- 1Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China
- 3Department of Neurosurgery, Tianjin Fifth Central Hospital, Tianjin, China
- 4Department of Neurosurgery, Sinopharm Tongmei General Hospital, Tianjin, Shanxi, China
- 5Department of Neurosurgery, Yangquan First People’s Hospital, Yangquan, Shanxi, China
- 6Department of Neurosurgery, Xi'an No 9 Hospital, Xi'an, Shaanxi, China
Choroid plexus papilloma (CPP) is a rare benign intracranial tumor origin that predominantly manifests in the lateral ventricle in children, accounting for 0.3%–0.6% of all primary intracranial tumors. It is extremely rare to have the CPP in the trigone of the lateral ventricle through the contralateral posterior interhemispheric transfalcine transprecuneus approach (PITTA). Herein, we report this rare case. A 7-year-old girl presented with headache. Magnetic resonance imaging of the brain showed periatrial lesions, and histopathological examination confirmed CPP (WHO grade I). The contralateral PITTA is a safe, effective, reasonable, and appropriate for some lesions in the trigone of the lateral ventricle. It provides a wider surgical angle (especially for the lateral extension) and reduces the risk of disturbance of the optic radiation compared with the conventional approaches. The use of multiple modern neurosurgical techniques, including interventional embolization, intraoperative navigation, microscope, and electrophysiological monitoring, make the procedure much easier and more accurate, and the neuroendoscope adds to the visualization of the microscope and can reduce surgical complications.
Introduction
Choroid plexus papilloma (CPP) is a rare benign intracranial tumor that arise from choroid plexus epithelium (1). They account for only 0.3%–0.6% of all primary intracranial tumors and just 2%–4% of brain tumors in children (2, 3). The commonest locations in children and adults of CPP is the atrium of the lateral ventricle and the fourth ventricle, respectively (4, 5). Pathological entities situated in this locale commonly manifest with symptoms such as hemorrhage, seizures, visual impairments, and intracranial hypertension (6). Here, we treated a case of CPP arising from the trigone of the lateral ventricle and presenting with typical symptom of headache. The headache disappeared after tumor resection.
Surgical approaches to the periatrial or peritrigonal lesions pose unique neurosurgical challenges because of their proximity to critical structures, including white matter fiber tracts and the overlying cortices (7). The highly functional cerebral cortex and white matter tracts in this region encompass the optic radiations positioned laterally to the ventricle, the supralateral aspect of the postcentral gyrus, and the anteroinferior portion of the thalamus (8, 9). The anterior and posterior choroidal, pericallosal, and splenial arteries supply blood flow to both the surrounding normal parenchyma and lesions within this area. Additionally, crucial deep venous drainage occurs through the internal cerebral veins, vein of Rosenthal, and the straight sinus (10). These structures impose considerable limitations on the surgical corridor to the atrium of the lateral ventricle. Several approaches, like the superior parietooccipital, transtemporal, lateral temporoparietal, posterior transcallosal, and posterior interhemispheric parieto-occipital approaches, have been proposed to safely expos the lesions while addressing the surrounding normal anatomy (7, 8, 10). However, most of these approaches are linked to varying degrees of neurological deficits or limited working corridors (10). There previously used the contralateral posterior interhemispheric transfalcine transprecuneus approach (PITTA) in meningiomas, glioblastoma, and arteriovenous malformation, in the atrium of the lateral ventricle and found that the approach could provide a wider surgical angle and reduce the incidence of complications compared with other conventional approaches (11–13). Thus, it has become a practical surgical approach for lesions in the atrium of the lateral ventricle.
To our knowledge, there are many case reports but no patient in the literature describing the operative detail of the contralateral PITTA for CPP in the trigone of the lateral ventricle. We present the case managed microsurgical via the contralateral PITTA utilizing a combination of multiple modern neurosurgical techniques, including interventional embolization, intraoperative navigation, microscope, neuroendoscopy, and electrophysiological monitoring. We found that the approach could provide a wider surgical angle and reduce the incidence of complications compared with conventional approaches. In this case report, we will describe the associated challenges and advantages of this operative route. The clinical details of this patient will also be presented. The operation was performed by the senior author (X.Y.).
Clinical presentation
Patient information
A 7-year-old girl was admitted to this hospital because of a 6-week history of headaches and right trigonal tumor. The weight was 40 kg and the body-mass index (BMI) was 23.6. Preoperative imaging demonstrated a right peritrigonal mass suspected to be a CPP (Figures 1A–E). Total resection of this histologically confirmed CPP was evident on Computed tomography (CT) of the head (Figure 1K) 1 day after surgery as well as no injury to the contralateral hemisphere. Postoperative pathology: choroid plexus papilloma (WHO I). Immunohistochemically, expression of GFAP (±), EMA (+), keratin (+++), CAM5.2 (+++), P53 (+), syn (++) was detectable. No immunopositivity was observed for olig-2. Ki-67LI was 0.6% (Figures 2A–H). MRI of the head, performed without the administration of intravenous contrast material, was reportedly negative for any new findings at 4 years of follow-up (Figures 1M–O).
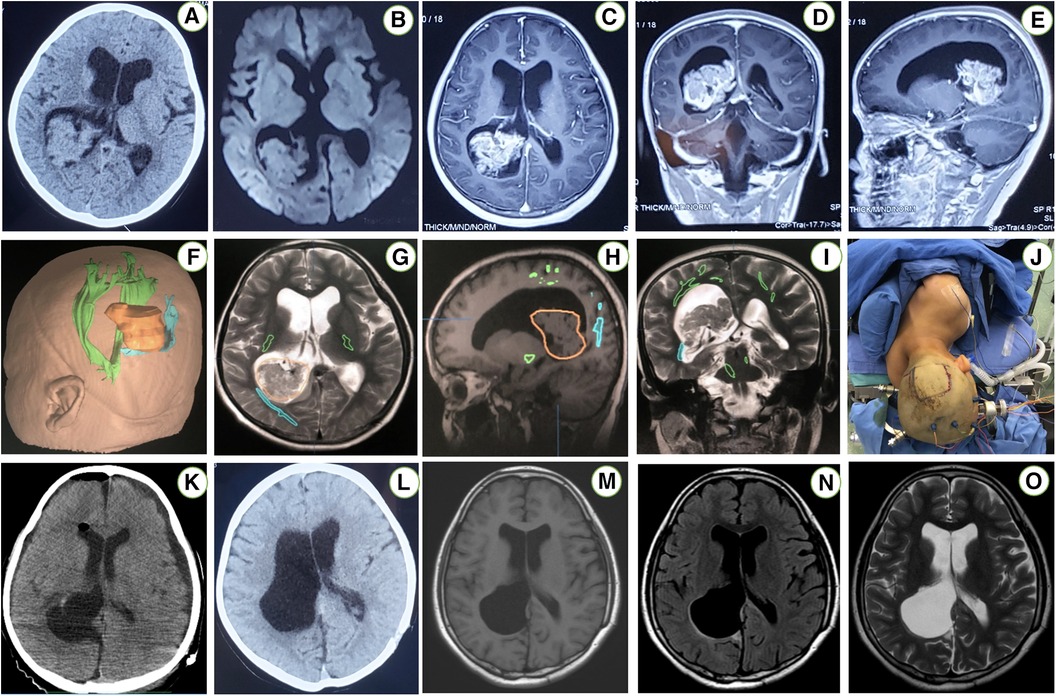
Figure 1 An axial CT scan of the head (A) reveals a right periatrial space occupying lesion. An axial T1-weighted MRI without contrast (B) demonstrates the exact location of the periatrial tumor. Enhanced images in sagittal, coronal, and transverse views of this patient brain MRI (C–E) demonstrated berry-like and irregular enhanced lesions in the right trigone of the lateral ventricle. Registration of the image-guided system (F–I) was performed. The patient position (J) in the contralateral PITTA. An axial postoperative CT scan (K) reveals a right atrial CPP that was resected via the left PITTA. An axial MRI T1-weighted sequence without contrast (L) excludes any unexpected injury to either posterior hemisphere. Postoperative axial T1-flair, T2-flair and T2-weighted without contrast images 4 years later (M–O) demonstrated complete resection via a contralateral approach.
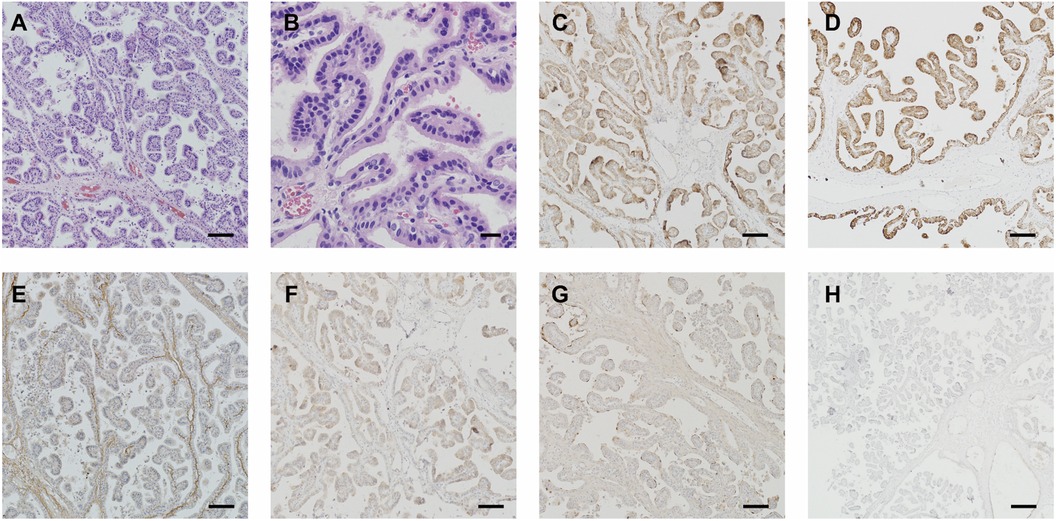
Figure 2 Postoperative pathology: choroid plexus papilloma (WHO I). Immunohistochemical expressions: GFAP (±), EMA (+), keratin (+++), CAM5.2 (+++), P53 (+), syn (++) and olig-2(−). Ki-67LI: 0.6% (A–H). Scale bar: (A,C–H) 100 μm, (B) 20 μm.
Operative technique
This patient was under general anesthesia. The endovascular access point was the right femoral artery. After securing access, a microcatheter was advanced into the right PchA for superselective angiography. The right PchA were identified prior to embolization (Figures 3A–D). After embolization, the patient is placed in a three-quarter prone position. The patient's head is rotated to align the axis of the superior sagittal sinus at a 45° angle with the floor. Subsequent to positioning, the image-guided system is registered (Figures 1F–I). The external ventricular drain (EVD) is strategically placed. Throughout the surgical procedure, neurophysiological monitoring is implemented for real-time assessment and guidance.
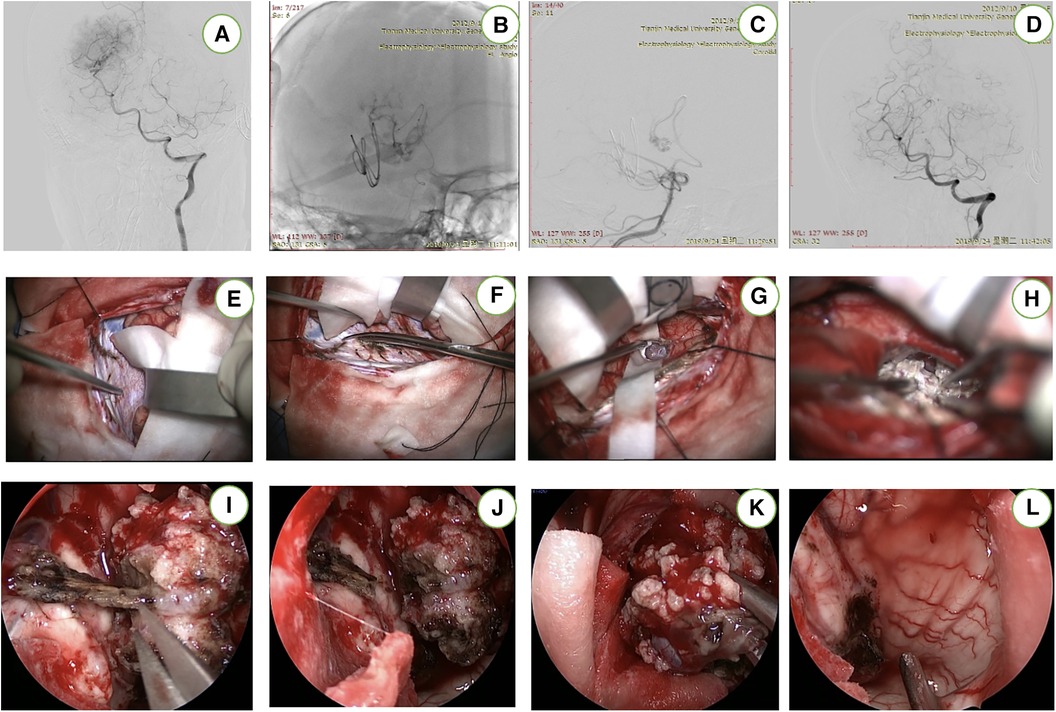
Figure 3 This patient underwent preoperative embolization (A–D). After cutting the occipital bone, the contralateral precuneus gyrus was located (E,F). The “T-shaped” incision in the falx has been completed and the tumor was completely resected (G,H). The endoscope (I–L) provided supplemental visualization of the temporal horn and the body of the lateral ventricle to check for any residual tumor and to remove the iatrogenic clot.
We employ a diminutive linear incision oriented perpendicular to the superior sagittal sinus, precisely situated over the occipital lobe (Figure 1J). The craniotomy, measuring 4 × 3 cm, is executed on the contralateral parietooccipital region while meticulously monitoring crucial bridging veins within the vicinity. Following the placement of two bur holes over the superior sagittal sinus, a parasagittal craniotomy is meticulously elevated, revealing the entire width and length of the dural sinus. The dura is incised in a horseshoe-shaped manner, aligning with the sinus morphology. Two retraction or tack-up sutures are carefully inserted through the superior portion of the falx, positioned just below the sinus. Microsurgical intervention may be required to release parasagittal bridging veins, enhancing the interhemispheric operative view in the anterior or posterior direction.
Approximately 50 ml of CSF is gradually removed through the EVD after dural opening. Cottonoid patties protect the contralateral unaffected hemisphere while a T-shaped incision in the falx is performed as guided by neuronavigation to provide access to the precuneus over the lesion (Figure 1I). The horizontal component of the “T-shaped” incision is made in proximity to the inferior aspect of the superior sagittal sinus, while the vertical segment of the incision is prolonged until the inferior sagittal sinus undergoes coagulation and sectioning (Figures 1J,K). Subsequent to this exposure, the resection of the lesion can proceed through a cortical incision within the precuneus, allowing access to the periatrial region for the removal of the pathological tissue and the exposure of the choroid plexus in the atrium (Figure 1L). Ultimately, the capsule is dissected, and the tumor is completely excised. We employed neuroendoscopy for the evacuation of intraventricular blood clots and comprehensive management of residual tumors or hemorrhage. Subsequently, the falx flap was meticulously closed using continuous sutures, followed by the sequential closure of the dura mater, bone flap, galea, and skin in layers. The (Figures 4A–E) is the Illustrations and intraoperative photographs of the procedure.
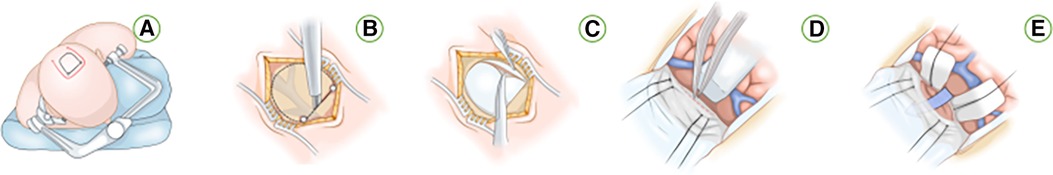
Figure 4 Illustrations and intraoperative photographs of the procedure. (A) Patient position and various incision options for approaching a left periatrial lesion while placing the contralateral “approach” hemisphere in the more dependent position to use gravity retraction. (B) Bur holes are placed on the superior sagittal sinus and a parasagittal craniotomy is completed while exposing the corresponding segment of the dural sinus. (C) A dural opening is made while avoiding the important bridging veins in the region. (D) The “T-shaped” incision within the falx cerebri to expose the contralateral medial hemisphere. (E) Placement of additional sutures on the falcine dural flaps to increase the transfalcine working angle and corridor to the contralateral atrium.
Headache was the primary preoperative symptom, and this patient showed disappear after surgery. The operative time was 3 h and 35 min. The estimated blood loss was less than 80 ml; she didn't require intra- or postoperative blood transfusion. Neurological outcomes as scored by the modified Rankin Scale were recorded during the 48 months of follow-up. Good outcomes (modified Rankin Scale scores of 0) were observed in this patient.
Discussion
The trigone or atrium of the lateral ventricle constitutes a triangular region demarcated by the convergence of the body, temporal, and occipital horns of the ventricle. While relatively infrequent (<1%), it is acknowledged as a distinct site for the occurrence of tumors, including CPPs and meningiomas (14, 15). Additionally, tumors like metastases and gliomas may originate in the peritrigonal region, extending into the atrium (16, 17).
The management of lesions situated in the periatrial region presents a formidable challenge due to their deep location and adjacency to eloquent structures and white matter tracts (7, 18). The determination of the optimal operative approach for this site remains a subject of controversy (19).
In generally, the ideal approach should enable comprehensive microsurgical resection while concurrently mitigating risks to the adjacent structures (20). Kawashima outlined three primary surgical routes: the anterior transsylvian, posterior transcortical/transcallosal, and lateral trans- or subtemporal (21). The anterior transsylvian approach, while providing a narrow corridor suitable for highly vascular lesions, poses a risk to motor fibers and optic radiations (7, 21). The ipsilateral posterior transcortical/transcallosal approach necessitates substantial brain retraction for accessing laterally situated atrial lesions (7, 21). Transtemporal approaches expose optic radiations along the trigone's lateral wall to potential risk (7, 21). An alternative route is the subtemporal corridor traversing the inferior temporal or occipitotemporal gyrus, which is associated with reduced risks of speech and visual field disturbances. However, challenges such as excessive temporal lobe retraction and traction on the vein of Labbe, particularly in the dominant hemisphere, are notable concerns (22, 23).
Alternative operators have supported the utilization of the posterior middle temporal gyrus approach (24). This approach enables prompt accessibility to anterior choroidal arteries and provides effective exposure to tumors that extend into the temporal horn. Nevertheless, the associated risks encompass potential harm to the optic radiations and language impairments in the dominant hemisphere, aphasia, agraphia, alexia, and visual-spatial apraxia (25).
Other surgeons have advocated an ipsilateral parietooccipital interhemispheric approach (24). Visual field impairments varying between 20% and 60% have been documented in association with this methodology. Additionally, Menon et al. identified additional complications, encompassing new motor deficits, seizures, and dysphasia (26). Others have preferred the posterior transcallosal approach (24, 25). The utilization of a contralateral transcallosal trajectory in this approach enables the surgeon to reach lesions with reduced transgression of the cortex and lateral white matter tracts. This approach also mitigates certain deficiencies and risks associated with alternative operative corridors. Nevertheless, the challenges inherent in traversing the corpus callosum for accessing the trigone involve limitations related to a narrow operative corridor and the potential risk of disconnection syndrome (7).
The ipsilateral posterior interhemispheric route is the standard and most commonly used approach for resection of medial periatrial lesions (7). This approach represents the established and frequently employed method for excising medial periatrial lesions. Placement of EVD into the occipital horn serves to deflate the ventricle, reducing hemispheric retraction and facilitating the exposure of the precuneus. However, the PITTA offers a trajectory that is more akin to a “cross-court” orientation towards the lateral aspect of the lesion, concurrently minimizing the extent of hemispheric retraction (7, 23).
One modification to the posterior transcortical/transcallosal approach is the posterior interhemispheric ipsilateral transprecuneus approach (27, 28). While this approach enables the surgeon to avoid optic radiations and the infringement upon the functional temporal cortex, it provides only a narrow operative corridor and necessitates ipsilateral brain retraction (29).
One solution to address the constraints of the narrow operative corridor is the contralateral PITTA (7, 23). This approach involves a modification of the traditional interhemispheric transprecuneus approach by executing a contralateral craniotomy and accessing the ipsilateral precuneus through a transfalcine method (7). By adopting this method, the associated risks of visual and speech deficits linked with the temporal transcortical approach and the potential complications related to somatosensory and visual deficits associated with the transcortical parietooccipital approach are reduced (7). The innovative PITTA route enhances the working angle and avoids excessive retraction of the ipsilateral hemisphere—both crucial elements in minimizing adverse effects and managing vascular lesions within the region (7, 23, 30). We have employed this approach in the treatment of the patient to facilitate the expansion of the operative corridor while mitigating brain transgression.
Several considerations must be carefully addressed to mitigate complications during the preparation of the contralateral PITTA. Particular attention is essential to prevent injury to the straight sinus when executing the “T-shaped” incision within the falx, especially when exposing lesions located more posteriorly. When performing the vertical incision within the falx under the guidance of neuronavigation, it is crucial to execute this incision obliquely from posterior to anterior to protect the straight sinus along the inferior aspect of the vertical falcine incision. This procedural step ensures extensive exposure of the more posterior regions of the precuneus as the falcine flaps are retracted. Following the resection of the lesion, the falcine retraction sutures are removed, and the falcine dural flaps are not sutured together to reconstruct the falx. Emphasis is placed on the pivotal role of neuronavigation in planning the location of the craniotomy flap, ensuring the protection of parasagittal bridging veins, and verifying the operative trajectory. Tractography, integrated with neuronavigation, is employed for navigation around subcortical functional white matter tracts. Subsequently, the removal of blood products and debris is conducted from the temporary EVD.
Conclusion
In accordance with our empirical observations, the posterior interhemispheric transfalcine transprecuneus approach to trigonal lesions presents itself as a rational, secure, and viable alternative to previously delineated methodologies. This adaptation of the interhemispheric approach mitigates the necessity for substantial ipsilateral cortical resection or retraction, concurrently enhancing the operational angles available to the operator. The exemplified case underscores the proposition that, with judicious patient selection, this alternative trajectory can be judiciously and efficiently applied to a diverse spectrum of pathologies situated within and medial to the trigone of the lateral ventricle.
Limitation
While this approach demonstrated success in the presented case, less experienced surgeons and even some experienced practitioners may encounter challenges associated with the contralateral midline venous drainage, aberrant deep drainage in the posterior falx, retraction-related injuries to the deep venous system, bilateral visual deficits, and contralateral deep ventricular bleeding without proximal control. Conducting future extensive studies, encompassing similar pathologies, would be imperative to thoroughly explore the potential advantages of the PITTA.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Methodology. ZW: Writing – original draft, Formal Analysis, Software. JZ: Conceptualization, Writing – original draft. XC: Writing – original draft, Methodology. ZW: Writing – original draft, Data curation. ZZ: Writing – original draft. YC: Writing – original draft. SZ: Writing – original draft. XZ: Writing – original draft. ZW: Project administration, Writing – review & editing. HZ: Writing – original draft, Investigation. CG: Writing – review & editing. SY: Writing – review & editing, Project administration, Resources, Supervision. YZ: Writing – original draft. XY: Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by funds from the Tianjin Medical University Clinical Research Program (Grant No. 2018kylc008), the Tianjin Medical University General Hospital Clinical Research Program (Grant No. 22ZYYLCCG07) and the Natural Science Foundation of Tianjin Municipality (Grant No. 20JCZDJC00300).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thomas C, Soschinski P, Zwaig M, Oikonomopoulos S, Okonechnikov K, Pajtler KW, et al. The genetic landscape of choroid plexus tumors in children and adults. Neuro Oncol. (2021) 23:650–60. doi: 10.1093/neuonc/noaa267
2. Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. (2010) 119:75–88. doi: 10.1007/s00401-009-0627-8
3. Mangham WM, Elijovich L, Lee-Diaz JA, Orr BA, Gienapp AJ, Boop FA. Pre-operative embolization for staged treatment of infantile choroid plexus papilloma. Childs Nerv Syst. (2022) 38(2):429–33. doi: 10.1007/s00381-021-05212-w
4. Tan LA, Fontes RB, Byrne RW. Retrosigmoid approach for resection of an extraventricular choroid plexus papilloma in the cerebellopontine angle. Neurosurg Focus. (2014) 36(1 Suppl):1. doi: 10.3171/2014.V1.FOCUS13271
5. Kennedy BC, Cloney MB, Anderson RC, Feldstein NA. Superior parietal lobule approach for choroid plexus papillomas without preoperative embolization in very young children. J Neurosurg Pediatr. (2015) 16(1):101–6. doi: 10.3171/2014.11.PEDS14281
6. Zhou F, Li Y, Shen L, Yao H, Hou X. Infantile epileptic spasms syndrome as an initial presentation in infantile choroid plexus papilloma: a case report. Front Pediatr. (2022) 10:1035621. doi: 10.3389/fped.2022.1035621
7. Yaşargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. (2008) 62(6 Suppl 3):1029–40; discussion 1040–1. doi: 10.1227/01.neu.0000333768.12951.9a
8. Xie T, Sun C, Zhang X, Zhu W, Zhang J, Gu Y, et al. The contralateral transfalcine transprecuneus approach to the atrium of the lateral ventricle: operative technique and surgical results. Neurosurgery. (2015) 11(Suppl 2):110–7; discussion 117–8. doi: 10.1227/NEU.0000000000000643
9. McDermott MW. Intraventricular meningiomas. Neurosurg Clin N Am. (2003) 14(4):559–69. doi: 10.1016/s1042-3680(03)00055-x
10. Bohnstedt BN, Kulwin CG, Shah MV, Cohen-Gadol AA. Posterior interhemispheric transfalcine transprecuneus approach for microsurgical resection of periatrial lesions: indications, technique, and outcomes. J Neurosurg. (2015) 123(4):1045–54. doi: 10.3171/2015.3.JNS14847
11. Wang S, Salma A, Ammirati M. Posterior interhemispheric transfalx transprecuneus approach to the atrium of the lateral ventricle: a cadaveric study. J Neurosurg. (2010) 113:949–54. doi: 10.3171/2010.1.JNS091169
12. Zaidi HA, Chowdhry SA, Nakaji P, Abla AA, Spetzler RF. Contralateral interhemispheric approach to deep-seated cavernous malformations: surgical considerations and clinical outcomes in 31 consecutive cases. Neurosurgery. (2014) 75:80–6. doi: 10.1227/NEU.0000000000000339
13. Zhu W, Xie T, Zhang X, Ma B, Wang X, Gu Y, et al. A solution to meningiomas at the trigone of the lateral ventricle using a contralateral transfalcine approach. World Neurosurg. (2013) 80(1–2):167–72. doi: 10.1016/j.wneu.2012.08.010
14. Delfini R, Acqui M, Oppido PA, Capone R, Santoro A, Ferrante L. Tumors of the lateral ventricles. Neurosurg Rev. (1991) 14:127–33. doi: 10.1007/BF00313037
15. Ellenbogen RG. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. (2001) 10(6):E2. doi: 10.3171/foc.2001.10.6.3
16. Barrow DL, Dawson R. Surgical management of arteriovenous malformations in the region of the ventricular trigone. Neurosurgery. (1994) 35:1046–54. doi: 10.1227/00006123-199412000-00005
17. Batjer H, Samson D. Surgical approaches to trigonal arteriovenous malformations. J Neurosurg. (1987) 67(4):511–7. doi: 10.3171/jns.1987.67.4.0511
18. Xie T, Zhou L, Zhang X, Sun W, Ding H, Liu T, et al. Endoscopic supracerebellar transtentorial approach to atrium of lateral ventricle: preliminary surgical and optical considerations. World Neurosurg. (2017) 105:805–11. doi: 10.1016/j.wneu.2017.06.093
19. Izci Y, Seçkin H, Ateş O, Başkaya MK. Supracerebellar transtentorial transcollateral sulcus approach to the atrium of the lateral ventricle: microsurgical anatomy and surgical technique in cadaveric dissections. Surg Neurol. (2009) 72:509–14; discussion 514, doi: 10.1016/j.surneu.2009.01.025
20. Jeelani Y, Gokoglu A, Anor T, Al-Mefty O, Cohen AR. Transtentorial transcollateral sulcus approach to the ventricular atrium: an endoscope-assisted anatomical study. J Neurosurg. (2017) 126(4):1246–52. doi: 10.3171/2016.3.JNS151289
21. Kawashima M, Li X, Rhoton AL Jr, Ulm AJ, Oka H, Fujii K. Surgical approaches to the atrium of the lateral ventricle: microsurgical anatomy. Surg Neurol. (2006) 65:436–45. doi: 10.1016/j.surneu.2005.09.033
22. Santoro A, Salvati M, Frati A, Polli FM, Delfini R, Cantore G. Surgical approaches to tumours of the lateral ventricles in the dominant hemisphere. J Neurosurg Sci. (2002) 46:60–5. discussion 65.12232550
23. Wang X, Cai BW, You C, He M. Microsurgical management of lateral ventricular meningiomas: a report of 51 cases. Minim Invasive Neurosurg. (2007) 50(6):346–9. doi: 10.1055/s-2007-993205
24. Jun CL, Nutik SL. Surgical approaches to intraventricular meningiomas of the trigone. Neurosurgery. (1985) 16:416–20. doi: 10.1227/00006123-198503000-00025
25. Tew JM, Larson JJ. Intraventricular meningioma. In: Kaye AH, Black PM, editors. Operative Neurosurgery. London: Churchill Livingstone (2000). p. 575–85.
26. Menon G, Nair S, Sudhir J, Rao R, Easwer HV, Krishnakumar K. Meningiomas of the lateral ventricle—a report of 15 cases. Br J Neurosurg. (2009) 23(3):297–303. doi: 10.1080/02688690902721862
27. Nishizaki T, Ikeda N, Nakano S, Okamura T, Abiko S. Occipital inter-hemispheric approach for lateral ventricular trigone meningioma. Acta Neurochir (Wien). (2009) 151:1717–21. doi: 10.1007/s00701-009-0310-9
28. Pescatori L, Tropeano MP, Torregrossa F, Grasso G, Ciappetta P. The ipsilateral interhemispheric transprecuneal approach to the atrium: technical considerations and clinical outcome on a series of 7 patients. Brain Sci. (2022) 12(11):1453. doi: 10.3390/brainsci12111453
29. Tokunaga K, Tamiya T, Date I. Transient memory disturbance after removal of an intraventricular trigonal meningioma by a parieto-occipital interhemispheric precuneus approach: case report. Surg Neurol. (2006) 65(2):167–9. doi: 10.1016/j.surneu.2005.06.036
Keywords: lateral ventricle, trigone, tumor, contralateral, interhemispheric approach, microsurgical resection, surgical technique
Citation: Song Y, Wang Z, Zhang J, Cui X, Wu Z, Zhao Z, Chen Y, Zhang S, Zhu X, Wang Z, Zhang H, Gao C, Yang S, Zhao Y and Yang X (2024) Resection of the tumor in the trigone of the lateral ventricle via the contralateral posterior interhemispheric transfalcine transprecuneus approach with multi-modern neurosurgery technology: a case report. Front. Surg. 11:1371983. doi: 10.3389/fsurg.2024.1371983
Received: 19 January 2024; Accepted: 6 June 2024;
Published: 24 June 2024.
Edited by:
Mirza Pojskic, University Hospital of Giessen and Marburg, GermanyReviewed by:
Erika Carrassi, Hospital Santa Maria della Misericordia of Rovigo, ItalyMarcelo Galarza, University of Murcia, Spain
Matias Baldoncini, University of Buenos Aires, Argentina
© 2024 Song, Wang, Zhang, Cui, Wu, Zhao, Chen, Zhang, Zhu, Wang, Zhang, Gao, Yang, Zhao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyu Yang, ZHJ5YW5neGlueXVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yunfei Song
Yunfei Song Zhen Wang1,†
Zhen Wang1,† Jun Zhang
Jun Zhang Xiaopeng Cui
Xiaopeng Cui Zhitao Wang
Zhitao Wang Yan Zhao
Yan Zhao