- 1Department of Biomedical Sciences, Kansas City School of Medicine, University of Missouri, Kansas City, MO, United States
- 2College of Osteopathic Medicine, Kansas City University of Medicine and Biosciences, Kansas City, MO, United States
- 3Lake Erie College of Osteopathic Medicine, Erie, PA, United States
- 4Department of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, United States
A Commentary on
By Lu T, Lin B, Zhang Y-p, Zhang J-h, Luo J-W, Tang Y, Fang Z-T. (2023). Front Surg. 10:1106682. doi:10.3389/fsurg.2023.1106682
Introduction
Renal artery aneurysms account for approximately 1 in every 5 visceral aneurysms (1, 2). While these vascular malformations are often found incidentally on imaging, they can also symptomatically present with flank pain, hypertension, and their rupture can lead to fatal massive hemorrhage requiring immediate intervention (1, 3). Historically, open surgery was indicated for the management of renal artery aneurysms until the advent of interventional methodologies in the renal vasculature (3–5).
The growing use of these endovascular interventions have transformed both diagnostic and therapeutic patient care worldwide (1, 3). Among those with diseases of vascular etiology, endovascular interventions consistently demonstrate a lower mean cost per hospital admission compared to open surgical interventions (6). This understanding has been backed by years of clinical data that demonstrates the utility of endovascular interventions across numerous vascular pathologies (6, 7). However, there remains a paucity of this data comparably for endovascular interventions on renal artery aneurysms. The authors of this article aim to comment on a recent study by Lu et al. which provides a cohort of individuals undergoing management of renal artery aneurysms via the endovascular methodology (1).
Summary
The retrospective study by Lu et al. demonstrated a cohort of 18 patients with a total of 23 renal artery aneurysms (1). Renal artery aneurysms were found to be asymptomatic. Among these 18 patients, 13 underwent endovascular intervention for 14 total renal artery aneurysms. There were 5 interventions of interest: stent implantation, coil embolization, parent artery embolization, stent-assisted coiling embolization, and liquid embolic agent embolization. Among interventions, the most used procedure was parent artery embolization (n = 4), followed by simple coil embolization (n = 3), and all other endovascular interventions respectively (n = 2). The average diameter of aneurysms was approximately 2.2 ± 1.5 cm. Additionally, among this cohort undergoing endovascular intervention, there were four patients which experienced what the study classified to be as mild complications within the context of pain. Follow-up was performed among these patients, and there was no noted technical stent displacement or aneurysm recurrence.
Discussion
This study provides an overview of the interventional operations for renal artery aneurysm management. It formidably describes the utilization and recommended indication of five types of interventions. Beyond these descriptions, the authors of this commentary believe the data in this study can inspire a potential starting point for the discussion of future clinical investigations which compares the use of these various endovascular techniques. To the best of our knowledge, there are minimal clinical guideline recommendations that demonstrate an algorithmic approach to determine which intervention is best suited for a patient. Let alone, there remains a paucity of literature that effectively demonstrates the postoperative outcomes within each procedure. While this study does not directly compare the utility of the interventions (i.e., parent artery embolization vs. simple coil embolization), a secondary analysis of this cohort could be performed that specifically analyzed additional operative factors such as procedure time, access site, time to coil deployment, estimated blood loss, and post-operative hospital course (1, 8–10). This study proposal is shown in Figure 1.
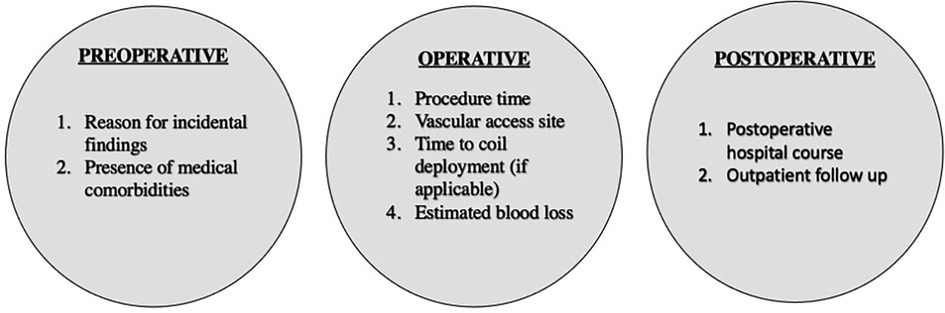
Figure 1. Proposed study variables for future clinical investigations at the preoperative, operative, and postoperative stages.
In addition to the operative factors, this study characterizes some preoperative variables such as classifying whether the renal artery aneurysms were incidental finding on imaging or symptomatic. With regards to incidental findings, the authors of this commentary encourage future studies to further classify what the initial intervention was that led to the incidental finding. This can play a key role in determining the rationale behind the course of undergoing the endovascular operation (i.e., patient deemed high risk for open surgery) (8). Likewise, another study design could be to stratify pre-operative risk to create a risk calculator for endovascular renal artery aneurysm management (8–11).
Additionally, patients were followed in this study after procedural intervention, with some found to have been followed several years post-operation. This data in unclear regarding the number of follow up visits performed by each patient but does provide post-operative imaging results. In future studies, the post-operative setting could also provide the number of follow visits and be able to implement longitudinal variables that could be measured over a post-operative course (11–15). Although following a study of this label may require extensive patient adherence to follow up in its sample size.
Conclusion
Overall, the study design by Lu et al. is sound in methodology and can be used as a framework for future clinical investigation. This commentary discusses suggests and discusses preoperative, operative, and postoperative variables that can be added to similar study designs. The goal of these variables is to generate a stronger clinical body of literature that can help clinicians and patients better understand and innovate endovascular intervention for renal artery aneurysms.
Author contributions
SS: Conceptualization, Writing – original draft, Writing – review & editing. UQ: Methodology, Writing – review & editing. FQ: Writing – review & editing. FQ: Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lu T, Lin B, Zhang YP, Zhang J, Luo J, Tang Y, et al. Eighteen cases of renal aneurysms: clinical retrospective analysis and experience of endovascular interventional treatment. Front Surg. (2023) 10:1106682. doi: 10.3389/fsurg.2023.1106682. (Published 2023 Feb 28).36925508
2. Etezadi V, Gandhi RT, Benenati JF, Rochon P, Gordon M, Benenati MJ, et al. Endovascular treatment of visceral and renal artery aneurysms. J Vasc Interv Radiol. (2011) 22(9):1246–53. doi: 10.1016/j.jvir.2011.05.012
3. Finch LM, Spiers HVM, Chinnadurai R, Herwadkar A, Anantha-Krishnan G, Augustine T. Endovascular coiling in the treatment of patients with renal artery aneurysms. J Vasc Surg Cases Innov Tech. (2021) 7(2):307–10. doi: 10.1016/j.jvscit.2021.03.006. (Published 2021 Apr 18).34027245
4. Jibiki M, Inoue Y, Kudo T, Toyofuku T. Surgical procedures for renal artery aneurysms. Ann Vasc Dis. (2012) 5(2):157–60. doi: 10.3400/avd.oa.11.00055
5. Titze N, Ivanukoff V, Fisher T, Pearl G, Grimsley B, Shutze WP. Surgical repair of renal artery aneurysms. Proc (Bayl Univ Med Cent). (2015) 28(4):499–501. doi: 10.1080/08998280.2015.11929322
6. van den Berg LA, Berkhemer OA, Fransen PSS, Beumer D, Lingsma H, Majoie CBM, et al. Economic evaluation of endovascular treatment for acute ischemic stroke. Stroke. (2022) 53(3):968–75. doi: 10.1161/STROKEAHA.121.034599
7. Li S, Li F, Liu Z, Zeng R, Ye W, Shao J, et al. Blood pressure and renal outcomes after renal artery aneurysm intervention: single-center experience and review of literature. Front Cardiovasc Med. (2023) 10:1127154. doi: 10.3389/fcvm.2023.1127154. (Published 2023 Apr 21)37153466
8. Sef D, Birdi I. Clinically significant incidental findings during preoperative computed tomography of patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. (2020) 31(5):629–31. doi: 10.1093/icvts/ivaa160
9. Lee C, Columbo JA, Stone DH, Creager MA, Henkin S. Preoperative evaluation and perioperative management of patients undergoing major vascular surgery. Vasc Med. (2022) 27(5):496–512. doi: 10.1177/1358863X221122552
10. Bossone E, Cademartiri F, AlSergani H, Chianese S, Mehta R, Capone V, et al. Preoperative assessment and management of cardiovascular risk in patients undergoing non-cardiac surgery: implementing a systematic stepwise approach during the COVID-19 pandemic era. J Cardiovasc Dev Dis. (2021) 8(10):126. doi: 10.3390/jcdd8100126. (Published 2021 Oct 3).34677195
11. Choussat R, Black A, Bossi I, Fajadet J, Marco J. Vascular complications and clinical outcome after coronary angioplasty with platelet IIb/IIIa receptor blockade. Comparison of transradial vs. transfemoral arterial access. Eur Heart J. (2000) 21(8):662–7. doi: 10.1053/euhj.1999.1945
12. Zhan HT, Purcell ST, Bush RL. Preoperative optimization of the vascular surgery patient. Vasc Health Risk Manag. (2015) 11:379–85. doi: 10.2147/VHRM.S83492. (Published 2015 Jul 1).26170688
13. Taslakian B, Sridhar D. Post-procedural care in interventional radiology: what every interventional radiologist should know-part II: catheter care and management of common systemic post-procedural complications. Cardiovasc Intervent Radiol. (2017) 40(9):1304–20. doi: 10.1007/s00270-017-1709-y
14. Reis PEO, Abrão GP, Roever L. Endovascular treatment of wide-neck saccular renal artery aneurysm with waffle-cone technique. J Vasc Bras. (2021) 20:e20200116. doi: 10.1590/1677-5449.200116. (Published 2021 May 10).34093681
Keywords: renal artery aneurysm, endovascular, coil embolism, stenting, study design
Citation: Singh SP, Qureshi U, Qureshi F and Qureshi F (2024) Commentary: Eighteen cases of renal aneurysms: clinical retrospective analysis and experience of endovascular interventional treatment. Front. Surg. 11:1352880. doi: 10.3389/fsurg.2024.1352880
Received: 9 December 2023; Accepted: 10 January 2024;
Published: 29 January 2024.
Edited by:
Tristan Lane, Cambridge University Hospitals NHS Foundation Trust, United KingdomReviewed by:
Gianluigi Fino, University of Perugia, Italy© 2024 Singh, Qureshi, Qureshi and Qureshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Som P. Singh c29tc2luZ2hAbWFpbC51bWtjLmVkdQ==
 Som P. Singh
Som P. Singh Ursula Qureshi2
Ursula Qureshi2