- Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD, United States
Background and importance: Vestibular schwannomas are benign tumors and are the most common tumor found in the cerebellopontine angle. Surgical management of these lesions involves consideration of various operative approaches, which can have profound effects on procedural course and patient outcomes. Therefore, a comprehensive understanding of the location of the tumor and surrounding anatomical structures is vital for a positive outcome. We present a case of a 47-year-old female patient with vestibular schwannoma. A novel mixed reality (MR) system was used to register patient-specific 3D models onto the patient's head for operative planning and anatomical visualization.
Case description: A 47-year-old female presented with a history of left-sided hearing loss, tinnitus, and episodic left facial tingling. Magnetic Resonance Imaging (MRI) demonstrated a 3.3 cm enhancing lesion in the left cerebellopontine angle at the with mass effect on the brachium pontis and medulla. Surgical resection was performed via retrosigmoid craniotomy.
Conclusions: In this study, we report the use of Augmented Reality (AR) visualization for planning of vestibular schwannoma resection. This technology allows for efficient and accurate registration of a patient's 3D anatomical model onto their head while positioned in the operating room. This system is a powerful tool for operative planning as it allows the surgeon to visualize critical anatomical structures where they lie on the patient's head. The present case demonstrates the value and use of AR for operative planning of complex cranial lesions.
Introduction
Vestibular schwannomas are benign tumors and are the most common tumor found in the cerebellopontine angle (1). Symptoms of vestibular schwannoma include sensorineural hearing loss, tinnitus, unsteadiness and less commonly may include vertigo, facial pain, numbness and weakness (2). The goal of vestibular schwannoma management is to reduce mass effect and preserve nerve function (3). The major treatment options for vestibular schwannomas are surgery, radiation therapy and observation (3). When surgery is indicated, various approaches can be performed such as translabyrinthine, retrosigmoid and middle fossa craniotomy (2). Each of these approaches involve critical neuroanatomy that require delicate management to optimize functional outcomes and reduce complications. Complications of surgical removal of vestibular schwannomas can include CSF leak, headache and worsened or persistent neurologic deficit including cranial neuropathies (3). In order to minimize these complications, surgeons must have a good understanding of the tumor's location and surrounding structures. Improvements in MRI imaging have allowed for the ability to visualize the relationship of these tumors with surrounding neurovascular structures for more efficient and targeted treatment plans (1). Although MRI provides a great deal of information about the anatomy, traditional surgical planning with 2D MRI-based imaging can be limited in providing a complete understanding of the tumor and its surroundings in 3D space.
Augmented Reality (AR) is a technology that can superimpose 3D virtual models onto the user's real-world environment. AR has the potential to improve the accuracy and effectiveness of surgical planning by providing surgeons with a 3D view of the tumor and its surrounding structures that can be used to plan the ideal surgical approach. AR provides the benefits of both 3D visualization, and the ability to visualize these models superimposed onto the real environment and patient. The purpose of this study is to assess the benefit of a novel AR registration system for operative planning in the setting of a retrosigmoid craniotomy for resection of a vestibular schwannoma.
Case description
A 47-year-old female presented for neurosurgical consultation with a history of hearing loss, tinnitus, and episodic left facial tingling. Magnetic Resonance Imaging (MRI) demonstrated a 3.3 cm contrast-enhancing left cerebellopontine angle (CPA) lesion with notable mass effect on the brachium pontis and medulla (Figure 1). Management strategies were discussed with patient, and plan was made for surgical resection via retrosigmoid craniotomy approach.
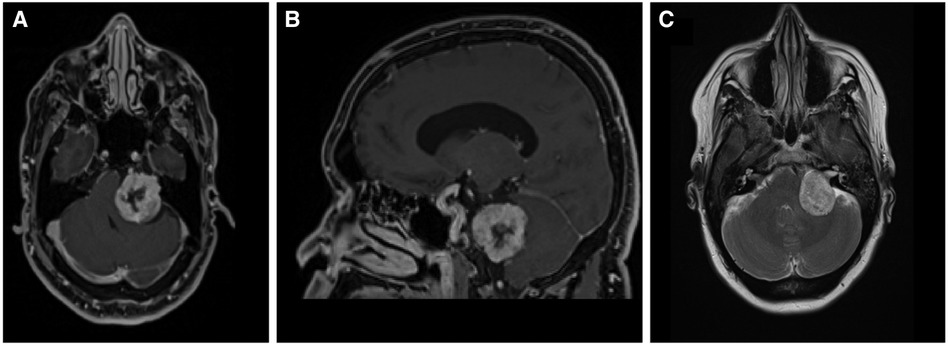
Figure 1. Diagnostic MRI study demonstrating the vestibular schwannoma (A) axial T1 with contrast; (B) sagittal T1 with contrast (C) axial T2.
Segmenting anatomical structures
3D models were generated based on the patient's preoperative MRI scan. Anatomy of interest—tumor, ventricles, vasculature, and whole brain—were manually segmented using ITKsnap. Figure 2 demonstrates the segmented MRI with schwannoma shown in red, ventricles in green, whole brain in turquoise/yellow, and vessels in blue. The patient's face was segmented and reconstructed for registration via auto segmentation algorithms (Hoth Intelligence Inc, Philadelphia, Pennsylvania). All segmented structures were merged and converted into a 3D digital model. The digital models underwent basic processing to reduce the file size to optimize performance, visual quality, and usability for an AR head-mounted display (HMD).
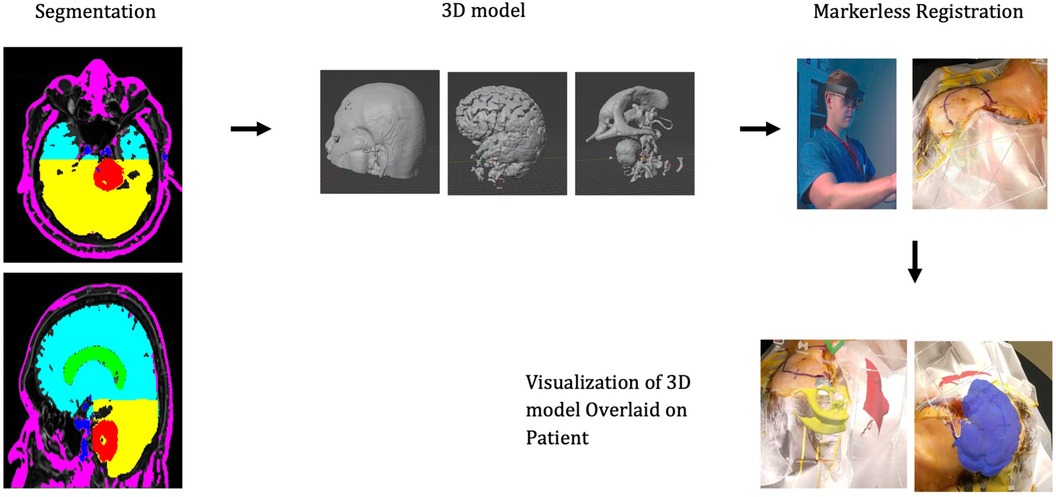
Figure 2. Workflow of the AR technology platform. The patient anatomy is first segmented and converted into a 3D model. The model is loaded onto the AR headset. While wearing the headset, the surgeon scans the patients’ head. Registration algorithms are processed on the headset and the 3D model can be visualized aligned/overlaid with the patients’ head.
AR technology platform
The AR application was developed by Hoth Intelligence (Philadelphia, Pennsylvania) to operate on the Microsoft Hololens 2 HMD (Redmond, Washington). The Microsoft Hololens 2 is an untethered optical see-through head-mounted display that superimposes virtual content (i.e., holograms, images, screens) onto the users' real-world field of view. A clinical workflow of the AR system is described in Figure 2.
3D model registration to patient
The application used in this study superimposes 3D digital models onto the head using an ultra-fast, fiducial-less registration process. Information from the headset sensors (depth sensor, RBG camera, and stereosensor) are merged with coordinate data from preoperative MRI scan to align the 3D model to the patient's head. After the patient was pinned in the operative position, the patient-specific 3D model was registered onto the head via the AR headset.
Mixed reality viewing
Once registered, the surgeon was able to freely move around the patient to visualize the 3D model overlaid onto the patients' head (Figure 3 and Supplementary Video S1). The added AR visualization allowed the surgeon to confirm proper approach and incision/craniotomy locations. Additionally, the ability to hide and show different layers of the model allowed the surgeons to discuss other operative considerations such as nearby vascular structures surrounding the lesion and proximity to brainstem.
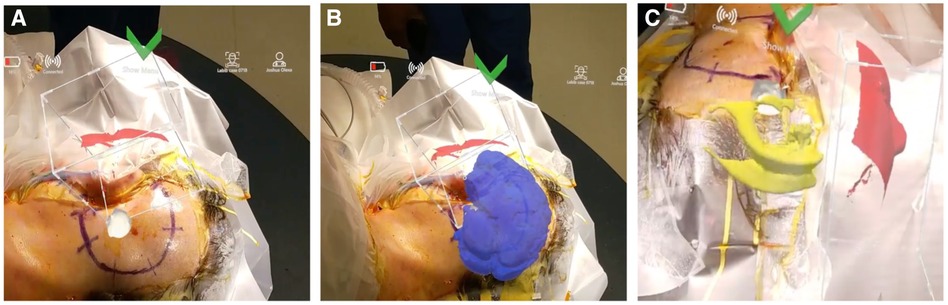
Figure 3. Surgeons’ view through the AR headset displaying 3D model overlaid on patients’ head. (A) Visualization of the tumor in white. (B) Whole brain shown in blue. (C) Ventricles in yellow.
Surgical strategy
To relieve brainstem compression, resection with a retrosigmoid craniectomy was performed. With the help of neuro-navigation, the sigmoid sinus and transverse sigmoid junction were identified and skeletonized. Cisterna magna was opened to decompress the cerebellum and access both the CPA and internal auditory canal (IAC) porus. The tumor was identified caudally above the jugular foramen and debulked. The posterior fossa dura was further incised to access the IAC, which revealed intact tumor from the most distal aspect of the canal into the CPA. To preserve nerve function, a small segment of tumor overlying the facial nerve was left in place before duraplasty and cranioplasty. Postoperatively, facial numbness resolved, and the patient was otherwise neurologically stable.
Discussion
We describe a novel use of AR for use in surgical planning for retrosigmoid craniotomy for resection of a cerebellopontine angle lesion. The use of virtual reality (VR) for surgical planning in neurosurgery has been shown to increase understanding of complex anatomic relationships to improve surgical planning (4). AR allows for even further advancement in surgical planning by integrating 3D visualization onto the real-world environment. We describe our experience using AR to overlay patient-specific 3D models onto the patient's head with a marker-less registration system. Previous studies have shown AR to be effective for both surgical planning and intraoperative guidance using standard patient registration methods (5). These standard registration methods are time consuming and require expensive systems that have a large amount of hardware. This novel technology allows for the benefits of increased visualization and understanding of patient specific anatomy with an efficient, marker-less registration system. Use of the AR system allowed the surgeon to plan surgical approaches such as incision and craniotomy location as well as discuss the spatial relationship between the lesion and other anatomical structures.
The use of AR for visualizing and overlaying patient anatomy has been shown to be an effective surgical planning tool (6). Reports have described the use of AR for craniotomy and trajectory planning using pre-operative MRI of cranial meningiomas. Additionally, other reports have described the use of AR for navigation during procedures such as needle biopsy and external ventricular drain placement. AR systems used in these other reports typically utilize fiducial markers and/or require multiple camera systems to register anatomy with the patients (7–9). This is a case report showing the use of a novel markerless registration AR system for visualization of patient-specific anatomy overlaid onto the patient in the operating room for surgical planning. The system does not require fiducial markers and operates entirely our of the headset without the need for external camera systems. For this case, while wearing an augmented reality headset, the surgeon was able to overlay a 3D model of the patients' vestibular schwanomma and brain onto the patients' head in the proper location and orientation. This allowed the surgeon to plan the craniotomy location and optimize proper head position. As such the system serves as an effective planning tool prior to making an incision. Right now the system—given its size and speed—serves as an effective adjunct to traditional neuronavigation however, in the future this technology has the potential to serve as a primary image guidance system. Further study of this technology is needed to characterize its benefits and accuracy compared with traditional surgical planning approaches.
Conclusion
In this study, we report the use of AR visualization for planning of retrosigmoid craniotomy for removal of a vestibular schwannoma. This technology is advantageous in surgical planning as it allows for enhanced visualization with a fast, user-friendly setup. Future studies are needed to further characterize the accuracy of this novel technology and benefits such as faster setup time, operative time, improved surgical outcomes and possible cost savings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to JO,am9sZXhhQHNvbS51bWFyeWxhbmQuZWR1.
Ethics statement
The studies involving humans were approved by University of Maryland Medical Center Institutional Review Board (HP-00104849). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because IRB approval ruled no increased risk to patient. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because IRB (HP-00104849) waived need for written informed consent.
Author contributions
JO: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. AT: Data curation, Methodology, Writing – original draft. RF: Conceptualization, Data curation, Writing – original draft. ML: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1304039/full#supplementary-material
Supplementary Video S1
Footage of 3D model overlaid onto patients' head as viewed through the headset.
References
1. Dang L, Tu NCY, Chan EY. Current imaging tools for vestibular schwannoma. Curr Opin Otolaryngol Head Neck Surg. (2020) 28(5):302. doi: 10.1097/MOO.0000000000000647
2. Lin EP, Crane BT. The management and imaging of vestibular schwannomas. Am J Neuroradiol. (2017) 38(11):2034–43. doi: 10.3174/ajnr.A5213
3. Halliday J, Rutherford SA, McCabe MG, Evans DG. An update on the diagnosis and tratment of vestibular schwannoma. Expert Rev Neurother. (2018) 18(1):29–39. doi: 10.1080/14737175.2018.1399795
4. .Ferroli P, Tringali G, Acerbi F, Schiariti M, Broggi M, Aquino D, et al. Advanced 3-dimensional planning in neurosurgery. Neurosurgery. (2013) 72:A54. doi: 10.1227/NEU.0b013e3182748ee8
5. Louis RG, Steinberg GK, Duma C, Britz G, Mehta V, Pace J, et al. Early experience with virtual and synchronized AR platform for preoperative planning and intraoperative navigation: a case series. Oper Neurosurg. (2021) 21(4):189–96. doi: 10.1093/ons/opab188
6. Dey A, Billinghurst M, Lindeman RW, Swan JE. A systematic review of 10 years of augmented reality usability studies: 2005 to 2014. Front Robot AI. (2018) 5:37. doi: 10.3389/frobt.2018.00037
7. Park YG, Woo HJ, Kim E, Park J. Accuracy and safety of bedside external ventricular drain placement at two different cranial sites : Kocher’s point versus forehead. J Korean Neurosurg Soc. (2011) 50(4):317–21. doi: 10.3340/jkns.2011.50.4.317
8. Skyrman S, Lai M, Edström E, Burström G, Förander P, Homan R, et al. Augmented reality navigation for cranial biopsy and external ventricular drain insertion. Neurosurg Focus. (2021) 51(2):E7. doi: 10.3171/2021.5.focus20813
9. Domínguez-Velasco CF, Tello-Mata IE, Guinto-Nishimura G, Martínez-Hernández A, Alcocer-Barradas V, Pérez-Lomelí JS, et al. Augmented reality simulation as training model of ventricular puncture: evidence in the improvement of the quality of punctures. Int J Med Robot Comput Assist Surg. (2023) 19(5):e2529. doi: 10.1002/rcs.2529
Keywords: augmented reality, vestibular schwannoma, presurgical planning, mixed reality, navigation
Citation: Olexa J, Trang A, Flessner R and Labib M (2024) Case Report: Use of novel AR registration system for presurgical planning during vestibular schwannoma resection surgery. Front. Surg. 11:1304039. doi: 10.3389/fsurg.2024.1304039
Received: 28 September 2023; Accepted: 21 February 2024;
Published: 4 March 2024.
Edited by:
Nicola Montano, Fondazione Policlinico Universitario Agostino Gemelli IRCCS—Università Cattolica del Sacro Cuore, ItalyReviewed by:
Emanuela Crobeddu, Azienda Ospedaliero Universitaria Maggiore della Carità, ItalyPrajwal Ghimire, King’s College Hospital NHS Foundation Trust, United Kingdom
© 2024 Olexa, Trang, Flessner and Labib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joshua Olexa am9sZXhhQHNvbS51bWFyeWxhbmQuZWR1
 Joshua Olexa
Joshua Olexa Annie Trang
Annie Trang Rebecca Flessner
Rebecca Flessner