- 1Department of Trauma Surgery, University Hospital Regensburg, Regensburg, Germany
- 2Department of Orthopaedic Surgery, University Hospital of Regensburg, Asklepios Klinikum Bad Abbach, Bad Abbach, Germany
Background: Pyogenic vertebral osteomyelitis (VO) represents a clinical challenge and is linked to substantial morbidity and mortality. This study aimed to examine mortality as well as potential risk factors contributing to in-hospital mortality among patients with VO.
Methods: This retrospective analysis involved patients receiving treatment for VO at University Regensburg in Germany from January 1, 2000, to December 3, 2020. It included in-hospital mortality rate, comorbidities and pathogens. Patients were identified using ICD-10 diagnosis codes: M46.2, M46.3, M46.4, and M46.5. Kaplan–Meier probability plots and odds ratios (OR) for mortality were calculated.
Results: Out of the total cohort of 155 patients with VO, 53 patients (34.1%) died during a mean follow-up time of 87.8 ± 70.8 months. The overall mortality was 17.2% at one year, 19.9% at two years and 28.3% at five years. Patients with congestive heart failure (p = 0.005), renal disease (p < 0.001), symptoms of paraplegia (p = 0.029), and sepsis (p = 0.006) demonstrated significantly higher overall mortality rates. In 56.1% of cases, pathogens were identified, with Staphylococcus aureus (S. aureus) and other unidentified pathogens being the most common. Renal disease (OR 1.85) and congestive heart failure (OR 1.52) were identified as significant risk factors.
Conclusion: Early assessment of the specific risk factors for each patient may prove beneficial in the management and treatment of VO to reduce the risk of mortality. These findings demonstrate the importance of close monitoring of VO patients with underlying chronic organ disease and early identification and treatment of sepsis. Prioritizing identification of the exact pathogens and antibiotic sensitivity testing can improve outcomes for patients in this high-risk group.
1 Introduction
Musculoskeletal infections present a significant challenge in orthopedic trauma surgery, particularly when involving the spine (1). These infections can occur as pyogenic vertebral osteomyelitis (VO), encompassing spondylitis, discitis and vertebral osteomyelitis (2, 3), requiring hospitalization for the affected patients (4, 5). The predominant cause are infectious organisms, with hemolytic Staphylococcus aureus (S. aureus) being the most frequent (6). VO is a relatively rare form of osteomyelitis, making up only about 3% to 5% of all infectious bone diseases (4).
Epidemiological studies have consistently indicated a rising incidence of VO and spondylodiscitis in Europe, underscoring the ongoing challenge for healthcare systems (7–9). In Germany the highest in-hospital mortality rate was recorded at 64.7 per 1,000 patients and intensive care unit (ICU) treatment was documented in 27.7% of cases (8). Intriguingly, in contrast to diagnoses such as major amputations, VO demonstrates comparatively lower mortality rates (10, 11). The treatment of elderly patients, who are particularly vulnerable to these infections, is becoming increasingly important due to epidemiological development and the growing prevalence of comorbidities in an aging population (12). Furthermore, secondary diagnoses contributing to frailty, such as liver cirrhosis, congestive heart failure, and kidney insufficiency requiring dialysis, have been associated with a higher risk (13). VO treatment also affects patients’ quality of life. Remarkably, even seven years on average following the completion of VO treatment, patients continue to experience diminished quality of life compared to the reverence population (14).
The hidden characteristics of these infections, especially when triggered by low-virulence pathogens, often result in delayed diagnoses, resulting in significant morbidity and mortality (15, 16). Notably, infections associated with coagulase-negative staphylococci (CoNS) are gaining importance in this context (17). Recent literature has summarized updated diagnostic and therapeutic approaches for treatment (18, 19). However, significant heterogeneity persists in reports on the epidemiology of these conditions, with limited analyses of nationwide databases and an incomplete understanding of in-hospital mortality rates (4, 7, 20). The mortality rate ranges between 2% and 12.4% (21–23).
In response to these challenges, this study aims to (1) investigate the mortality rates of patients with VO stratified for comorbidities. Second (2) to identify potential risk factors for in-hospital mortality among VO patients.
2 Material and methods
We conducted a retrospective study involving patients treated for VO at a university trauma center in Germany. The study period extended from January 1, 2000, to December 3, 2020. Patients were identified based on specific international disease classification (ICD-10) diagnosis codes, which included M46.2, M46.3, M46.4, and M46.5. We screened patients’ medical records, surgical records, radiological findings, laboratory results, microbiological reports, and histopathological reports to determine eligibility for inclusion in the study. We specifically included patients who had completed their treatment for VO, and this completion was well-documented in their medical charts. We collected patient characteristics, including sex, age, body mass index (BMI) at the time of admission, mortality and details of the treatments received (surgery, revision surgery, and changes in antibiotic regimens) by reviewing electronic medical records. The surgical indications for VO in this retrospective study were based on hospital-internal standard operating procedures (SOPs) and tailored individually for each case. Given the study's retrospective nature, some heterogeneity in surgical indications across cases cannot be excluded. In general indications for surgical treatment of pyogenic spondylodiscitis are sepsis, an epidural abscess, neurological deficits/complications, and instabilities/deformities in the affected motion segment (24). Preservation of vertebral body integrity, development of spinal deformities, and refractory back pain, inadequate patient compliance, and failure of conservative therapy are considered relative surgical indications (25). Segmental kyphosis >15°, vertebral body loss >50%, and/or translation >5 mm are considered instability criteria (26). Obesity is defined as a BMI ≥25. Comorbid conditions were evaluated by acquiring the Charlson Comorbidity Index (CCI) and the age-specific Charlson Comorbidity Index (ACCI) (27). To conduct subgroup analyses, we categorized the causes of VO into two groups: community-acquired vertebral osteomyelitis (CAVO) and healthcare-associated vertebral osteomyelitis (HAVO), as previously defined (16). The criteria were:
I. Onset of symptoms occurring after one month of hospitalization without signs of vertebral osteomyelitis upon admission.
II. Hospital admission within six months prior to symptom onset.
III. Outpatient diagnostic or therapeutic interventions within six months prior to symptom onset (long-term use of a central venous catheter, arteriovenous fistula for hemodialysis, invasive intravascular techniques, urological, gynecological, or gastrointestinal procedures, and skin procedures).
IV. Spondylodiscitis cases not meeting any of the above criteria were classified as CAVO
Exclusion criteria were defined as: tuberculous, fungal, or viral spondylodiscitis, brucella VO, implant-associated VO (IAVO), incomplete dataset, malignant or metastatic disease of the spine.
Further we contacted the patients by phone.
2.1 Statistical analysis
Data analysis was performed using SPSS Statistics version 28.0 (SPSS Inc, Chicago, IL, USA). We calculated descriptive statistics for all variables. Continuous variables were presented as mean values and standard deviations. The study presents the frequencies of secondary diagnoses, which were identified through coded data. These frequencies are presented as both the actual numbers and as proportions relative to the total number of cases. To make the data more understandable, these secondary diagnoses were categorized into two groups: comorbidities and complications. Additionally, the coded data related to pathogens was carefully analyzed.
Also, a univariate analysis approach was employed to individually assess each variable for its potential role as a risk factor linked to in-hospital mortality among patients diagnosed with VO. The dataset used for this analysis covered a wide range of patient outcomes, including cases with and without documented in-hospital deaths.
We evaluated a Kaplan-Meier analysis with the follow-ups as endpoints. The statistical significance of the association between each parameter and the recurrence-free survival was performed with the ANOVA test.
For various comorbidities and complications of interest, odds ratios (OR) were calculated. In addition to ORs, lower and upper 95% Confidence Intervals (CI) were determined to provide an estimate of the range within which the true OR is likely to fall with a 95% probability.
A multifactorial analysis was conducted between the laboratory values and factors like blood cultures and clinical sepsis syndrome.
The strength of the statistical relationship between exposure to specific factors and the occurrence of a particular outcome, which in this case is in-hospital mortality, was measured using OR.
To investigate the relationship between in-hospital mortality and each variable, a Chi-square test of independence was performed. A significance level (alpha) of 0.05 was chosen, meaning that a p-value less than this threshold would indicate a statistically significant association between the variable and in-hospital mortality. The level of statistical significance was set at p < 0.05.
2.2 Ethical considerations
This study adhered to the principles outlined in the Declaration of Helsinki and received approval from the local university ethics committee (Institutional Review Board Number 12-218_2-101; Amendment 08/2021). Written informed consent was obtained from all individual participants who were included in the study.
3 Results
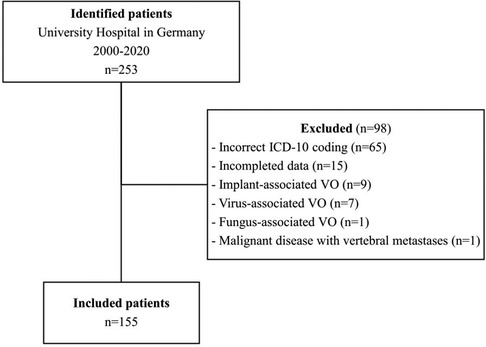
Out of the 253 patients with relevant ICD-10 codes, 155 were found to have VO and met the inclusion criteria. Ninety-eight patients were excluded from the study due to the following exclusion criteria (Figure 1): Brucellar or mycobacterial spondylodiscitis, virus- or fungus-associated spondylodiscitis, IAVO, malignant disease with vertebral metastases, incomplete data documentation. In 65 cases, the criteria for diagnosing were not met according to the medical records, so they were excluded from the study due to incorrect ICD-10 coding. Seven patients had tuberculous spondylodiscitis, and nine had IAVO. Fifteen were excluded due to incomplete data, and two were excluded for other reasons (fungal-associated spondylodiscitis and malignant disease of the spine).
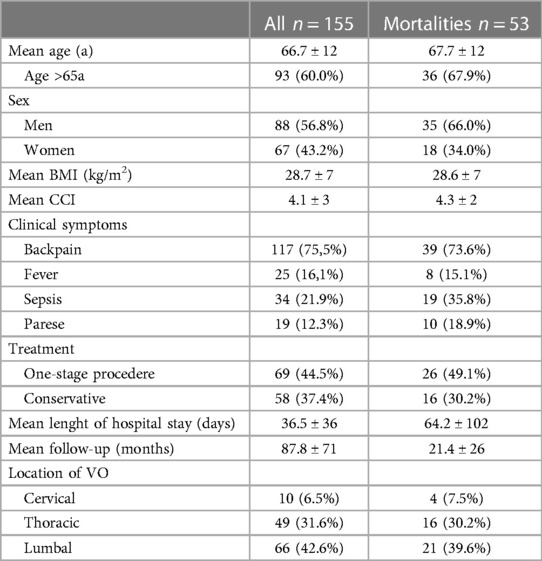
So, a total of 155 patients, included 88 men and 67 women, who were treated for VO, were collected in the study between 2010 and 2020 at a University Hospital in Germany. The average age of these patients was 66.7 ± 12.4 (26–93) years.
The mean BMI of these patients was 28.7 ± 7. The mean CCI stood at 4.1 ± 3, while the mean ACCI was recorded at 2.1 ± 1. The mean time from the onset of symptoms to the day of hospitalization was 93.7 ± 290.9 days. The average length of hospital stay for these patients was 36.5 ± 36.3 (1–343) days (Table 1).
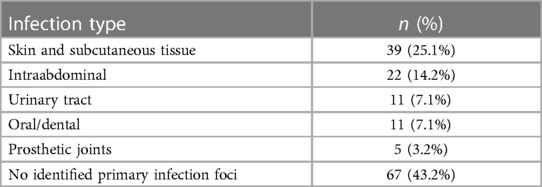
The majority of patients were treated surgically (n = 96, 61.9%). All cervical spondylodiscitis cases (n = 10) were managed surgically, while 36 thoracic cases (75%) and 34 lumbar cases (52%) received surgical treatment. Thereof 69 patients (71.9%) got a one-stage procedure. A one-stage operative approach was predominantly undertaken in thoracic spondylodiscitis cases (n = 32, 89%; p = 0.007). Surgically treated thoracic spondylodiscitis cases frequently received (partial) corpectomy and a vertebral body replacement (VBR; n = 28, 57.1%; p < 0.001). Patients exhibiting symptoms of paraplegia were significantly more likely to undergo surgical treatment (n = 15, 93.8%; p = 0.007) and received a VBR (n = 11, 68.8%; p = 0.002). Surgical intervention was notably more common among patients experiencing paralysis (n = 19, 98.5%; p = 0.010) and back pain (n = 67, 57.8%; p = 0.024). Septic patients showed a significantly higher tendency to undergo surgery (n = 27, 79.4%; p = 0.020). All patients diagnosed with peptic ulcer disease received surgical intervention (n = 8; p = 0.024). Patients diagnosed with periprosthetic joint infection (PJI) were significantly more likely to received dorsal instrumentation (n = 4, 80%; p = 0.031). Spondylodiscitis cases associated with S. aureus were significantly more likely to undergo surgery (n = 34, 75.6%; p = 0.030). Skin and subcutaneous tissue infections were the predominant focus in 25.1% of cases, while no identifiable focus was observed in 43.2% of cases (Table 2).
3.1 Midterm survival of patients with VO
In total, 53 patients, constituting 34.1% of the cohort, did not survive. In the case of 41 patients (26.5%), the age at death was determined through patient records and discussions with family members, averaging 69.21 ± 11.7 years. Additionally, 22 patients (14.2%) passed away in the hospital. The cause of death was identified for 18 patients. Among them, four patients expired due to cardiac arrest, three succumbed to multiorgan failure, seven were attributed to sepsis (accompanied by multiorgan failure), one to drug intoxication, one to respiratory failure, one to graft-vs.-host reaction, and one to fungal pneumonia. Ten patients (6.5%) were lost to follow-up. The mean duration of follow-up was 87.8 ± 70.8 months, ranging from 0 to 214 months.
The one-year survival rate was 82.8%, the two-year survival rate was 80.9%, and the five-year survival rate was 71.7%. The overall mortality was 17.2% at one year, 19.9% at two years and 28.3% at five years.
Patients who presented with congestive heart failure (38.1%; p = 0.005), renal disease (32.9%; p < 0.001), symptoms of paraplegia (10.3%; p = 0.029), and clinical sepsis syndrome (22%; p = 0.006) demonstrated a significant higher rate of mortality than patients without these conditions (Figure 2). Patients with renal disease had a one-year mortality rate of 24%. Otherwise, previous hospitalization, age over 65 years, surgery, abscesses, sex, other comorbidities such as diabetes mellitus, liver disease, were not associated with an elevated mortality rate during the follow-up (Table 3). Half of the patient cohort (n = 69) underwent blood culture analysis, and these individuals showed a significantly higher mortality rate (p = 0.002) compared to those who did not have blood cultures taken. There is a statistically significant difference between leukocyte values and clinical sepsis (p = 0.032). However, there is no other statistically significant difference between CRP values and leukocyte values for the combined dependent variables such as blood cultures, fever, and B-symptoms.
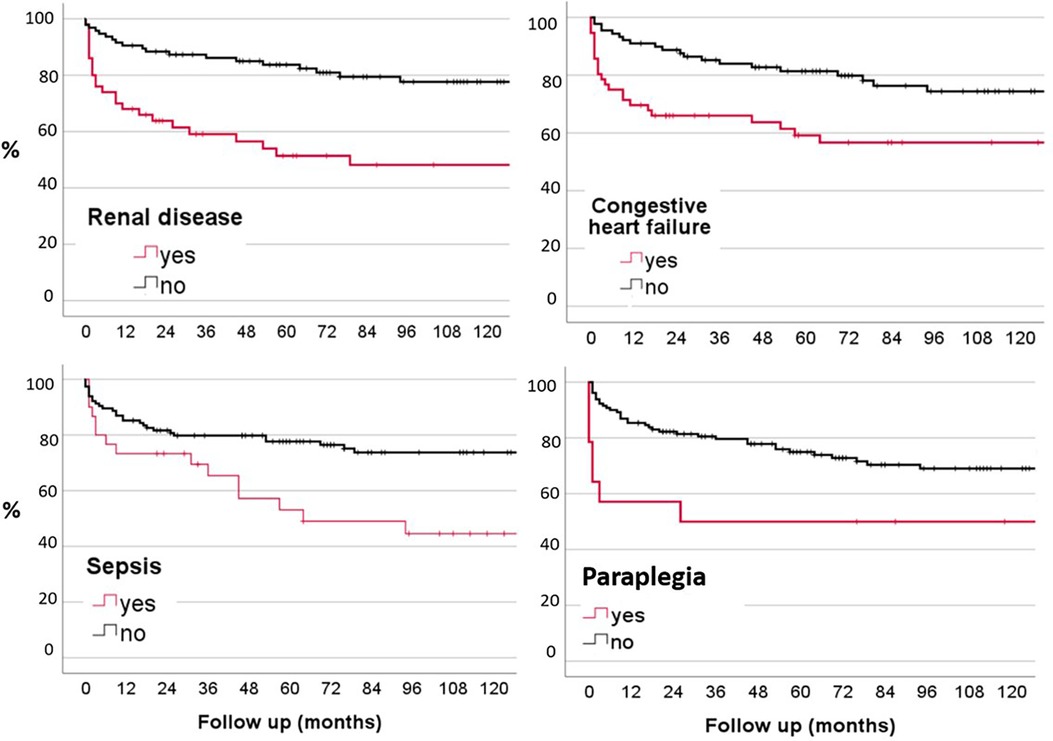
Figure 2. Kaplan-Meier probability plots of mortality related to renal disease, congestive heart failure, paraplegia and sepsis.
3.2 Risk factors for the in-hospital mortality of VO patients
Certain underlying health conditions were found to be associated with in-hospital mortality. Renal disease was identified as a risk factor (OR = 1.85; 95% CI 0.97–3.51; p = 0.006), as well as congestive heart failure (OR = 1.52; 95% CI 0.82–2.80; p = 0.042) (Figure 3).
There was no statistically significant correlation found between mortality and CCI (ρ=−0.05; p = 0.387) or ACCI (ρ=−0.07; p = 0.805).
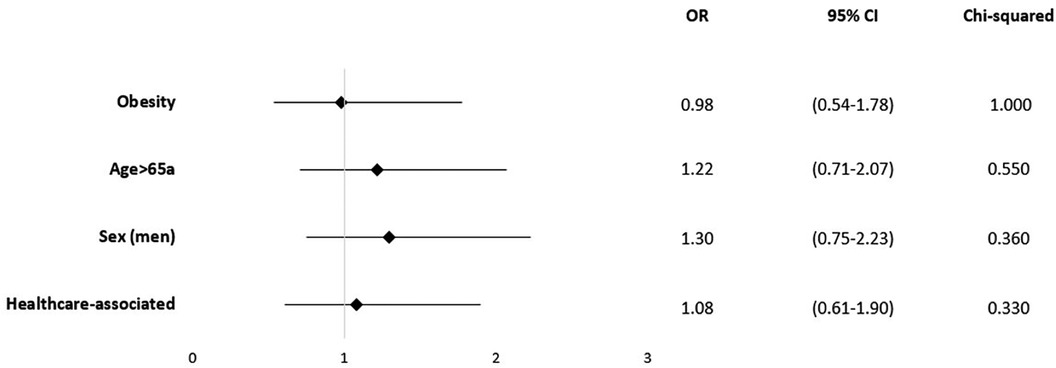
In line, obesity (OR = 0.98; 95% CI 0.54–1.78; p = 1.00) and age over 65 years (OR = 1.25; 95% CI 0.732.12; p = 0–.418) were not significantly associated with in-hospital mortality (Figure 4). Based on the patient records, 78 cases were classified as HAVO (50.3%), while 77 cases (49.7%) were categorized as CAVO. Neither HAVO nor CAVO cases demonstrated an increased in-hospital mortality rate (Figure 4).
Clinical sepsis was found to have a significant association with mortality (OR = 2.44; 95% CI 1.15–5.18; p = 0.003). In the cohort, in which blood cultures had been taken the following symptoms were significantly more often documented compared to the cohort without blood cultures: Fever >38°C (p = 0.034), clinical sepsis (p < 0.001), paravertebral abscess (p = 0.003), urinary tract infection (p = 0.025), intra-abdominal infection (p = 0.008), vascular access infection (p = 0.021) and endocarditis (p = 0.006). There was no correlation between dental infection, PJI, skin and subcutaneous tissue infection, epidural infection, and psoas abscess.
Pathogens were identified, with S. aureus (n = 45; 29%) and “other unidentified pathogens” (n = 19; 12.3%) and CoNS (n = 15; 9.7.%) being the most common. In both cohorts, S. aureus was the most frequently identified pathogen (46.8% CAVO vs. 51.2% HAVO). This was followed by CoNS (20% CAVO vs. 80% HAVO; p = 0.016). All cases of vascular access infection were attributed to HAVO (n = 5; p = 0.024).
When examining the impact of these pathogens, documented infection with S. aureus showed the highest risk of mortality (OR = 1.28; 95% CI 0.65–2.54; p = 0.333), although it did not reach statistical significance (Figure 5). There was no correlation between the detection of pathogens and the presence of positive blood cultures. In our cohort, only in cases with S. aureus infections psoas-abscess were documented (p = 0.023).
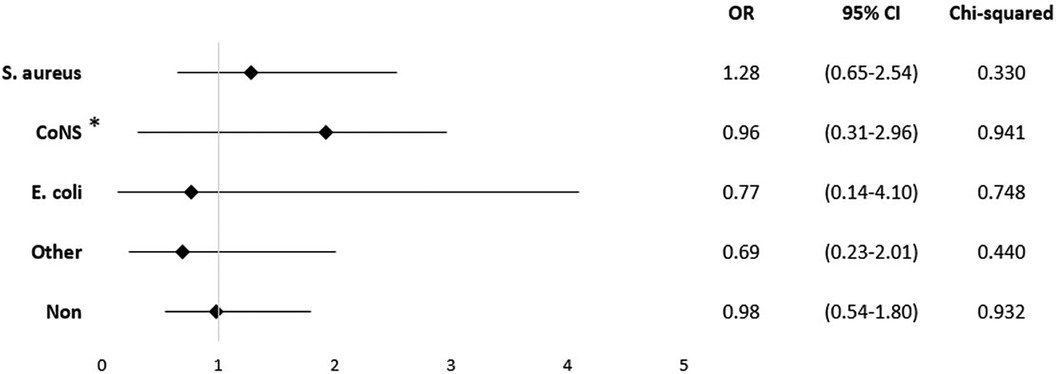
Figure 5. Mortality odds ratio for pathogens. *All other staphylococci without S. aureus, for example S. epidermidis, S. haemolyticus, and S. saprophyticus.
4 Discussion
The present case series demonstrates that VO is linked with a high rate of in-hospital mortality and a substantial 5-year mortality rate. Regarding outcomes, the 5-year survival rate was 71.7%, with an overall mortality of 28.3% at five years. Patients with congestive heart failure (38.1%), renal disease (32.9%), paraplegia symptoms (10.3%), and sepsis (22%) had notably higher mortality rates. In summary, the study detailed significant associations between surgical intervention and specific conditions, highlighted risk factors for mortality, and emphasized the impact of certain pathogens on patient outcomes in VO cases.
4.1 Midterm survival of patients with VO
The overall mortality rates in our patient cohort were 17.2% at one year, 19.9% at two years, and 28.3% at five years. The 5-year-survival rate was 71.7%. Comorbidities that contribute to frailty were linked to an increased risk for mortality in patients with VO. Kidney disease (OR = 1.85) was identified as a high-risk factor as well as congestive heart failure (OR = 1.52).
The observed mortality rate of this study is consistent with the findings reported in existing literature. Mortality from VO has been described with discrepancy ranging from 11% to 20% at one year (15, 28–30). Yagdiran et al. found that the mortality rates at 1 year and 2 years were 20% and 23%, respectively (31). Meanwhile, Vettivel et al, in their single-center study involving 76 patients with pyogenic VO, reported a 5.2% mortality rate at 30 days and a 22.3% mortality rate at 1 year (32). In a multi-center study concentrating on spine surgery, it was evident that infectious diseases substantially increased the risk of in-hospital mortality (33).
4.2 Risk factors for the in-hospital mortality of VO patients
Kehrer et al. (28) identified several significant risk factors in their study. They associated severe neurological deficits on admission and the presence of an epidural abscess with a higher risk of short-term mortality. Consistent with our findings, they observed that patients with symptoms of paraplegia had a higher mortality rate. The most commonly types of abscesses, identified in this study were psoas abscesses (27.1%), followed by paravertebral abscesses (12.3%), and epidural abscesses (10.3%). Interestingly, in our study the location of abscesses, regardless of their level, did not significantly affect mortality.
Some studies focused on risk factors of in-hospital mortality after VO. Ziarko et al. (13) analysed risk factor of 9,753 VO cases in Germany in 2020. Apart from age, several comorbidities were associated with an elevated risk of mortality. Notably, heart failure (OR = 2.80) and chronic kidney disease (OR = 1.83) were identified as significant risk factors for mortality. Among the complications studied, clinical sepsis syndrome (OR = 5.94), was associated with increased in-hospital mortality. These results are similar to the current study. Another study by Lenz et al. showed a significant correlation between preoperative glomerular filtration rate (GFR) values with in-hospital mortality in patients with spondylodiscitis (34). A matched case-control study by Kushioka et al. using a multicenter database examined 26.604 patients that underwent spine surgery at 27 Japanese institutions between 2012 and 2018. They identified preoperative renal comorbidity as a significant risk factor for increased mortality (33). Joerger et al. focused on the trend of early mortality among spondylodiscitis patients despite receiving treatment. Among the 430 patients included in the study, a distressing 7.4% (n = 32) passed away during their hospital stay. Advanced age, Diabetes mellitus, Previous steroid medication, CCI, and GFR (Glomerular Filtration Rate) level at admission were significant risk factors for in-hospital death (35). Vettivel et al., identified frailty (OR = 13.62) and chronic renal failure (OR = 13.40) as factors associated with a higher risk of 30-day mortality (32). In summary, chronic renal disease is recognized as a well-documented risk factor in the literature. The utilization of contrast media in CT scans and its potential nephrotoxic effects may be one factor contributing to the elevated mortality rates.
Notably, in our analysis, age over 65 years (OR = 1.25) did not reach statistical significance for elevating the risk for mortality. Liver disease showed a clear association (OR = 2.20) but without significancy. In the literature, age and liver disease have already been described as risk factors for mortality (13, 21, 32, 36–38). The mean age in our study is comparable with these studies. However, the mean age at the time of death in our study is lower. Specifically, in our study the mean age in the mortality group was 67.7 ± 12.1 years, whereas in the study by Heuer et al., was 73.8 ± 9.9 years.
Another significant risk factor for increased mortality was the presence of sepsis, which was linked to a 2.44-fold rise in in-hospital mortality. Sepsis is widely recognized for its high mortality rates (39). A systematic review and meta-analysis highlighted a notably elevated mean sepsis mortality rate, reaching 24.4% at 30 days and 32.2% at 90 days (40). Furthermore, sepsis can lead to acute renal failure (AKI), a condition affecting roughly 40%–50% of AKI patients (41). Kidney diseases also contribute to overall mortality, as evidenced in this study.
Sepsis-related acute kidney injury is a frequently encountered complication in critically ill patients, and it is associated with significant health complications and death. Conversely, acute kidney injury has been recognized as a risk factor for sepsis and its associated negative outcomes. In the cohort of patients, from which blood cultures were taken symptoms of complicated clinical courses were significantly more often seen, compared to the cohort without this diagnostic measurement: Fever >38°C, sepsis, paravertebral abscess, urinary tract infection, intraabdominal infection, vascular access infection, and endocarditis. This finding indicated, that blood cultures in our clinical practice have been predominantly obtained in patients with severe symptoms, such as fever, sepsis, or abscesses. However, this underscores the need for a more comprehensive diagnostic approach that includes obtaining blood cultures from afebrile patients as well, to improve pathogen identification and treatment outcomes in cases of VO. Bacterial detection in cases of spondylodiscitis has been reported to be successful in approximately 50%–83% of cases (42). Blood culture significantly enhanced diagnostic accuracy, highlighting the importance of a systematic diagnostic approach. Therefore, it is important to ensure that blood cultures are taken.
Another interesting aspect of VO is the pathogens that have been identified. The most frequently identified pathogens were S. aureus (29%), followed by the S epidermidis (5%) as already known in the literature (20, 42, 43). The notable predominance of other staphylococci strongly indicated a significant presence of CoNS. This is consistent with recent findings on the increasing importance of HAVO, particularly those associated with devices that cause bloodstream infections and contribute to the development of spondylodiscitis (44, 45). Furthermore, in our study, spondylodiscitis with CoNS detection demonstrated a significantly higher incidence in the HAVO group compared to the CAVO group. A retrospective study involving 586 cases of spondylodiscitis identified that S. aureus was predominantly present in individuals younger than 60 years old. On the other hand, gram-negative bacteria were more prevalent among those aged over 60 years. Additionally, there was an association observed between the presence of gram-negative pathogens and patients diagnosed with solid tumors or liver cirrhosis (46). Michels et al. highlighted the significant antimicrobial resistance profile seen in CoNS, particularly in healthcare environments (47). Due to CoNS’ lower virulence in comparison to S. aureus in VO (48), these infections might frequently go unnoticed and receive delayed diagnoses. Intravascular catheter-related infections are considered responsible for up to 34% of HAVO VO cases (45). Another study described S. aureus infections, the presence of gram-negative pathogens with higher mortality (13, 36, 43). In our cohort, infection with S. aureus as the only pathogen demonstrated a significantly higher risk of psoas abscess, a complication that could contribute to a severe course of the disease. Interestingly, Priest et al. (49) reported that 60% of all cases with S. aureus detection either progressed to developing an epidural, paraspinous, or psoas abscess (44).
4.3 Operative treatment
Surgical debridement stands as a widely acknowledged treatment for infectious diseases. Its primary goal is to reduce the spread of infection, speed up the control of the infection, and at the same time, provide tissue samples that could help adjust additional antibiotic treatment (50).
In a meta-analysis that involved 21 studies and a total of 10.954 patients, researchers compared early surgical intervention to conservative management. The analysis evaluated that opting for early surgery showed a correlation with decreased mortality rates (8% vs. 13% in conservative treatment) and lower rates of relapse/failure (15% vs. 21%). Furthermore, early surgical intervention led to a notably shorter hospital stay, mean 7.8 days. These consistent findings provide strong evidence in favor of prioritizing early surgical management for cases involving pyogenic spondylodiscitis (51).
Similar to our findings, a systematic review included 64 studies revealed that 54.7% of cases underwent surgical treatment. Moreover, a substantial majority of cases involving neurological complications (83.9%, totaling 120 cases) underwent surgery (52).
Literature indicates that there is evidence favoring surgical intervention, particularly in cases of cervical spondylodiscitis compared to thoracolumbar conditions. Cervical infections have been associated with notably higher morbidity and mortality rates. Often cervical spondylodiscitis is linked to neurological symptoms (53, 54). For instance, in one cohort of 15 cases with cervical spondylodiscitis, 67% of patients displayed neurological deficits at the time of diagnosis (55), with similar findings in a study of 19 patients at a different medical center (56). Contrary to the established literature, this study did not find an association between cervical spondylodiscitis and the presence of neurological symptoms. All in all, patients presenting with paraplegia, paralysis, sepsis and back pain underwent general surgical treatment more frequently. Blecher et al. initiated non-surgical therapy for 35 patients diagnosed with vertebral osteomyelitis. They suggested that fever and a history of intravenous drug abuse and fever as well as the extent of osseous and posterior element involvement could serve as valuable indicator advocating for early surgical intervention in the treatment of spinal infections (57).
Regarding limitations, it is important to note that VO may not only depend on the identified risk factors, but could be influenced by several other factors not measured in this study. The assertion that VO is only influenced by the known risk factors is difficult, as there may be additional variables at the same time that still need to be investigated. Furthermore, similar risk factors for other infections such as PJI appeared in this study. It is significant that these identified risk groups may need customised therapies and adaptations. However, it's important to note that this study has certain limitations. The retrospective design of this study, coupled with its small sample size and single-center scope, limits the generalizability of the findings, as it may not adequately capture the diverse clinical presentations and management strategies in a broader patient population. A notable constraint is that the occurrence of positive blood culture results, may be attributed to other sources of infection (such as infected central venous catheters, pneumonia, etc.). In this retrospective study, blood cultures were apparently obtained mainly from patients with severe symptoms, potentially skewing results to suggest a higher correlation between positive cultures and severe disease courses. Additionally, it should be noted that the standardized protocol for blood culture collection is currently lacking, warranting further attention in clinical practice. The lack of pathogen identification in patients not subjected to blood cultures hinders targeted antibiotic therapy, underscoring the need for more systematic blood culture usage across varying symptom severities in VO cases.
5 Conclusion
In conclusion, the evaluation of individual patient risk factors, including the presence of comorbidities, is essential for the effective management and treatment of VO. Our findings underscore the necessity of vigilant monitoring, especially for VO patients with chronic organ disease, swift identification, and management of sepsis, as well as the prioritization of accurate pathogen detection and antibiotic sensitivity testing, to enhance survival prospects in this vulnerable patient cohort.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Local university ethics committee Regensburg (Institutional Review Board Number 12-218_2-101; Amendment 08/2021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MS: Conceptualization, Data curation, Formal Analysis, Writing – original draft. NW: Methodology, Writing – review & editing. JR: Formal Analysis, Writing – review & editing. SP: Software, Writing – review & editing. DS: Supervision, Writing – review & editing. VA: Supervision, Writing – review & editing. MR: Supervision, Validation, Writing – review & editing. SL: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alt V, Giannoudis PV. Musculoskeletal infections - A global burden and a new subsection in injury. Injury. (2019) 50(12):2152–3. doi: 10.1016/j.injury.2019.11.001
2. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. (2010) 65(Suppl 3):iii11–24. doi: 10.1093/jac/dkq303
3. Rupp M, Walter N, Baertl S, Lang S, Lowenberg DW, Alt V. Terminology of bone and joint infection. Bone Joint Res. (2021a) 10(11):742–3. doi: 10.1302/2046-3758.1011.BJR-2021-0371
4. Issa K, Diebo BG, Faloon M, Naziri Q, Pourtaheri S, Paulino CB, et al. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg. (2018) 31(2):E102–8. doi: 10.1097/BSD.0000000000000597
5. Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. (2010) 362(11):1022–9. doi: 10.1056/NEJMcp0910753
6. Bonfiglio M, Lange TA, Kim YM. The classic: pyogenic vertebral osteomyelitis: disk space infections. 1973. Clin Orthop Relat Res. (2006) 444:4–8. doi: 10.1097/01.blo.0000201172.64764.bb
7. Conan Y, Laurent E, Belin Y, Lacasse M, Amelot A, Mulleman D, et al. Large increase of vertebral osteomyelitis in France: a 2010–2019 cross-sectional study. Epidemiol Infect. (2021) 149:e227. doi: 10.1017/S0950268821002181
8. Lang S, Walter N, Schindler M, Baertl S, Szymski D, Loibl M, et al. The epidemiology of spondylodiscitis in Germany: a descriptive report of incidence rates, pathogens, in-hospital mortality, and hospital stays between 2010 and 2020. J Clin Med. (2023) 12(10):3373. doi: 10.3390/jcm12103373
9. Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. The Journal of Infection. (2014) 68(4):313–20. doi: 10.1016/j.jinf.2013.11.011
10. Szymski D, Walter N, Krull P, Melsheimer O, Schindler M, Grimberg A, et al. Comparison of mortality rate and septic and aseptic revisions in total hip arthroplasties for osteoarthritis and femoral neck fracture: an analysis of the German arthroplasty registry. J Orthop Traumatol. (2023) 24(1):29. doi: 10.1186/s10195-023-00711-9
11. Schindler M, Baertl S, Walter N, Lang S, Szymski D, Alt V, et al. Retrospective analysis of mortality and quality of life after hip disarticulation or hemipelvectomy: a report on 15 patients. Arch Orthop Trauma Surg. (2023) 143(8):4943–9. doi: 10.1007/s00402-023-04783-4
12. Rupp M, Walter N, Pfeifer C, Lang S, Kerschbaum M, Krutsch W, et al. The incidence of fractures among the adult population of Germany–an analysis from 2009 through 2019. Dtsch Arztebl Int. (2021) 118(40):665–9. doi: 10.3238/arztebl.m2021.0238
13. Ziarko TP, Walter N, Schindler M, Alt V, Rupp M, Lang S. Risk factors for the in-hospital mortality in pyogenic vertebral osteomyelitis: a cross-sectional study on 9753 patients. J Clin Med. (2023) 12:14. doi: 10.3390/jcm12144805
14. Lang S, Walter N, Froemming A, Baertl S, Szymski D, Alt V, et al. Long-term patient-related quality of life outcomes and ICD-10 symptom rating (ISR) of patients with pyogenic vertebral osteomyelitis: what is the psychological impact of this life-threatening disease? Eur Spine J. (2023) 32(5):1810–7. doi: 10.1007/s00586-023-07616-5
15. McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. (2002) 34(10):1342–50. doi: 10.1086/340102
16. Lang S, Frömming A, Walter N, Freigang V, Neumann C, Loibl M, et al. Is there a difference in clinical features, microbiological epidemiology and effective empiric antimicrobial therapy comparing healthcare-associated and community-acquired vertebral osteomyelitis? Antibiotics (Basel). (2021) 10(11):1410. doi: 10.3390/antibiotics10111410
17. Benavent E, Kortajarena X, Sobrino-Diaz B, Gasch O, Rodríguez-Pardo D, Escudero-Sanchez R, et al. Vertebral osteomyelitis after spine instrumentation surgery: risk factors and management. J Hosp Infect. (2023) 140:102–9. doi: 10.1016/j.jhin.2023.07.008
18. Lang S, Rupp M, Hanses F, Neumann C, Loibl M, Alt V. Infektionen der wirbelsäule: pyogene spondylodiszitis und implantatassoziierte vertebrale osteomyelitis. Unfallchirurg. (2021) 124(6):489–504. doi: 10.1007/s00113-021-01002-w
19. Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. (2015) 61(6):e26–46. doi: 10.1093/cid/civ482
20. Doutchi M, Seng P, Menard A, Meddeb L, Adetchessi T, Fuentes S, et al. Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France. New Microbes New Infect. (2015) 7:1–7. doi: 10.1016/j.nmni.2015.04.008
21. Loibl M, Stoyanov L, Doenitz C, Brawanski A, Wiggermann P, Krutsch W, et al. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection. (2014) 42(3):503–10. doi: 10.1007/s15010-013-0582-0
22. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. (2008) 56(6):401–12. doi: 10.1016/j.jinf.2008.02.005
23. Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the national surgical quality improvement program. J Bone Joint Surg Am. (2011) 93(17):1577–82. doi: 10.2106/JBJS.J.01048
24. Almansour H, Pepke W, Akbar M. Pyogene spondylodiszitis: die suche nach einer klinisch-radiologischen klassifikation. Orthopade. (2020) 49(6):482–93. doi: 10.1007/s00132-019-03836-0
25. Dietze DD, Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg. (1997) 86(6):981–9. doi: 10.3171/jns.1997.86.6.0981
26. Fleege C, Wichelhaus TA, Rauschmann M. Systemische und lokale antibiotikatherapie bei konservativ und operativ behandelten spondylodiszitiden. Orthopade. (2012) 41(9):727–35. doi: 10.1007/s00132-012-1920-0
27. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. (1994) 47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5
28. Kehrer M, Pedersen C, Jensen TG, Hallas J, Lassen AT. Increased short- and long-term mortality among patients with infectious spondylodiscitis compared with a reference population. Spine J. (2015) 15(6):1233–40. doi: 10.1016/j.spinee.2015.02.021
29. Aagaard T, Roed C, Dragsted C, Skinhøj P. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006–2011. Scand J Infect Dis. (2013) 45(6):417–24. doi: 10.3109/00365548.2012.753160
30. Brummerstedt M, Bangstrup M, Barfod TS. High mortality from pyogenic vertebral osteomyelitis: a retrospective cohort study. Spinal Cord Ser Cases. (2018) 4:59. doi: 10.1038/s41394-018-0099-y
31. Yagdiran A, Otto-Lambertz C, Lingscheid KM, Sircar K, Samel C, Scheyerer MJ, et al. Quality of life and mortality after surgical treatment for vertebral osteomyelitis (VO): a prospective study. Eur Spine J. (2021) 30(6):1721–31. doi: 10.1007/s00586-020-06519-z
32. Vettivel J, Bortz C, Passias PG, Baker JF. Pyogenic vertebral column osteomyelitis in adults: analysis of risk factors for 30-day and 1-year mortality in a single center cohort study. Asian Spine J. (2019) 13(4):608–14. doi: 10.31616/asj.2018.0295
33. Kushioka J, Takenaka S, Makino T, Sakai Y, Kashii M, Iwasaki M, et al. Risk factors for in-hospital mortality after spine surgery: a matched case-control study using a multicenter database. Spine J. (2020) 20(3):321–8. doi: 10.1016/j.spinee.2019.10.008
34. Lenz M, Harland A, Egenolf P, Horbach M, Hodenberg Cv, Brinkkoetter PT, et al. Correlation between kidney function and mortality in pyogenic spondylodiscitis: the glomerular filtration rate (GFR) as new predictive parameter? Eur Spine J. (2023) 32(4):1455–62. doi: 10.1007/s00586-023-07578-8
35. Joerger AK, Albrecht C, Lange N, Meyer B, Wostrack M. In-hospital mortality from spondylodiscitis: insights from a single-center retrospective study. J Clin Med. (2023) 12(23):7228. doi: 10.3390/jcm12237228
36. Heuer A, Strahl A, Viezens L, Koepke LG, Stangenberg M, Dreimann M. The Hamburg Spondylodiscitis Assessment Score (HSAS) for immediate evaluation of mortality risk on hospital admission. J Clin Med. (2022) 11(3):660. doi: 10.3390/jcm11030660
37. Kim D, Kim J, Kim T. Clinical characteristics of patients with pyogenic vertebral osteomyelitis and concurrent infections and their clinical outcomes. J Pers Med. (2022) 12(4). doi: 10.3390/jpm12040541
38. Lawson McLean A, Senft C, Schwarz F. Management of lumbar pyogenic spondylodiscitis in Germany: a cross-sectional analysis of spine specialists. World Neurosurg. (2023) 173:e663–8. doi: 10.1016/j.wneu.2023.02.128
39. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. (2018) 392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2
40. Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Critical Care (London, England). (2020) 24(1):239. doi: 10.1186/s13054-020-02950-2
41. Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol. (2015) 35(1):2–11. doi: 10.1016/j.semnephrol.2015.01.002
42. Pola E, Taccari F, Autore G, Giovannenze F, Pambianco V, Cauda R, et al. Multidisciplinary management of pyogenic spondylodiscitis: epidemiological and clinical features, prognostic factors and long-term outcomes in 207 patients. Eur Spine J. (2018) 27(Suppl 2):229–36. doi: 10.1007/s00586-018-5598-9
43. Stangenberg M, Mende KC, Mohme M, Krätzig T, Viezens L, Both A, et al. Influence of microbiological diagnosis on the clinical course of spondylodiscitis. Infection. (2021) 49(5):1017–27. doi: 10.1007/s15010-021-01642-5
44. Renz N, Haupenthal J, Schuetz MA, Trampuz A. Hematogenous vertebral osteomyelitis associated with intravascular device-associated infections - A retrospective cohort study. Diagn Microbiol Infect Dis. (2017) 88(1):75–81. doi: 10.1016/j.diagmicrobio.2017.01.020
45. Pigrau C, Rodríguez-Pardo D, Fernández-Hidalgo N, Moretó L, Pellise F, Larrosa M-N, et al. Health care associated hematogenous pyogenic vertebral osteomyelitis: a severe and potentially preventable infectious disease. Medicine (Baltimore). (2015) 94(3):e365. doi: 10.1097/MD.0000000000000365
46. Kim DY, Kim UJ, Yu Y, Kim S-E, Kang S-J, Jun K-I, et al. Microbial etiology of pyogenic vertebral osteomyelitis according to patient characteristics. Open Forum Infect Dis. (2020) 7(6):ofaa176. doi: 10.1093/ofid/ofaa176
47. Michels R, Last K, Becker SL, Papan C. Update on coagulase-negative staphylococci-what the clinician should know. Microorganisms. (2021) 9(4):830. doi: 10.3390/microorganisms9040830
48. Lopez J, Tatar Z, Tournadre A, Couderc M, Pereira B, Soubrier M, et al. Characteristics of spontaneous coagulase-negative staphylococcal spondylodiscitis: a retrospective comparative study versus Staphylococcus aureus spondylodiscitis. BMC Infect Dis. (2017) 17(1):683. doi: 10.1186/s12879-017-2783-0
49. Priest DH, Peacock JE. Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J. (2005) 98(9):854–62. doi: 10.1097/01.smj.0000168666.98129.33
50. Wagner JM, Zöllner H, Wallner C, Ismer B, Schira J, Abraham S, et al. Surgical debridement is superior to sole antibiotic therapy in a novel murine posttraumatic osteomyelitis model. PLoS One. (2016) 11(2):e0149389. doi: 10.1371/journal.pone.0149389
51. Thavarajasingam SG, Vemulapalli KV, Vishnu K S, Ponniah HS, Vogel AS-M, Vardanyan R, et al. Conservative versus early surgical treatment in the management of pyogenic spondylodiscitis: a systematic review and meta-analysis. Sci Rep. (2023) 13(1):15647. doi: 10.1038/s41598-023-41381-1
52. Gentile L, Benazzo F, de Rosa F, Boriani S, Dallagiacoma G, Franceschetti G, et al. A systematic review: characteristics, complications and treatment of spondylodiscitis. Eur Rev Med Pharmacol Sci. (2019) 23(2 Suppl):117–28. doi: 10.26355/eurrev_201904_17481
53. Heyde CE, Boehm H, El Saghir H, Tschöke SK, Kayser R. Surgical treatment of spondylodiscitis in the cervical spine: a minimum 2-year follow-up. Eur Spine J. (2006) 15(9):1380–7. doi: 10.1007/s00586-006-0191-z
54. Stangenberg M, Mohme M, Mende KC, Thiesen DM, Krätzig T, Schoof B, et al. Impact of the localization on disease course and clinical management in spondylodiscitis. Int J Infect Dis. (2020) 99:122–30. doi: 10.1016/j.ijid.2020.07.028
55. Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. (2002) 15(2):110–7. doi: 10.1097/00024720-200204000-00004
56. Urrutia J, Zamora T, Campos M. Cervical pyogenic spinal infections: are they more severe diseases than infections in other vertebral locations? Eur Spine J. (2013) 22(12):2815–20. doi: 10.1007/s00586-013-2995-y
Keywords: vertebral osteomyelitis (VO), mortality, pathogens, spine infection, comorbidities
Citation: Schindler M, Walter N, Reinhard J, Pagano S, Szymski D, Alt V, Rupp M and Lang S (2024) Midterm survival and risk factor analysis in patients with pyogenic vertebral osteomyelitis: a retrospective study of 155 cases. Front. Surg. 11:1357318. doi: 10.3389/fsurg.2024.1357318
Received: 17 December 2023; Accepted: 10 May 2024;
Published: 21 May 2024.
Edited by:
Panagiotis Kerezoudis, Mayo Clinic, United StatesReviewed by:
Vicki Marie Butenschoen, Technical University of Munich, GermanyWerner Zimmerli, Cantonal Hospital Baselland (KSBL), Switzerland
© 2024 Schindler, Walter, Reinhard, Pagano, Szymski, Alt, Rupp and Lang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melanie Schindler, bWVsYW5pZS5zY2hpbmRsZXJAdWtyLmRl
 Melanie Schindler
Melanie Schindler Nike Walter1
Nike Walter1