurrent lesions (Figure 3A). In patients with recurrent CRC, those of Black race (P = 0.0030), age over 65 years (P < 0.0001), with a tumor size of >5 and ≤10 cm (P = 0.0035), T2 stage (P = 0.0005), and positive/elevated CEA levels (P < 0.0001) exhibited a significantly poorer survival (Figure 3). The Kaplan–Meier survival curve analysis, conducted based on gender (P = 0.1932), grade (P = 0.8925), chemotherapy (P = 0.4963), radiotherapy (P = 0.5098), and radiation recode (P = 0.8758), revealed no significant differences (Supplementary Figure S1).
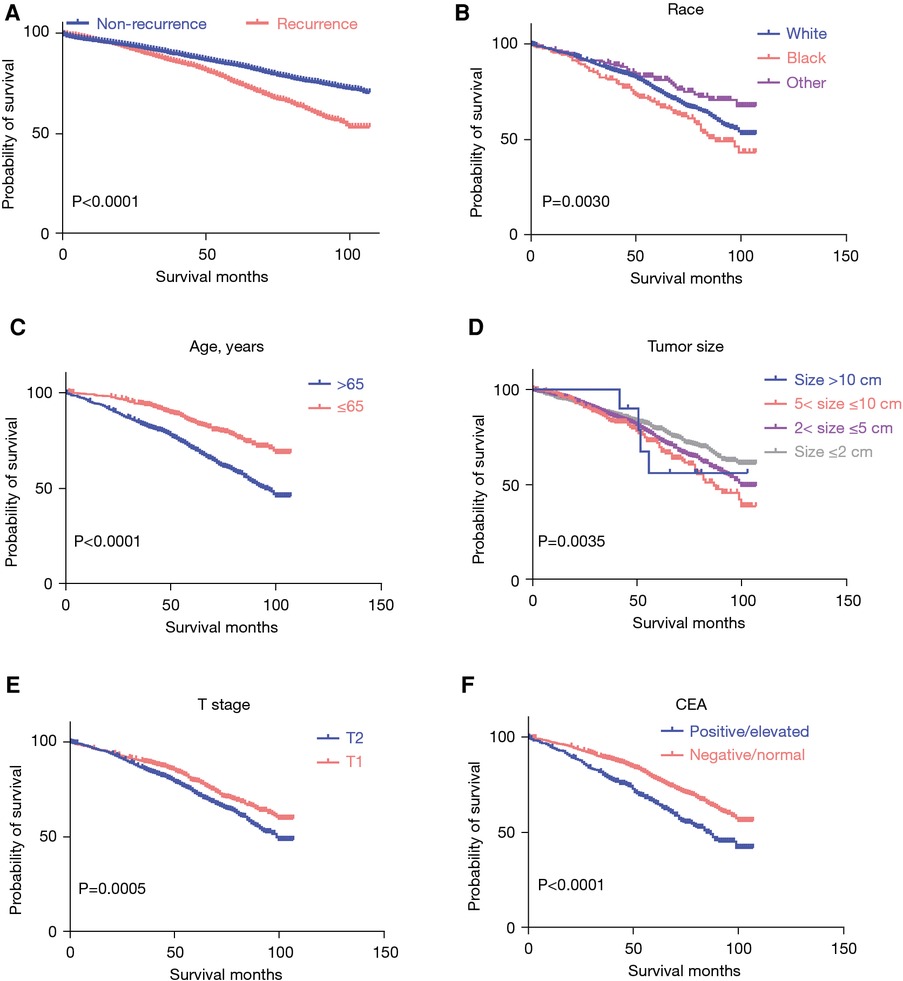
Figure 3. The Kaplan–Meier survival curve of patients with stage I CRC. (A) The Kaplan–Meier survival curve by recurrence status; (B-F) the Kaplan–Meier survival curve by race, age, tumor size, T stage, and CEA levels. CRC, colorectal cancer; CEA, carcinoembryonic antigen.
Recurrence risk analysis of patients with stage I CRC
A subsequent Cox regression analysis was conducted to assess the risks associated with survival in patients with CRC recurrence. The findings revealed that several factors were independently associated with the survival of patients with recurrent stage I CRC: age (<65 vs. ≥65 years: HR = 0.462; 95% CI: 0.3379–0.564; P < 0.001), race (Black vs. White: HR = 1.435; 95% CI: 1.121–1.837, P = 0.004; other vs. White: HR = 0.705; 95% CI: 0.504–0.985, P = 0.041), T stage (T1 vs. T2: HR = 0.82; 95% CI: 0.690–0.969, P = 0.020), and CEA status (negative/normal vs. positive/elevated: HR = 0.641; 95% CI: 0.537–0.766; P < 0.0001) (Figure 4).
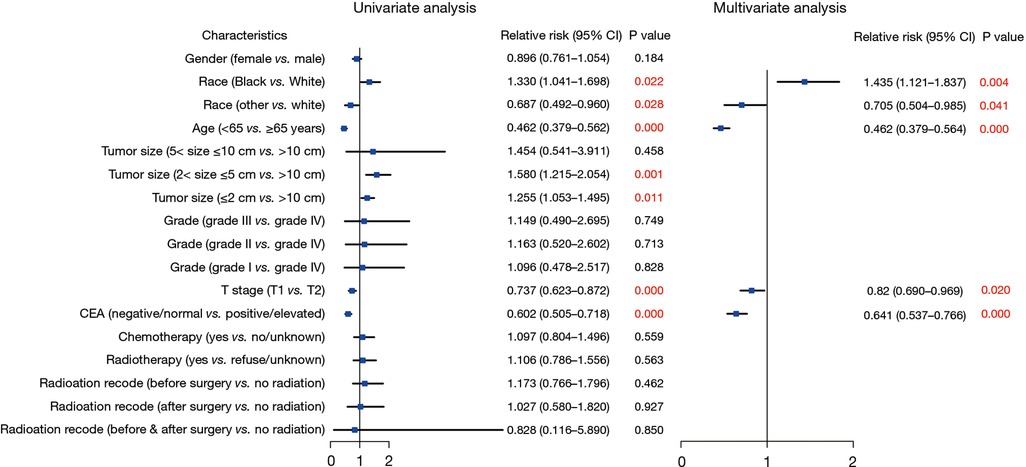
Figure 4. Forest plot of Cox regression analysis to assess the risks associated with survival in patients with recurrence.
Next, to explore risks associated with tumor recurrence among patients with stage I CRC, we conducted univariate and multivariate logistic regression analyses. Our findings indicated that female gender (HR = 0.781; 95% CI: 0.707–0.863; P < 0.01), Black race (HR = 0.812; 95% CI: 0.679–0.971; P = 0.022), age under 65 years (HR = 0.654; 95% CI: 0.590–0.726; P < 0.01), a negative/normal CEA level (HR = 0.816; 95% CI: 0.723–0.920; P = 0.001), and radiotherapy (HR = 0.809; 95% CI: 0.654–0.924; P = 0.041) were associated with a decreased likelihood of recurrence among patients with stage I CRC (Figure 5).
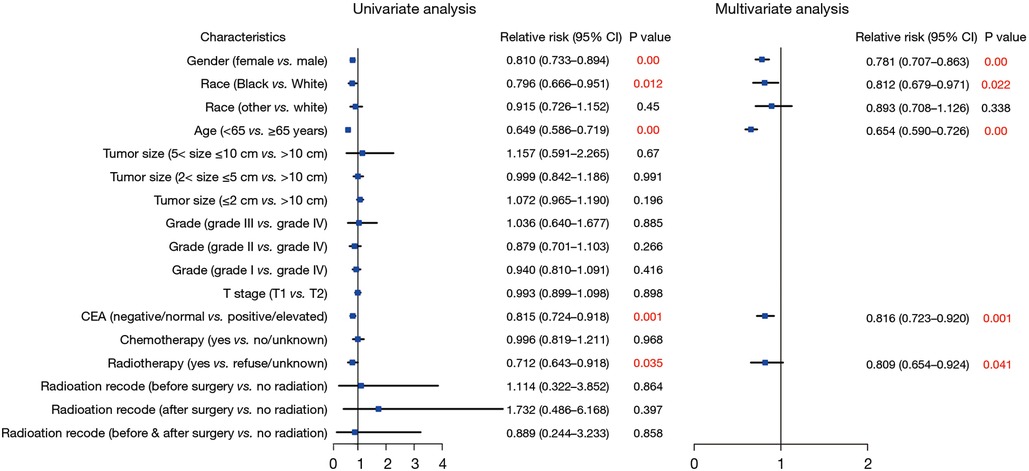
Figure 5. Forest plot of univariate and multivariate logistic regression analysis to assess the risks associated With recurrence.
Discussion
Cancer recurrence stands as a primary concern during the follow-up phase for patients diagnosed with CRC. More than 50% of patients with CRC experience recurrence within 2 years after surgery (13, 14), which is considered one of the main factors affecting patient prognosis. Preventing tumor recurrence can significantly improve the prognosis of these patients. While the consensus holds that patients with stage I CRC typically do not require postoperative adjuvant treatment but should be followed up every 6 months postsurgery, clinical observations often reveal postoperative recurrences among these patients. Hence, there is a need to analyze the current landscape of recurrence patterns and clinicopathological characteristics among patients with stage I CRC to inform appropriate follow-up and therapeutic strategies for early recurrence detection and curative treatment.
In previous research, the recurrence rate of CRC was reported as infrequent, ranging between 2.4% and 10% (15–19). In our study group, we observed a recurrence rate of 15% in stage I CRC, notably surpassing the rates reported in earlier research. Our results suggest that the recurrence rate of stage I CRC has increased, which may mean a larger burden on the treatment of CRC. Moreover, our study notably identified that Black and other non-White races, age over 65 years, and T2 stage were independent risk factors for tumor recurrence. These findings underscore the necessity for heightened vigilance during follow-up to mitigate recurrence or to promptly detect its onset in patients with these characteristics. Despite Nicholson et al.'s research indicating that CEA alone lacks the requisite sensitivity, even with a low threshold for recurrence detection in CRC patients (20), elevated CEA levels emerged as an independent risk factor for stage I CRC recurrence in our study. Interestingly, we identified a tumor size ≤5 cm as an independent risk factor for tumor recurrence in stage I CRC, which is somewhat counterintuitive. According to the NCCN Clinical Practice Guidelines in Oncology, patients with stage I CRC usually do not need to receive radiotherapy (9, 10). Another noteworthy finding of our study was that although radiotherapy could not reduce tumor recurrence in patients, it was significantly associated with an improved prognosis. This discovery offers a new perspective on whether radiotherapy should be recommended for patients with early-stage CRC.
Radiotherapy alone may be considered a curative approach in select cases of early-stage rectal cancer, particularly for T1N0M0 tumors, where it can achieve significant local control with minimal adverse effects. This treatment strategy allows for the preservation of the rectum and avoids the morbidity associated with surgery. Furthermore, we acknowledge the limitations of this approach, including the potential for local recurrence and the need for vigilant follow-up and management strategies to monitor and address any adverse effects.
In conclusion, we identified certain specific clinicopathological features for patients with stage I CRC and demonstrated the survival benefits of radiotherapy. These findings provide us with a new perspective on stage I CRC follow-up and treatment recommendations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JW: Writing – original draft. ZS: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was funded by the Zhejiang Provincial Natural Science Foundation of China (No. LY23H160022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1388250/full#supplementary-material
Supplementary Figure S1The Kaplan–Meier survival curve of patients with stage I CRC by gender, grade, chemotherapy, radiotherapy, and radiation recode. CRC, colorectal cancer.References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. (2023) 44:222–36. doi: 10.1016/j.tips.2023.01.003
3. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. (2020) 158:341–53. doi: 10.1053/j.gastro.2019.07.055
4. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. (2015) 150:17–22. doi: 10.1001/jamasurg.2014.1756
5. Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clin Gastroenterol Hepatol. (2017) 15:903–9.e906. doi: 10.1016/j.cgh.2016.08.037
6. Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. (2017) 109(8):djw322. doi: 10.1093/jnci/djw322
7. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
8. Renouf DJ, Woods R, Speers C, Hay J, Phang PT, Fitzgerald C, et al. Improvements in 5-year outcomes of stage II/III rectal cancer relative to colon cancer. Am J Clin Oncol. (2013) 36:558–64. doi: 10.1097/COC.0b013e318256f5dc
9. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2021) 19:329–59. doi: 10.6004/jnccn.2021.0012
10. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2022) 20:1139–67. doi: 10.6004/jnccn.2022.0051
11. Wu Q, Chen P, Shu C, Chen L, Jin Z, Huang J, et al. Survival outcomes of stage I colorectal cancer: development and validation of the ACEPLY model using two prospective cohorts. BMC Med. (2023) 21:3. doi: 10.1186/s12916-022-02693-7
12. Chakrabarti S, Peterson CY, Sriram D, Mahipal A. Early stage colon cancer: current treatment standards, evolving paradigms, and future directions. World J Gastrointest Oncol. (2020) 12:808–32. doi: 10.4251/wjgo.v12.i8.808
13. Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. (1997) 40:15–24. doi: 10.1007/BF02055676
14. Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. (2009) 27:872–7. doi: 10.1200/JCO.2008.19.5362
15. Gilardoni E, Bernasconi DP, Poli S, Garancini M, Luperto M, Zucchini N, et al. Surveillance for early stages of colon cancer: potentials for optimizing follow-up protocols. World J Surg Oncol. (2015) 13:260. doi: 10.1186/s12957-015-0674-7
16. Kang SI, Kim DW, Kwak Y, Lee HS, Kim MH, Kim MJ, et al. The prognostic implications of primary tumor location on recurrence in early-stage colorectal cancer with no associated risk factors. Int J Colorectal Dis. (2018) 33:719–26. doi: 10.1007/s00384-018-3031-9
17. Kim C, Kim WR, Kim KY, Chon HJ, Beom SH, Kim H, et al. Predictive nomogram for recurrence of stage I colorectal cancer after curative resection. Clin Colorectal Cancer. (2018) 17:e513–8. doi: 10.1016/j.clcc.2018.03.011
18. Leijssen LGJ, Dinaux AM, Kinutake H, Bordeianou LG, Berger DL. Do stage I colorectal cancers with lymphatic invasion require a different postoperative approach? J Gastrointest Surg. (2019) 23:1884–92. doi: 10.1007/s11605-018-4054-9
19. Teloken PE, Ransom D, Faragher I, Jones I, Gibbs P, Platell C. Recurrence in patients with stage I colorectal cancer. ANZ J Surg. (2016) 86:49–53. doi: 10.1111/ans.13254
Keywords: stage I colorectal cancer (stage I CRC), recurrence, survival, radiotherapy, colorectal cancer (CRC)
Citation: Wang J and Song Z (2024) Analysis of risk factors for postoperative recurrence of stage I colorectal cancer: a retrospective analysis of a large population. Front. Surg. 11:1388250. doi: 10.3389/fsurg.2024.1388250
Received: 19 February 2024; Accepted: 8 April 2024;
Published: 19 April 2024.
Edited by:
Vito D'Andrea, Sapienza University of Rome, ItalyReviewed by:
Andee Dzulkarnaen Zakaria, Universiti Sains Malaysia, MalaysiaRoberta Gelmini, University of Modena and Reggio Emilia, Italy
Lucia Mangone, IRCCS Local Health Authority of Reggio Emilia, Italy
© 2024 Wang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhangfa Song c29uZ3poYW5nZmFAMTYzLmNvbQ==
 Jiawei Wang
Jiawei Wang Zhangfa Song1,2,3*
Zhangfa Song1,2,3*