- 1The Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Oslo, Norway
- 2Department of Thoracic and Vascular Surgery, Akershus University Hospital, Lørenskog, Norway
- 3The Intervention Centre, Oslo University Hospital, Oslo, Norway
- 4The Faculty of Medicine, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway
- 5Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway
Objectives: The aim of this study was to assess the potential of electrodermal activity (EDA) as a diagnostic tool for preoperative evaluation in hyperhidrosis patients. EDA levels and patterns in different skin areas were investigated before and after endoscopic thoracic sympathicotomy (ETS) and was compared to healthy subjects.
Methods: Thirty-seven patients underwent two days of measurements before and after the operation. Twenty-five (67.5%) of the patients also had a third measurement after six months. Non-invasive EDA measurements, involving skin conductance, were sampled from five different skin areas while patients were at rest in supine and sitting positions or when subjected to stimuli such as deep inspirations, mental challenge, and exposure to a sudden loud sound.
Results: Prior to the operation, hyperhidrosis patients showed higher spontaneous palm EDA variations at rest and stronger responses to stimuli compared to healthy subjects. Patients with facial blushing/hyperhidrosis or combined facial/palmar hyperhidrosis showed minimal spontaneous activity or responses, particularly during mental challenge and sound stimulus. Notably, palm EDA response was abolished shortly following sympathicotomy, although a minor response was observed after six months. Minimal EDA responses were also observed in the back and abdomen postoperatively.
Conclusion: Hyperhidrosis patients showed stronger EDA response to stimuli compared to healthy subjects. Sympathicotomy resulted in the complete elimination of palm EDA responses, gradually returning to a limited extent after six months. These findings suggest that EDA recordings could be utilized in preoperative assessment of hyperhidrosis patients.
Introduction
Primary hyperhidrosis is a disorder characterized by sweating in excess of physiological requirements for thermoregulation and heat dissipation. This condition is particularly exacerbated by both physiological and mental stimuli (1–3). Hyperhidrosis primarily affects the palms, soles, axillae, or forehead (face) (1, 2, 4, 5) and is equally prevalent in both genders, with an incidence ranging from 0.6 to 1.0% in the general population (1, 6). The disorder can significantly impact the quality of life for affected individuals (1, 2, 5, 7–9).
Several treatment modalities have been attempted with limited success (1, 7). Endoscopic thoracic sympathicotomy (ETS) is considered the treatment of choice for severe palmar hyperhidrosis (7, 10–13) and facial blushing/hyperhidrosis (14–20). ETS yields satisfactory results for palmar hyperhidrosis, with most severe hand sweating cases being effectively resolved (9, 10, 21, 22). However, the ETS procedure is associated with potentially undesirable side effects, particularly compensatory hyperhidrosis (CH) (23, 24). An expert consensus for the surgical treatment of hyperhidrosis has been published (25), and Weksler et al. reported that R2 sympathicotomy is a suitable surgical option for patients suffering from facial blushing or hyperhidrosis, while R3 sympathicotomy is recommended for palmar hyperhidrosis (26).
Hyperhidrosis is thought to be primarily caused by overactivity of the sympathetic nervous system (27–31), and electrodermal activity (EDA) reflects activity of the sympathetic nervous system. We have previously described EDA responses in healthy subjects using a standardized protocol (32). The aim of the present study was to investigate steady-state levels, dynamics, and recurrence rate of EDA in different skin locations in response to minor physiological and mental stimuli before and after sympathicotomy in hyperhidrosis patients. Additionally, we compared the EDA recordings in these patients to EDA recordings in healthy subjects to assess the protocol’s possible suitability for preoperative diagnostic workup in hyperhidrosis patients.
Materials and methods
Subjects
A total of thirty-seven patients were included in the study, all referred from primary healthcare to our hospital from all parts of the country. Patients were scheduled for ETS due to facial blushing (seventeen patients), facial hyperhidrosis (six patients), palmar hyperhidrosis (four patients), and combined facial/palmar hyperhidrosis (ten patients). Twenty patients were not included due to reoperations, missing recordings, or Harlequin sign.
The indication for surgery was disabling hyperhidrosis or facial blushing based on the patients’ complaints. All patients underwent anamnestic and clinical examinations by one of three experienced operating surgeons. Seven patients were smokers. Five patients used medications for comorbidities such as hypertension, asthma, and epilepsy, and two for anxiety. Neither had recordings that differed from the rest.
Surgical technique
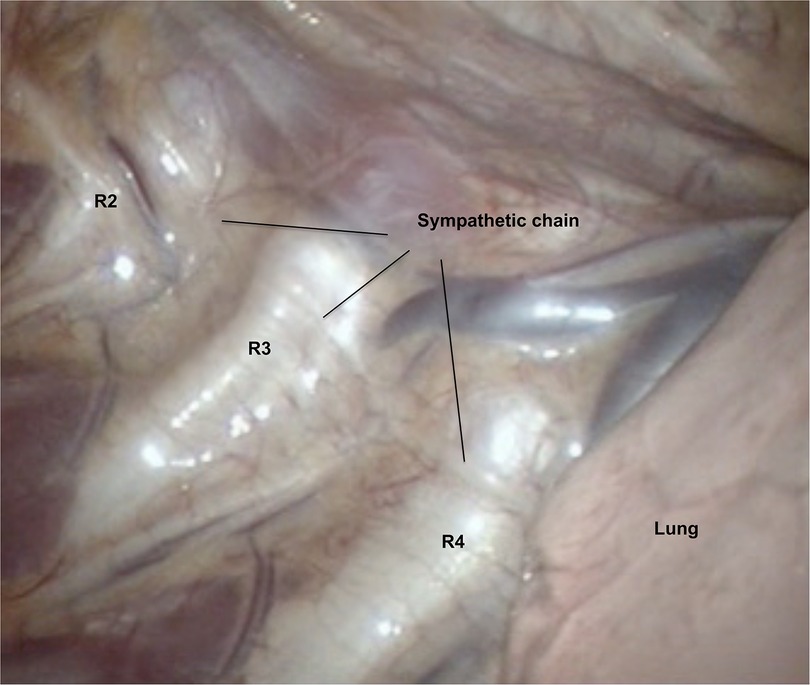
Patients were positioned in a beach-position (back elevated approximately 45 degrees from horizontal), and with abduction of both arms. The surgical procedure was conducted under general anesthesia using single-lumen endotracheal intubation. Video-assisted thoracoscopic surgery was used for all patients. Two 5 mm ports were utilized, with the first positioned in the anterior axillary line in the second to fourth intercostal space, and the second located one to two ribs below the first port. Upon the introduction of thoracoscopy into the thoracic cavity, CO2 gas with positive pressure was administered to deflate the lung. Rib-orientation was used to describe the transected position on the sympathetic chain (Figure 1). The costal pleura covering the sympathetic chain was transected using diathermy or ultra-scission scalpel (Ethicon/Johnson & Johnson, New-Brunswik, NJ, USA) at the R2-level (R2 sympathicotomy), R3-level (R3 sympathicotomy) or both R2/R3-level (R2/R3 sympathicotomy). After the insertion of a 10 Fr female urinary catheter into the pleural cavity through the lower port, the lung was reinflated, and the catheter was removed upon cessation of air leakage. If air leakage persisted, the catheter remained in place and was connected either to passive drainage (Heimlich valve) or to a Thopaz pump (Medela, Baar, Switzerland) with active drainage. An identical procedure was then performed on the contralateral side. All patients underwent postoperative chest x-ray before discharge. In the event of a clinically significant pneumothorax or the detection of a large residual gas postoperatively, it was managed with a Tru-close Thoracic Vent (Uresil, Skokie, IL, USA) with passive or active suction, inserted under local anesthesia.
Experimental design
The present study adopted an identical experimental protocol to that previous described in a study on sweat activity in healthy subjects (32). EDA measurements were obtained continuously over 30 min, with 10 min in a supine position (SUP) and 20 min in a sitting position (SIP). During the last 10 min in the sitting position, the subjects were exposed to a series of stimuli. The first was to take a deep inspiration three times every minute (INSP1, INSP2, INSP3) interspersed with normal respiration between the deep inspirations. This was followed by a mental challenge (MC) in which subjects were required to perform a calculation (subtracting 7 from 100 each time) over the course of one minute. Finally, the subjects were exposed to a sudden (< 0.5 s) sound stimulus (SS). The SS was a single hand clap behind the subject that created a sound of approximately 90 dB.
The subjects wore t-shirt and shorts, and the ambient temperature was maintained at 27 ± 1°C to ensure the subjects were in the thermoneutral zone. They were resting in the supine position for 30 min before each experimental session. All subjects underwent pre- and postoperative measurements. Postoperative measurements were performed four hours to four weeks after ETS. In addition, twenty-five subjects also underwent a third day of measurement six months later.
Measurements
A multichannel Sudologger (BioGauge AS, Oslo Innovation Center, Oslo, Norway) was used to collect non-invasive continuous measurements of electrodermal activity. EDA was simultaneously measured in five locations on both palms, forehead (named face in tables and figures), back, and abdomen (Figure 2) (33). The Sudologger uses skin surface electrodes for unipolar conductance measurements in the stratum corneum. The method was introduced and described in detail by Tronstad et al. (33).
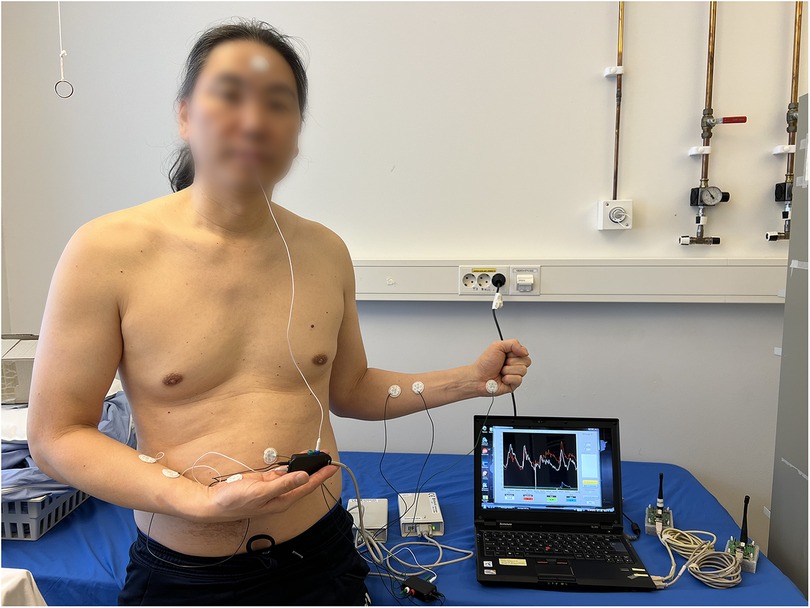
Figure 2. Two Sudologger devices with electrodes attached to a subject. The electrodermal activity was measured in five different skin areas, including the palms, forehead (face), back and abdomen. Additionally, two electrodes on each forearm (reference electrodes) were used for calibrating the measurements of electrodermal activity. The subject has given written consent for publication.
Data analysis and statistics
Two Sudologger units were used, and recordings of EDA measurements were saved in the Sudologger program. Due to the unavailability of a second instrument, only one Sudologger was used for the first ten patients, and EDA measurements from the left palm, back, face, and abdomen were recorded. Previously, synchronous sweat patterns in both palms have been shown (32, 34), thus measurement from just one palm was considered to be sufficient for further analysis. Data analysis was performed using customized computer programs with Python 2.4 and MATLAB 2021a (MathWork Inc., Natick, MA, USA).
To eliminate random variations, averaged responses from different sweat areas of the skin from thirty-seven patients were calculated (i.e., coherent averaging) (35). Outliers were identified during manual data inspection, including two patients with missing signals over a short period of four to five minutes. The missing signals were attributed to inadequate electrode placement. Instead of removing the entire data points of these outliers, only four to five minutes of missing signal were excluded from the analysis. To ensure consistency in future analysis, these missing data points were replaced with NaN (not a number) values, which would not affect calculation of means or other statistical measures (MATLAB 2021a). This approach allows the remaining data from these patients to contribute to the overall analysis while accounting for the missing information appropriately.
Based on our pilot measurements and observations from similar studies of patients undergoing endoscopic thoracic sympathicotomy, we expected to observe a change of at least 80% in EDA in response to physiological and mental stimuli. A sample size of thirty-seven patients with hyperhidrosis would have 80% power to detect a difference in means of 80% with a standard deviation of 1.05 using a two-sided paired sample t-test with an α of 0.05.
For all patients, the statistical significance of differences in sweat patterns between pre- and postoperative days was analyzed using paired-samples t-tests in IBM SPSS Statistics 29 (Armonk, NY, USA) (36). Values are expressed as means and 95% confidence intervals (CIs), and differences are considered statistically significant at p < 0.05.
Results
The study population included twenty-one males and sixteen females, with a median age of 32.4 years (range 19–61 years). In our study, 46% of the patients (n = 17) had facial blushing, and 16% (n = 6) had facial hyperhidrosis and underwent sympathetic chain transection at R2. Four patients (11%) with palmar hyperhidrosis had sympathetic nerve interruption at R3. Ten patients (27%) had sympathetic nerve interruption at R2 and/or R3 for both conditions. One patient with facial blushing underwent sympathetic block by clipping, as requested. This patient had similar EDA recordings as the rest of the group. The ETS procedure was completed within a mean of 20 min (range 16–30 min), with most patients being discharged the next morning. A few patients were discharged in the evening after the operation. Five patients (13.5%) experienced mild perioperative complications such as pneumonia, pneumothorax, and a small lung lesion, but all were discharged within 1–2 days.
Figure 3 shows EDA recordings from a patient with facial hyperhidrosis. Preoperatively, the EDA in both left and right palms increased rapidly and synchronously with every stimulus. Additionally, several spontaneous minor electrodermal variations were observed in the palms in the sitting position. However, no EDA responses were observed in the face. The palm responses were completely abolished shortly after the operation, which in this patient was recorded after seven days. However, minor EDA responses were observed in the palms after six months in response to position change, MC, and SS. A gradual increase in facial EDA was observed throughout the experiment after seven days, but there were no measurable responses to individual stimuli. No response recordings to stimuli in EDA were observed from the back and abdominal locations, neither pre- nor postoperatively.
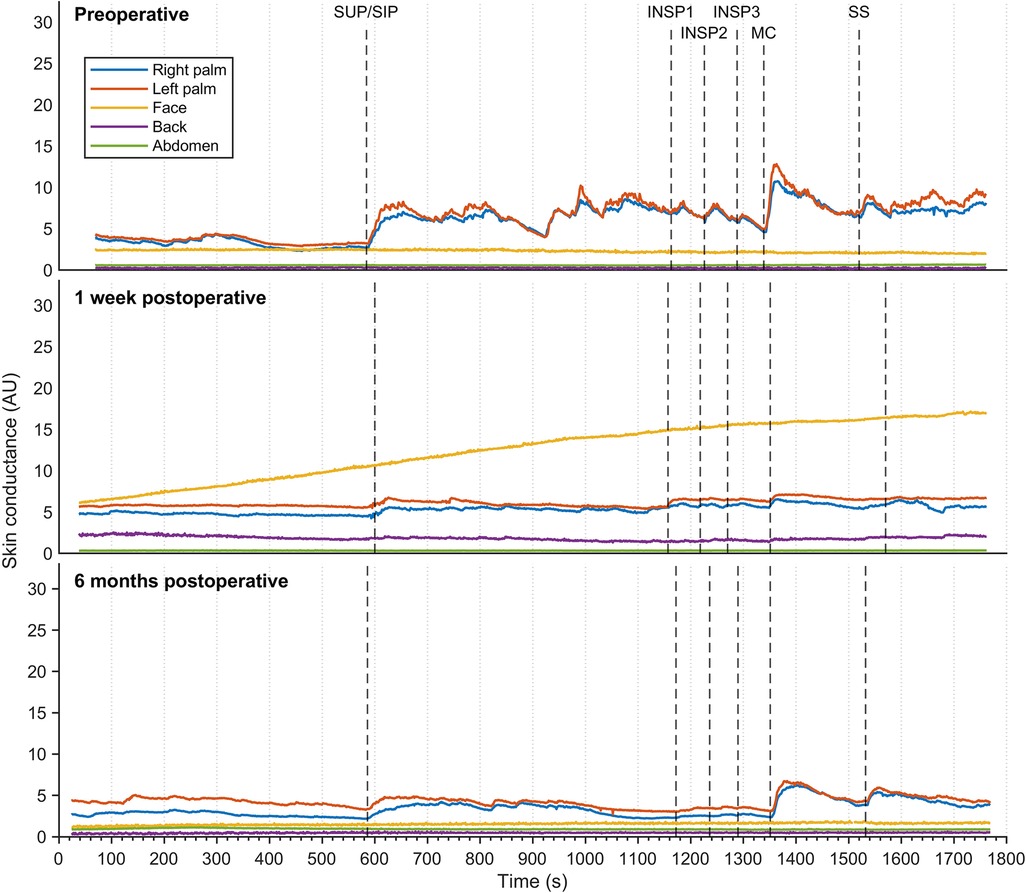
Figure 3. Thirty minutes of continuous electrodermal activity was recorded from five different skin locations in a patient suffering from facial hyperhidrosis, but who was otherwise healthy. Recordings were captured preoperatively (A), one week postoperatively (B), and six months postoperatively (C) The color coding of the traces is explained on the left. The x-axis represents time in seconds, while the y-axis represents arbitrary units of skin conductance recorded in the right and left hypothenar region of the palm, back, face, and abdomen. Vertical dashed lines indicate the initiation of different stimuli, corresponding to position change from supine (SUP) to sitting (SIP) (SUP-SIP), three deep inspirations (INSP1-3), mental challenge (MC), and sound stimulus (SS).
Another sweat pattern in one patient suffering from facial blushing showed increased palm EDA in response to every stimulus preoperatively (Figure 4), and several small EDA variations were also observed in the supine position. There were no changes in EDA in the face, back, or abdomen. Palm EDA showed lower response to stimuli shortly after ETS (four weeks), but this increased six months postoperatively and was observed in the supine position and in response to every stimulus. However, the amplitude was not as high as it had been preoperatively. Four weeks postoperatively, this patient also presented an EDA response in the face, back, and abdomen upon MC and SS. While the facial EDA response was abolished six months postoperatively, back and abdominal EDA responses to MC and SS stimuli persisted.

Figure 4. Thirty minutes of continuous EDA was recorded from five different skin areas in a patient suffering from facial blushing, but who was otherwise healthy. Recordings were captured preoperatively (A), four weeks postoperatively (B), and six months postoperatively (C) The color coding, axes, and lines are identical to those in Figure 3.
Figure 5 shows sweat patterns in a patient with combined facial/palmar hyperhidrosis. During preoperative assessment, a slight increase in EDA response was observed in the face, back, and abdomen in response to MC and SS. Shortly after ETS (five days), EDA responses to MC and SS in the back and abdomen were still noticeable and had increased after six months.
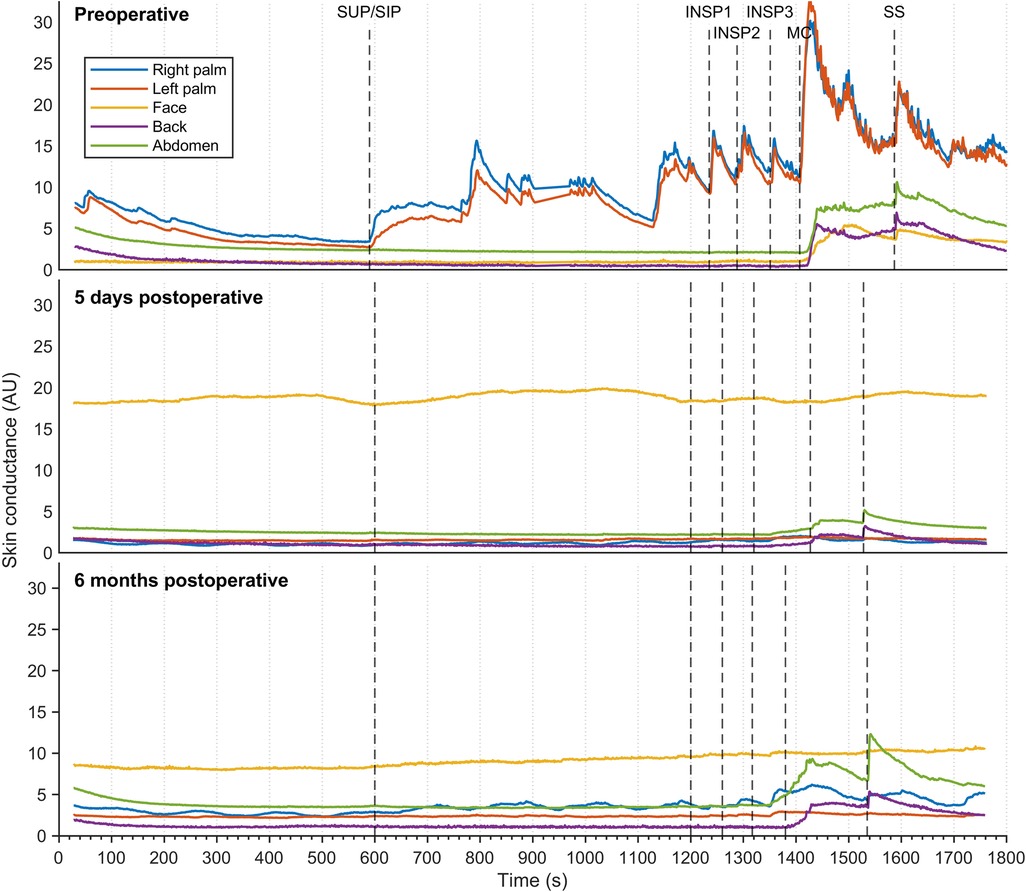
Figure 5. Thirty minutes of continuous EDA was recorded from five different skin areas in a patient suffering from combined palmar/facial hyperhidrosis, but who was otherwise healthy. Recordings were captured preoperatively (A), five days postoperatively (B), and six months postoperatively (C) The color coding, axes, and lines are identical to those in Figure 3.
The mean values of continuous EDA recordings from five different skin locations in thirty-seven patients with facial hyperhidrosis, facial blushing, palmar hyperhidrosis, and combined facial/palmar hyperhidrosis are shown in Figure 6. Postoperatively, the EDA pattern differed from preoperative recordings. Before the surgery, EDA peaks varied in magnitude in response to physiological stimuli and mental activity. Recordings from the palms showed a temporary and synchronous response with each stimulus, with the greatest EDA response being recorded during position change, MC, and SS. A slight increase in EDA was also observed in the face, back, and abdomen during MC and upon exposure to SS. Six months postoperatively, an increase in EDA was observed in the palms during position change, MC, and SS, although the amplitude was lower than preoperative recordings. The sweat pattern in the back and abdomen persisted postoperatively, with a somewhat lower EDA amplitude compared to preoperative recordings. After the operation, no EDA response was observed in the face. Supplementary Figure S1 shows the mean EDA response curves, similar to those in Figure 6, for three different groups: patients with facial blushing (seventeen patients), facial hyperhidrosis (six patients), and combined facial/palmar hyperhidrosis (ten patients). The mean recordings in these three groups are very similar.
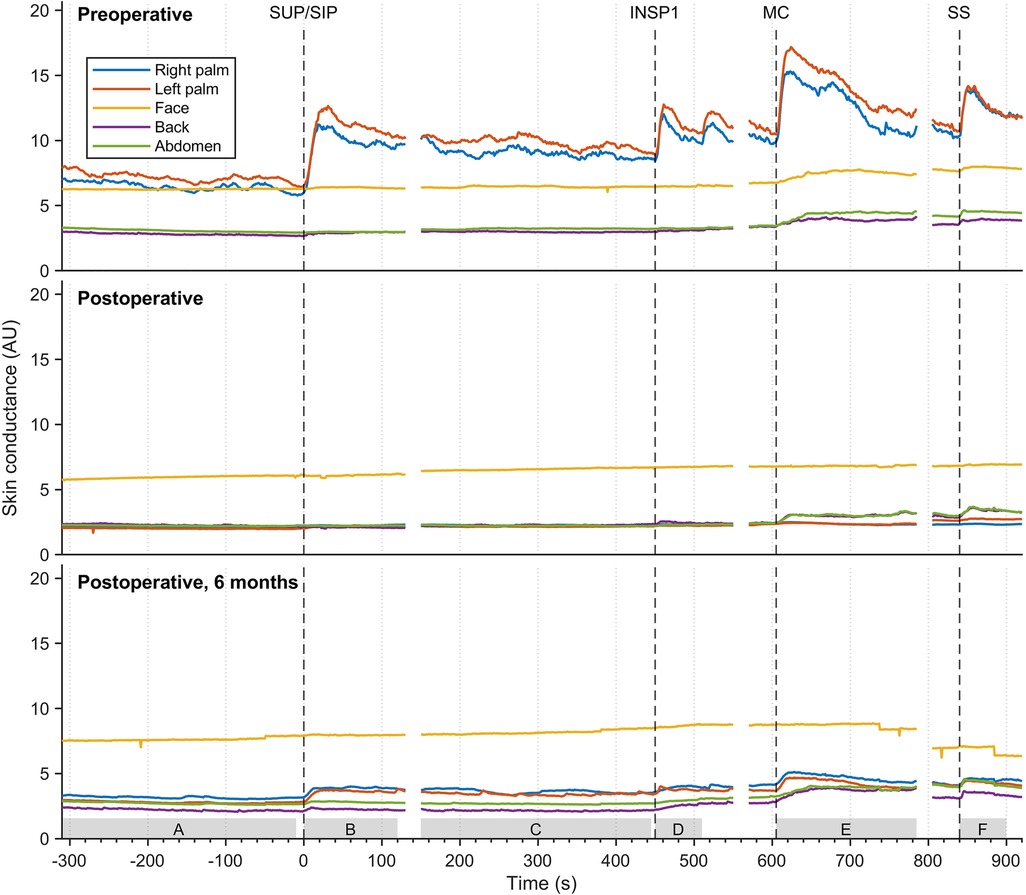
Figure 6. The mean EDA responses of thirty-seven patients with facial blushing/hyperhidrosis, palmar hyperhidrosis, and combined palmar/facial hyperhidrosis preoperatively (A), four hours to four weeks postoperatively (B), and six months postoperatively (C) The color coding, axes, and lines are identical to those in figure 3. The grey boxes on the x-axis (A-F) indicate the period from which the values for the different stimuli were calculated in Table 1.
Table 1 shows a comparison of postoperative EDA responses to preoperative responses. The table shows a significant difference in postoperative EDA recordings compared to preoperative EDA recordings in the palms (p < 0.05) for all thirty-seven patients in response to every stimulus. A significant change after the operation was observed in facial EDA responses to MC. However, no significant differences in EDA were observed between preoperative EDA and shortly postoperative EDA in the face, back, and abdomen in response to the other stimuli (p > 0.05). The table also shows that there was a significant increase in amplitude of MC-induced EDA in the back six months after the operation, compared to EDA responses recorded shortly after the operation.
Discussion
One major finding of this study is the complete abolition of palm EDA responses to stimuli immediately after ETS at the R2-/R3-level, followed by a gradual return of EDA responses at six months. The response was most pronounced during position change, MC, and SS, but with a lower amplitude compared to preoperative recordings. This phenomenon may be attributed to the ongoing activity of EDA responses at the R4-level, as suggested in previous studies (13, 37–48).
Prior to sympathicotomy, the EDA responses were synchronous in both palms, and the responses were comparable to previous findings in healthy subjects, as described by Ho and colleagues (32). However, the patients showed much higher variations in spontaneous palm EDA at rest than the healthy subjects. The responses to stimuli were similar but higher in amplitude. Prior to ETS, there were significant differences in palmar EDA between the hyperhidrosis patients and the thirteen healthy subjects (Table 1) in response to each stimulus. The spontaneous variations in palm EDA during rest in hyperhidrosis patients’ needs further investigation and may be used for diagnostic purposes.
Prior to ETS, minimal EDA responses were observed in the face, back, and abdomen in hyperhidrosis patients, whereas no such EDA responses were seen in healthy subjects.
Only minor spontaneous activity or responses to stimuli were observed in the forehead of patients with facial blushing/hyperhidrosis or combined facial/palmar hyperhidrosis. This observation may be attributed to the method and experimental setup used, as EDA is based on an increase in skin conductance due to sweat secretion, whereas facial blushing and hyperhidrosis may not sufficiently moisten the skin to the same extent as in the palms. Additionally, it is possible that the applied stimuli are not sufficiently strong to elicit EDA responses. Further, a minor response in facial EDA was elicited by MC and SS stimuli in both pre- and postoperative settings in a few patients, as shown in Figure 4. An explanation could be that MC and SS stimuli might elicit a stronger psychological response in facial EDA than exposure to other physiological stimuli.
Recurrence of minor postoperative EDA responses was observed in the back and abdomen in half of the patients, but there were no statistically significant differences in EDA pre- and postoperatively in these locations (Table 1). Previous studies have predominantly relied on patient-reported symptom questionnaires, rather than objective measurements, to evaluate hyperhidrosis and compensatory hyperhidrosis (7, 9, 16, 17, 37, 49–54). Although two different methods for objectively recording sweat have been used in two hyperhidrosis studies, they were limited to 10-second recordings (34, 43).
None of the patients in our study complained of postoperative compensatory hyperhidrosis. However, the present study only lasted six months, and our aim was not to study such phenomena. Long-term studies are needed to address the problem of postoperative compensatory hyperhidrosis.
Limitations
The study has certain limitations. Firstly, the heterogeneity within the study group, which included individuals with symptoms as blushing and hyperhidrosis in different areas as face, palms, and combinations thereof, represents a challenge. Although presenting results for specific subgroups defined by symptom types and hyperhidrosis locations would have been preferable, the limited sample size of these subgroups prevented statistical evaluation. However, Supplementary Figure S1 shows that there is probably no major difference between the three groups. Secondly, this study was based on EDA recordings that measure sweating elicited by minor stimuli, and not skin blood flow. This might explain why facial blushing did not alter the EDA recordings. Thirdly, despite an identical setup and our efforts to expose hyperhidrosis patients and healthy subjects to the same experimental conditions, the recordings for these groups were not conducted during the same time period. Furthermore, the early postoperative EDA recordings ranged from four hours to four weeks, and thus introduces a variability which represents a limitation regarding the impact of sympathicotomy on EDA patterns in hyperhidrosis patients. The postoperative recordings of Figures 3–5 are very similar, so this is probably not a major limitation to the reliability of the study. The variability was the result of a busy and challenging clinical setting, where patients often sought early discharge due to factors such as long travel distances and high satisfaction with the operation. These limitations emphasize the need for caution in generalizing our findings and highlight opportunities for future research to address these challenges.
Conclusion
In conclusion, hyperhidrosis patients demonstrated significantly higher amplitudes of palmar EDA responses to stimuli compared to healthy subjects before sympathicotomy. The patients also showed more pronounced variations in palm EDA during rest. Palm EDA responses were completely abolished after ETS but reappeared to some extent at six months. EDA recordings could be useful for preoperative assessment of hyperhidrosis patients and should be explored in future studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Regional Ethics Committee (REC) (2009/132, and 13841). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AH: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. EØ: Writing – review & editing, Investigation. DL: Writing – review & editing, Software, Visualization. KT: Writing – review & editing, Funding acquisition, Resources. OG: Conceptualization, Funding acquisition, Resources, Writing – review & editing. KK: Writing – review & editing. JW: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This work was financially supported by the University of Oslo [271555/F20].
Acknowledgments
We would like to thank the subjects who participated in this study and the Department of Thoracic and Vascular Surgery at Akershus University Hospital, especially Inger Helene Nådland, for valuable discussions on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1358357/full#supplementary-material
References
1. Prasad A, Ali M, Kaul S. Endoscopic thoracic sympathectomy for primary palmar hyperidrosis. Surg Endosc. (2010) 24(8):1952–7. doi: 10.1007/s00464-010-0885-5
2. Krogstad AL, Mork C, Piechnik SK. Daily pattern of sweating and response to stress and exercise in patients with palmar hyperhidrosis. Br J Dermatol. (2006) 154(6):1118–22. doi: 10.1111/j.1365-2133.2006.07212.x
3. Stolman LP. Treatment of hyperhidrosis. Dermatol Clin. (1998) 16(4):863–9. doi: 10.1016/S0733-8635(05)70062-0
4. Cheng A, Johnsen H, Chang MY. Patient satisfaction after thoracoscopic sympathectomy for palmar hyperhidrosis: do method and level matter? Perm J. (2015) 19(4):29–31. doi: 10.7812/TPP/15-040
5. Raveglia F, Orlandi R, Guttadauro A, Cioffi U, Cardillo G, Cioffi G, et al. How to prevent, reduce, and treat severe post sympathetic chain compensatory hyperhidrosis: 2021 state of the art. Front Surg. (2022) 8:814916. doi: 10.3389/fsurg.2021.814916
6. Leung AK, Chan PY, Choi MC. Hyperhidrosis. Int J Dermatol. (1999) 38(8):561–7. doi: 10.1046/j.1365-4362.1999.00609.x
7. Turhan K, Kavurmaci O, Akcam TI, Ergonul AG, Ozdil A, Cakan A, et al. Long-term outcomes and course of compensatory sweating after endoscopic sympathicotomy. Thorac Cardiovasc Surg. (2022) 70(2):167–72. doi: 10.1055/s-0041-1728777
8. Fujimoto T. Pathophysiology and treatment of hyperhidrosis. Curr Probl Dermatol. (2016) 51:86–93. doi: 10.1159/000446786
9. Huang Y, Liu Y, Zou W, Mao N, Tang J, Jiang L, et al. Impact of endoscopic thoracic R4 sympathicotomy combined with R3 ramicotomy for primary palmar hyperhidrosis. Front Surg. (2023) 10:1144299. doi: 10.3389/fsurg.2023.1144299
10. Wolosker N, de Campos JRM, Kauffman P, da Silva MFA, Faustino CB, Tedde ML, et al. Cohort study on 20 years’ experience of bilateral video-assisted thoracic sympathectomy (VATS) for treatment of hyperhidrosis in 2,431 patients. Sao Paulo Med J. (2022) 140(2):284–9. doi: 10.1590/1516-3180.2021.0078.r1.23072021
11. Yazbek G, Ishy A, Alexandrino da Silva MF, Sposato Louzada AC, de Campos JRM, Kauffman P, et al. Evaluation of compensatory hyperhidrosis after sympathectomy: the use of an objective method. Ann Vasc Surg. (2021) 77:25–30. doi: 10.1016/j.avsg.2021.05.014
12. Soares TJ, Dias PG, Sampaio SM. Impact of video-assisted thoracoscopic sympathectomy and related complications on quality of life according to the level of sympathectomy. Ann Vasc Surg. (2020) 63:63–7 e1. doi: 10.1016/j.avsg.2019.07.018
13. Zhang W, Wei Y, Jiang H, Xu J, Yu D. T3 versus T4 thoracoscopic sympathectomy for palmar hyperhidrosis: a meta-analysis and systematic review. J Surg Res. (2017) 218:124–31. doi: 10.1016/j.jss.2017.05.063
14. Toolabi K, Parsaei R, Farid R, Zamanian A. Endoscopic thoracic sympathotomy for primary hyperhidrosis: predictors of outcome over a 10-year period. Surg Endosc. (2022) 36(5):3585–91. doi: 10.1007/s00464-021-08684-8
15. Herbst F, Plas EG, Fugger R, Fritsch A. Endoscopic thoracic sympathectomy for primary hyperhidrosis of the upper limbs. A critical analysis and long-term results of 480 operations. Ann Surg. (1994) 220(1):86–90. doi: 10.1097/00000658-199407000-00012
16. Dittberner FA, Jorgensen OD, Pilegaard HK, Ladegaard L, Licht PB. Sympathicotomy for isolated facial blushing: long-term follow-up of a randomized trial. Eur J Cardiothorac Surg. (2023). doi: 10.1093/ejcts/ezad414. [Epub ahead of print]38085236
17. Licht PB, Pilegaard HK, Ladegaard L. Sympathicotomy for isolated facial blushing: a randomized clinical trial. Ann Thorac Surg. (2012) 94(2):401–5. doi: 10.1016/j.athoracsur.2012.03.076
18. Licht PB, Pilegaard HK. Management of facial blushing. Thorac Surg Clin. (2008) 18(2):223–8. doi: 10.1016/j.thorsurg.2008.01.010
19. Girish G, D’Souza RE, D’Souza P, Lewis MG, Baker DM. Role of surgical thoracic sympathetic interruption in treatment of facial blushing: a systematic review. Postgrad Med. (2017) 129(2):267–75. doi: 10.1080/00325481.2017.1283207
20. Licht PB, Ladegaard L, Pilegaard HK. Thoracoscopic sympathectomy for isolated facial blushing. Ann Thorac Surg. (2006) 81(5):1863–6. doi: 10.1016/j.athoracsur.2005.12.017
21. Claes G. Indications for endoscopic thoracic sympathectomy. Clin Auton Res. (2003) 13(Suppl 1):I16–9. doi: 10.1007/s10286-003-1106-2
22. Hashmonai M, Assalia A, Kopelman D. Thoracoscopic sympathectomy for palmar hyperhidrosis. Ablate or resect? Surg Endosc. (2001) 15(5):435–41. doi: 10.1007/s004640080042
23. Pei G, Meng S, Yang Y, Wang X, Liu Q, Wang S, et al. Anatomical variations of the thoracic sympathetic ganglions and their effects on sympathicotomy for primary palmar hyperhidrosis. Clin Auton Res. (2023) 33(2):111–20. doi: 10.1007/s10286-023-00932-2
24. Loizzi D, Mongiello D, Bevilacqua MT, Raveglia F, Fiorelli A, Congedo MT, et al. Surgical management of compensatory sweating: a systematic review. Front Surg. (2023) 10:1160827. doi: 10.3389/fsurg.2023.1160827
25. Cerfolio RJ, De Campos JR, Bryant AS, Connery CP, Miller DL, DeCamp MM, et al. The society of thoracic surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. (2011) 91(5):1642–8. doi: 10.1016/j.athoracsur.2011.01.105
26. Weksler B, Luketich JD, Shende MR. Endoscopic thoracic sympathectomy: at what level should you perform surgery? Thorac Surg Clin. (2008) 18(2):183–91. doi: 10.1016/j.thorsurg.2008.01.006
27. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US Prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. (2004) 51(2):241–8. doi: 10.1016/j.jaad.2003.12.040
28. Hashmonai M, Kopelman D, Assalia A. The treatment of primary palmar hyperhidrosis: a review. Surg Today. (2000) 30(3):211–8. doi: 10.1007/s005950050047
29. Hu Y, Converse C, Lyons MC, Hsu WH. Neural control of sweat secretion: a review. Br J Dermatol. (2018) 178(6):1246–56. doi: 10.1111/bjd.15808
30. Tabet JC, Bay JW, Magdinec M. Essential hyperhidrosis. Current therapy. Cleve Clin Q. (1986) 53(1):83–8. doi: 10.3949/ccjm.53.1.83
31. Kuijpers M, van Zanden JE, Harms PW, Mungroop HE, Mariani MA, Klinkenberg TJ, et al. Minimally invasive sympathicotomy for palmar hyperhidrosis and facial blushing: current status and the hyperhidrosis expert center approach. J Clin Med. (2022) 11(3):786. doi: 10.3390/jcm11030786
32. Ho AVT, Toska K, Wesche J. Rapid, large, and synchronous sweat and cardiovascular responses upon minor stimuli in healthy subjects. Dynamics and reproducibility. Front Neurol. (2020) 11:51. doi: 10.3389/fneur.2020.00051
33. Tronstad C, Gjein GE, Grimnes S, Martinsen OG, Krogstad AL, Fosse E. Electrical measurement of sweat activity. Physiol Meas. (2008) 29(6):S407–15. doi: 10.1088/0967-3334/29/6/S34
34. Llado A, Leon L, Valls-Sole J, Mena P, Callejas MA, Peri JM. Changes in the sympathetic skin response after thoracoscopic sympathectomy in patients with primary palmar hyperhidrosis. Clin Neurophysiol. (2005) 116(6):1348–54. doi: 10.1016/j.clinph.2005.02.009
35. Toska K, Eriksen M, Walloe L. Short-term cardiovascular responses to a step decrease in peripheral conductance in humans. Am J Physiol. (1994) 266(1 Pt 2):H199–211. doi: 10.1152/ajpheart.1994.266.1.H199
37. Montessi J, Almeida EP, Vieira JP, Abreu Mda M, Souza RL, Montessi OV. Video-assisted thoracic sympathectomy in the treatment of primary hyperhidrosis: a retrospective study of 521 cases comparing different levels of ablation. J Bras Pneumol. (2007) 33(3):248–54. doi: 10.1590/S1806-37132007000300004
38. Chang YT, Li HP, Lee JY, Lin PJ, Lin CC, Kao EL, et al. Treatment of palmar hyperhidrosis: t(4) level compared with T(3) and T(2). Ann Surg. (2007) 246(2):330–6. doi: 10.1097/SLA.0b013e3180caa466
39. Liu Y, Yang J, Liu J, Yang F, Jiang G, Li J, et al. Surgical treatment of primary palmar hyperhidrosis: a prospective randomized study comparing T3 and T4 sympathicotomy. Eur J Cardiothorac Surg. (2009) 35(3):398–402. doi: 10.1016/j.ejcts.2008.10.048
40. Mahdy T, Youssef T, Elmonem HA, Omar W, Elateef AA. T4 sympathectomy for palmar hyperhidrosis: looking for the right operation. Surgery. (2008) 143(6):784–9. doi: 10.1016/j.surg.2008.01.007
41. Kim WO, Kil HK, Yoon KB, Yoon DM, Lee JS. Influence of T3 or T4 sympathicotomy for palmar hyperhidrosis. Am J Surg. (2010) 199(2):166–9. doi: 10.1016/j.amjsurg.2008.12.024
42. Joo S, Lee GD, Haam S, Lee S. Comparisons of the clinical outcomes of thoracoscopic sympathetic surgery for palmar hyperhidrosis: R4 sympathicotomy versus R4 sympathetic clipping versus R3 sympathetic clipping. J Thorac Dis. (2016) 8(5):934–41. doi: 10.21037/jtd.2016.03.57
43. Ishy A, de Campos JR, Wolosker N, Kauffman P, Tedde ML, Chiavoni CR, et al. Objective evaluation of patients with palmar hyperhidrosis submitted to two levels of sympathectomy: T3 and T4. Interact Cardiovasc Thorac Surg. (2011) 12(4):545–8. doi: 10.1510/icvts.2010.252015
44. Abd Ellatif ME, Hadidi AE, Musa AM, Askar W, Abbas A, Negm A, et al. Optimal level of sympathectomy for primary palmar hyperhidrosis: t3 versus T4 in a retrospective cohort study. Int J Surg. (2014) 12(8):778–82. doi: 10.1016/j.ijsu.2014.05.039
45. Chou SH, Kao EL, Li HP, Lin CC, Huang MF. T4 sympathectomy for palmar hyperhidrosis: an effective approach that simultaneously minimzes compensatory hyperhidrosis. Kaohsiung J Med Sci. (2005) 21(7):310–3. doi: 10.1016/S1607-551X(09)70126-3
46. Reisfeld R. Sympathectomy for hyperhidrosis: should we place the clamps at T2–T3 or T3–T4? Clin Auton Res. (2006) 16(6):384–9. doi: 10.1007/s10286-006-0374-z
47. Wolosker N, Yazbek G, Ishy A, de Campos JR, Kauffman P, Puech-Leao P. Is sympathectomy at T4 level better than at T3 level for treating palmar hyperhidrosis? J Laparoendosc Adv Surg Tech A. (2008) 18(1):102–6. doi: 10.1089/lap.2007.0030
48. Yang J, Tan JJ, Ye GL, Gu WQ, Wang J, Liu YG. T3/T4 thoracic sympathictomy and compensatory sweating in treatment of palmar hyperhidrosis. Chin Med J (Engl). (2007) 120(18):1574–7. doi: 10.1097/00029330-200709020-00003
49. Yazbek G, Wolosker N, de Campos JR, Kauffman P, Ishy A, Puech-Leao P. Palmar hyperhidrosis–which is the best level of denervation using video-assisted thoracoscopic sympathectomy: T2 or T3 ganglion? J Vasc Surg. (2005) 42(2):281–5. doi: 10.1016/j.jvs.2005.03.041
50. Doolabh N, Horswell S, Williams M, Huber L, Prince S, Meyer DM, et al. Thoracoscopic sympathectomy for hyperhidrosis: indications and results. Ann Thorac Surg. (2004) 77(2):410–4. discussion 4. doi: 10.1016/j.athoracsur.2003.06.003
51. Licht PB, Pilegaard HK. Severity of compensatory sweating after thoracoscopic sympathectomy. Ann Thorac Surg. (2004) 78(2):427–31. doi: 10.1016/j.athoracsur.2004.02.087
52. Fredman B, Zohar E, Shachor D, Bendahan J, Jedeikin R. Video-assisted transthoracic sympathectomy in the treatment of primary hyperhidrosis: friend or foe? Surg Laparosc Endosc Percutan Tech. (2000) 10(4):226–9. doi: 10.1097/00019509-200008000-00009
53. Han JW, Kim JJ, Kim YH, Kim IS, Jeong SC. New sympathicotomy for prevention of severe compensatory hyperhidrosis in patients with primary hyperhidrosis. J Thorac Dis. (2020) 12(3):765–72. doi: 10.21037/jtd.2019.12.91
Keywords: hyperhidrosis, palmar hyperhidrosis, facial blushing, facial hyperhidrosis, electrodermal activity, endoscopic thoracic sympathicotomy, sympathetic activity, compensatory hyperhidrosis
Citation: Ho AVT, Øvensen E, Lilja D, Toska K, Grenager O, Kristiansen K and Wesche J (2024) Changes in electrodermal activity following sympathicotomy in hyperhidrosis patients. Front. Surg. 11:1358357. doi: 10.3389/fsurg.2024.1358357
Received: 19 December 2023; Accepted: 22 February 2024;
Published: 11 March 2024.
Edited by:
Calvin Sze Hang Ng, The Chinese University of Hong Kong, ChinaReviewed by:
Moshe Hashmonai, Technion Israel Institute of Technology, IsraelGiulio Maurizi, Sapienza University of Rome, Italy
© 2024 Ho, Øvensen, Lilja, Toska, Grenager, Kristiansen and Wesche. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai Van Thuy Ho YWlfdmFuXzExQG1lLmNvbQ==
Abbreviations CH, compensatory hyperhidrosis; EDA, electrodermal activity; ETS, endoscopic thoracic sympathicotomy; INSP, inspiration; MC, mental challenge; SIP, sitting position; SS, sound stimulus; SUP, supine position.
 Ai Van Thuy Ho
Ai Van Thuy Ho Eirik Øvensen2
Eirik Øvensen2 Didrik Lilja
Didrik Lilja Jarlis Wesche
Jarlis Wesche