- Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
Background: Robotic bariatric surgery serves as an alternative to laparoscopy. The technology provides the surgeon with an accurate three-dimensional view, allowing complex maneuvers while maintaining full control of the operating room.
Hypothesis: We report our experience with this innovative surgery compared with laparoscopy during Roux-en-Y gastric bypass to demonstrate its safety and feasibility. The aim of this study is to evaluate potential differences between the robotic and laparoscopic techniques.
Materials and methods: Our study retrospectively identified 153 consecutive obese patients who underwent either laparoscopic or robotic gastric bypass (RGB) procedures over a 2-year period at the Department of Medical and Surgical Sciences, University of Foggia. Data on demographics, operative time, conversion rate, length of hospital stay, and mortality were collected and compared between two groups of patients: 82 patients who underwent laparoscopic procedures and 71 who underwent robotic procedures.
Results: We analyzed 153 patients who underwent gastric bypass with a mean age of 42.58 years, of whom 74 were female; 71 were treated with a robotic approach and 82 with a laparoscopic approach. The mean operative time was 224.75 ± 10.4 min for RGB (including docking time) and 101.22 min for laparoscopic gastric bypass (LGB) (p < 0.05), which is statistically significant. The median length of stay was 4.1 days for the RGB group and 3.9 days for the LGB group (p = 0.89). There is only one conversion to laparoscopy in the RGB group. We observed only one case of postoperative complications, specifically one episode of endoluminal bleeding in the laparoscopic group, which was successfully managed with medical treatment. No mortality was observed in either group.
Conclusion: The statistical analysis shows to support the robotic approach that had a lower incidence of complications but a longer operative duration. Based on our experience, the laparoscopic approach remains a technique with more haptic feedback than the robotic approach, making surgeons feel more confident.
This study has been registered on ClinicalTrial.gov Protocol Registration and Results System with this ID: NCT05746936 for the Organization UFoggia (https://clinicaltrials.gov/ct2/show/NCT05746936).
Introduction
Obesity is a chronic, relapsing disease associated with numerous complications and significant morbidity, mortality, and healthcare burden. Bariatric surgery now plays an important role in the treatment of obesity in addition to non-invasive conservative treatments such as lifestyle changes, pharmacotherapy, and behavioral therapy (1, 2). In 1991, the National Institutes of Health Consensus Statement on Gastrointestinal Surgery for Severe Obesity stated that bariatric surgery is the most effective treatment for obesity owing to its benefits in weight loss, glycemic control, and reduced mortality (3, 4).
Roux-en-Y gastric bypass (RYGB) is the most common procedure in Europe to treat severely obese people (5, 6), particularly in the presence of gastroesophageal reflux or type 2 diabetes cases (7–9).
The laparoscopic technique is the most widely used for Roux-en-Y gastric bypass, which is significantly superior to open surgery (10, 11), with a low complication rate but high technical requirements and a flat learning curve between 1,006 and 500 cases (12, 13).
However, the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA), which was introduced in 2000, successfully addressed certain technical limitations associated with laparoscopic surgery. One of the main advantages is the improved dexterity of the instrument (increased freedom of movement) and the filtering of tremors, which allow the surgeon to operate as if in open surgery, allowing delicate microsurgery and microanastomosis (14, 15). Other advantages include a three-dimensional view of the surgical field that provides better depth perception, the ability for the surgeon to control the field of view of the surgical field, and an ergonomically designed workstation that allows the surgeon a comfortable sitting position (16, 17).
In this study, we report our experience with this innovative surgery compared with laparoscopy during Roux-en-Y gastric bypass to demonstrate its safety and feasibility. The aim is to evaluate if any differences exist between the robotic and laparoscopic techniques.
Materials and methods
Our study retrospectively identified 153 consecutive obese patients who underwent either laparoscopic or robotic gastric bypass (RGB) procedures over a 2-year period (2020–2022) at the Department of Medical and Surgical Sciences, University of Foggia. Data on demographics, operative time, conversion rate, length of hospital stay, and mortality were collected and compared between two groups of patients: 82 patients who underwent laparoscopic procedures and 71 patients who underwent robotic procedures.
The unique protocol identification number (UIN) for the ClinicalTrial.gov Protocol Registration and Results System is NCT05746936 for the Organization UFoggia (https://clinicaltrials.gov/ct2/show/NCT05746936).
Inclusion criteria
Individuals who have a body mass index (BMI) of ≥ 35–39 kg/m2 and at least one obesity-associated comorbidity or a BMI of ≥ 40 kg/m2 and are 18 years of age or older were included in the study. Prior to surgery, the patients underwent a standardized psychological and physical evaluation that included blood chemistry tests, chest x-rays, electrocardiogram and cardiological examinations, nutritional evaluation, esophagogastroduodenoscopy, spirometry, and psychiatric evaluation.
Exclusion criteria
We excluded re-do surgery procedures from the study.
Statistical analysis
Continuous data were expressed as mean and standard deviation (SD), and statistical analysis was performed using the χ2 test with a p-value threshold of less than 0.05 (p < 0.05) and the Mann–Whitney U test to determine statistical significance.
Surgical technique
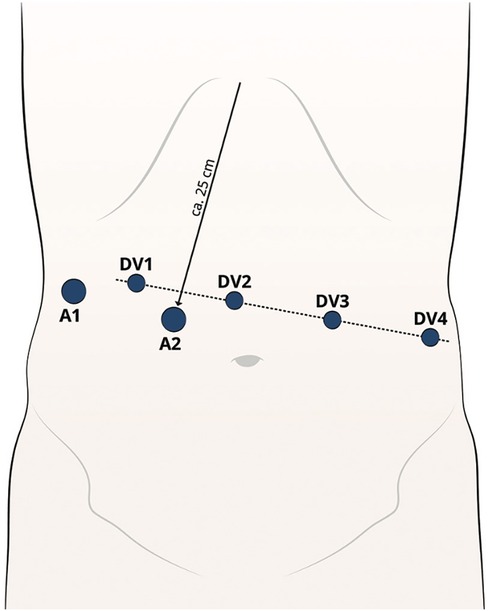
A pneumoperitoneum is established (15 mmHg) using a 12 mm First Entry trocar, positioned approximately 25 cm below the xiphoid [Assistant trocar 2 (A2)] on the right paramedian side. Another 12 or 5 mm (depending on the retractor) trocar [Assistant trocar 1 (A1)] is inserted on the right lateral flank. This is mainly used for liver retractions. From A1, a line is drawn across the upper abdomen at a 90° angle to the xiphoid-A2 line. All da Vinci (DV) ports are positioned along this line. Port 1 (8 mm) (DV1) is positioned 8–10 cm lateral to port A1. Port 2 (8 mm) (DV2) is positioned 8–10 cm to the side of port 1. This will be the camera port. Port 3 (8 mm) (DV3) and port 4 (8 mm) are positioned (DV4) 8–10 cm lateral to each other on the patient's left side (Figure 1) (18).
The liver paddle is first inserted through the A1, and the liver is retracted toward the right upper quadrant.
Starting from the ligament of Treitz, 120 cm of jejunum is measured aborally, and the jejunum is dissected by the assistant through a linear stapler from A2, creating the biliary limb. Another 120 cm is counted (alimentary limb), and the jejunum is opened antimesenterally on both limbs with Ultracision, and a side-to-side jejunojejunal anastomosis is created using the 60 mm linear stapler. The enterotomy is closed using a single suture with Stratafix 3-0 in a seromuscular suture.
To preserve the left gastric artery, a retrogastric tunnel is formed starting 6 cm below the lesser curvature gastroesophageal junction. In order to minimize the risk of stricture or dumping syndrome, the anesthesiologist inserts a 40 mm bougie orally to assure the appropriate size of the pouch. Using a linear stapler, the stomach pouch is formed with the bougie as a calibration.
Ultracision is used to open the pouch at its lowest point, away from the minor curvature.
The stapler is inserted into the gastric pouch and the alimentary limb, and then the lateral gastrojejunal anastomosis is contoured, ensuring good positioning of both ends to create only a small enterostomy.
A 15 cm unidirectional Stratafix 2-0 suture is used to close the enterotomy for a continuous seromuscular suture. Arm 4 is used to optimize the position of the small bowel loop, while arms 1 and 3 are used dynamically for suturing. Starting on each side of the enterostomy, two sutures are required to complete the anastomosis.
A methylene blue test is conducted to test the gastrojejunostomy. The systolic blood pressure is then raised above 130 mmHg to identify any signs of bleeding. Any bleeding is treated using either the bipolar forceps, with a clip application, or a suture.
A drainage is placed through the A1, and all trocars are retrieved under view. The pneumoperitoneum is released although the A2 and the patient cart can be undocked.
Results
We analyzed 153 patients who underwent gastric bypass, 74 of whom were female, with a mean age of 42.58 years. Among the patients, 71 were treated using a robotic approach, while 82 were treated using a laparoscopic approach. The initial mean weight was 130.50 kg, and the initial mean body mass index was 45.73 kg/m2, specifically 43.68 ± 6.1 kg/m2 for the robotic group and 45.53 ± 7.1 kg/m2 for the laparoscopic group.
The mean operative time was 224.75 ± 10.4 min for robotic gastric bypass (including docking time) and 101.22 min for laparoscopic gastric bypass (LGB) (p < 0.05), which is statistically significant (Table 1). The z-score was −5.92815, and the value of U was 0, indicating that the distribution is approximately normal.
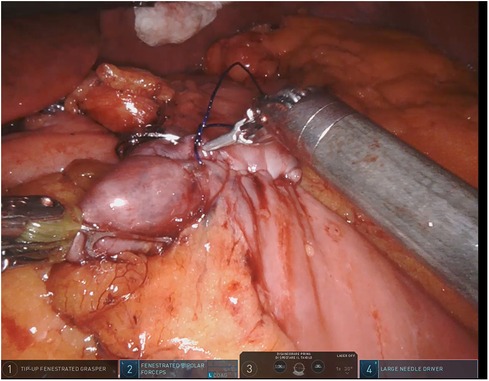
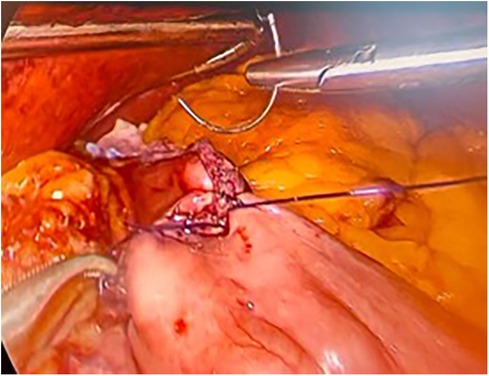
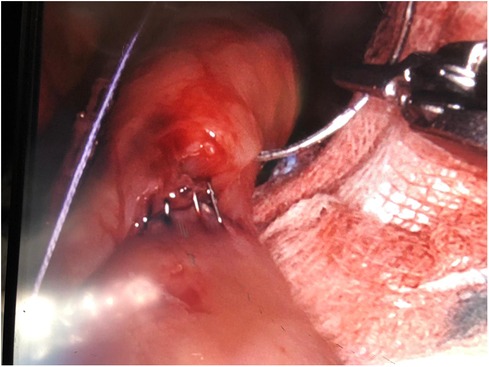
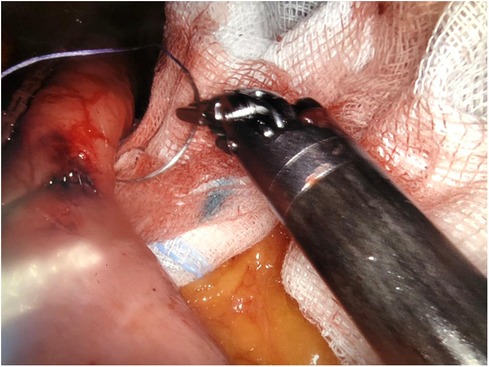
The surgical technique is the same, which provides for the creation of a gastric pouch, biliopancreatic limb, jejunojejunal anastomosis, alimentary limb, and gastrojejunal anastomosis (Figures 2,3).
The median length of hospital stay was 4.1 days for the RGB group and 3.9 days for the LGB group (p = 0.89). The z-score was 0.18301, and the value of U was 201.5, indicating that the distribution is approximately normal.
Patients were discharged on the third postoperative day after oral contrast gastrointestinal tract radiography.
There was only one case of converting to laparoscopy in the robotic group due to the detection of a positive intraoperative methylene blue test. The surgeon preferred to reinforce the gastrojejunal anastomosis with laparoscopic sutures due to the lack of haptic feedback with the robotic procedure. We observed only one case of postoperative complications: one occurrence of endoluminal bleeding in the laparoscopic group, which was successfully managed with medical treatment. There were no deaths observed in either group (Figures 4,5).
The operative time of the RGB group was longer than that of the LGB group, resulting in a significantly higher operating room time costs (median €18421.07 vs. €2088.09, respectively, p < 0.005).
Discussion
For approximately 20 years, robotic surgery has been successfully used in critical bowel, gastric, pancreatic, urological, and transplant procedures, where anastomoses are a critical part of the procedure as they are often time-sensitive, particularly when warm ischemia must be minimized, such as during transplant surgery. Organs are critical to transplant function and outcome (19–21). In the literature, robotic approaches have provided similar results to laparoscopic and open approaches in terms of anastomotic leaks or strictures. There was no significant difference found in leakage after RYGB when comparing robotic anastomosis with laparoscopic anastomosis. Robotic surgery is gaining popularity and offers solutions to the challenges of laparoscopy, including ergonomics, high-resolution 3D cameras, tremor filtering, the surgeon's third arm, and handheld instruments (22).
In the bariatric surgical field, for example, these features translate into the ability to perform better traction of a normally thick abdominal wall, relieving the surgeon's physical efforts to overcome the counterproductive forces, as well as a highly stable camera and better manipulation of the surgical structures (23).
In agreement with the literature data, our study did not show significant differences in terms of the length of hospital stay, reoperations, and mortality (24, 25).
The mean operative time was significantly shortened (101.22 min) in the laparoscopic group due to the time-consuming setup and docking phase featuring the robotic approach.
There was only one case of conversion from a robotic to a laparoscopic approach, probably due to the greater haptic feedback guaranteed by laparoscopy.
Compared with endoscopic or laparoscopic techniques, robotic surgical systems suffer from a complete loss of tactile feedback to the user (26). Consequently, surgeons rely on visual cues and their expertise in order to perform the accurate motor movements required for operations (27, 28). The absence of tactile feedback leads to prolonged procedural times and a greater risk of surgical error (29).
There was only one complication in the laparoscopic group, which can be explained by the better accuracy and precision of the intracorporeal suture during the robotic approach in comparison with the traditional laparoscopic approach.
Our results are consistent with the data presented by Wilson et al. (30) at the 2012 American Society for Metabolic and Bariatric Surgery Annual Meeting in San Diego. The study involved the enrollment of 1,695 patients who underwent robotic RYGB surgery. The postoperative complications included ileus in 17 patients, wound infection in five patients, and bleeding in 18 patients. The readmission rate was 4.8%, and the reoperation rate was 2.7%. The rates of leakage and anastomotic stenosis were extremely low, at 0.3% and 0.2%, respectively. There were no deaths reported. “This largest series of robotic bypass reports from three high-volume centers shows very low complication rates in the first 30 days. It shows zero 30-day mortality, very low leak rates, and provides a strong evidence that Robotic GB can deliver extremely safe and reproducible results.”
Kim et al. (31, 32) concluded that the use of the robot is associated with a shorter learning curve, particularly in performing delicate and precise maneuvers such as fine dissections and sutures. Indeed, it is widely recognized that robotic bariatric surgery, particularly RGB, has a steeper learning curve than the laparoscopic approach, and a minimum of 20 cases may be sufficient to successfully complete the basic learning phase.
The most important limitation of this study is the small sample size of patients, but despite this, it offers a preliminary statistical analysis between the two procedures. Furthermore, the learning curve can contribute to a decrease in the duration of a robotic gastric bypass procedure.
Conclusion
Our statistical analysis seems to favor the robotic approach due to a lower incidence of complications, but the major disadvantage of the robotic bariatric surgery still remains the long operative time.
In our experience, the laparoscopic approach continues to be a technique that provides greater haptic feedback compared with the robotic approach, making the surgeon feel more confident. RGB was found to be comparable with LGB in terms of safety and efficacy, but larger and longer studies are needed for a better comparative evaluation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee: “COMITATO ETICO AREA 1” (DDG n. 363 del 25/10/2016 e s.m.i. DDG n. 318 del 14/06/2019) of our institution (Policlinico Riuniti). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GP: Writing – original draft, Data curation, Validation. MP: Data curation, Methodology, Writing – review & editing. AG: Formal Analysis, Writing – review & editing. AQ: Data curation, Investigation, Writing – original draft. AA: Conceptualization, Supervision, Writing – original draft. NT: Supervision, Visualization, Writing – original draft.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bergmann NC, Davies MJ, Lingvay I, Knop FK. Semaglutide for the treatment of overweight and obesity: a review. Diabetes Obes Metab. (2023) 25(1):18–35. doi: 10.1111/dom.14863
2. Gastrointestinal surgery for severe obesity: National Institutes of Health consensus development conference statement. Am J Clin Nutr. (1992) 55(2):615S–9S. doi: 10.1093/ajcn/55.2.615s
3. Singhal R, Tahrani AA, Ludwig C, Mahawar K, GENEVA collaborators. Global 30-day outcomes after bariatric surgery during the COVID-19 pandemic (GENEVA): an international cohort study. Lancet Diabetes Endocrinol. (2021) 9(1):7–9. doi: 10.1016/S2213-8587(20)30375-2
4. Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. (2018) 28(12):3783–94. doi: 10.1007/s11695-018-3450-2
5. Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg. (2019) 29(3):782–95. doi: 10.1007/s11695-018-3593-1
6. Singhal R, Ludwig C, Rudge G, Gkoutos GV, Tahrani A, Mahawar K, et al. 30-day morbidity and mortality of bariatric surgery during the COVID-19 pandemic: a multinational cohort study of 7704 patients from 42 countries. Obes Surg. (2021) 31(10):4272–88. doi: 10.1007/s11695-021-05493-9
7. Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg Incl Laparosc Allied Care. (1994) 4(4):353–7. doi: 10.1381/096089294765558331
8. Banka G, Woodard G, Hernandez-Boussard T, Morton JM. Laparoscopic vs open gastric bypass surgery: differences in patient demographics, safety, and outcomes. Arch Surg. (2012) 147(6):550–6. doi: 10.1001/archsurg.2012.195
9. Di Lascia A, Tartaglia N, Petruzzelli F, Pacilli M, Maddalena F, Fersini A, et al. Right hemicolectomy: laparoscopic versus robotic approach. Ann Ital Chir. (2020) 91:478–85. 32543465.32543465
10. Schauer P, Ikramuddin S, Hamad G, Gourash W. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc. (2003) 17(2):212–5. doi: 10.1007/s00464-002-8857-z
11. Doumouras AG, Saleh F, Anvari S, Gmora S, Anvari M, Hong D. Mastery in bariatric surgery. Ann Surg. (2018) 267(3):489–94. doi: 10.1097/SLA.0000000000002180
12. Singhal R, Wiggins T, Super J, Alqahtani A, Nadler EP, Ludwig C, et al. 30-day morbidity and mortality of bariatric metabolic surgery in adolescence during the COVID-19 pandemic—the GENEVA study. Pediatr Obes. (2021) 16(12):e12832. doi: 10.1111/ijpo.12832
13. Ruurda JP, Can Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl. (2002) 84:223–6. doi: 10.1308/003588402320439621
14. Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. (2004) 239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d
15. Singhal R, Cardoso VR, Wiggins T, Super J, Ludwig C, Gkoutos GV, et al. 30-day morbidity and mortality of sleeve gastrectomy, Roux-en-Y gastric bypass and one anastomosis gastric bypass: a propensity score-matched analysis of the GENEVA data. Int J Obes. (2022) 46(4):750–7. doi: 10.1038/s41366-021-01048-1
16. Ferede AA, Walsh AL, Davis NF, Smyth G, Mohan P, Power R, et al. Warm ischemia time at vascular anastomosis is an independent predictor for delayed graft function in kidney transplant recipients. Exp Clin Transplant. (2020) 18(1):13–8. doi: 10.6002/ect.2018.0377
17. Pavone G, Gerundo A, Pacilli M, Fersini A, Ambrosi A, Tartaglia N. Bariatric surgery: to bleed or not to bleed? This is the question. BMC Surg. (2022) 22(1):331. doi: 10.1186/s12893-022-01783-w
18. Kersebaum JN, Möller T, von Schönfels W, Taivankhuu T, Becker T, Egberts JH, et al. Robotic Roux-en-Y gastric bypass procedure guide. JSLS. (2020) 24(4):e2020.00062. doi: 10.4293/JSLS.2020.00062
19. Heylen L, Pirenne J, Samuel U, Tieken I, Naesens M, Sprangers B, et al. The impact of anastomosis time during kidney transplantation on graft loss: a eurotransplant cohort study. Am J Transplant. (2017) 17:724–32. doi: 10.1111/ajt.2017.17.issue-3
20. Singhal R, Omar I, Madhok B, Ludwig C, Tahrani AA, Mahawar K, et al. Effect of BMI on safety of bariatric surgery during the COVID-19 pandemic, procedure choice, and safety protocols - an analysis from the GENEVA study. Obes Res Clin Pract. (2022) 16(3):249–53. doi: 10.1016/j.orcp.2022.06.003
21. Papasavas P, Seip RL, Stone A, Staff I, McLaughlin T, Tishler D. Robot-assisted sleeve gastrectomy and Roux-en-Y gastric bypass: results from the metabolic and bariatric surgery accreditation and quality improvement program data registry. Surg Obes Relat Dis. (2019) 15(8):1281–90. doi: 10.1016/j.soard.2019.04.003
22. Zhang Z, Miao L, Ren Z, Li Y. Robotic bariatric surgery for the obesity: a systematic review and meta-analysis. Surg Endosc. (2021) 35(6):2440–56. doi: 10.1007/s00464-020-08283-z
23. Singhal R, Omar I, Madhok B, Rajeev Y, Graham Y, Tahrani AA, et al. Safety of bariatric surgery in ≥ 65-year-old patients during the COVID-19 pandemic. Obes Surg. (2022) 32(7):1–13. doi: 10.1007/s11695-022-06067-z
24. Dreifuss NH, Mangano A, Hassan C, Masrur MA. Robotic revisional bariatric surgery: a high-volume center experience. Obes Surg. (2021) 31(4):1656–63. doi: 10.1007/s11695-020-05174-z
25. Talamini MA, Chapman S, Horgan S, Melvin WS; Academic Robotics Group. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc. (2003) 17:1521–4. doi: 10.1007/s00464-002-8853-3
26. Pinzon D, Byrns S, Zheng B. Prevailing trends in haptic feedback simulation for minimally invasive surgery. Surg Innov. (2016) 23:415–21. doi: 10.1177/1553350616628680
27. Pavone G, Fersini A, Pacilli M, Cianci P, Ambrosi A, Tartaglia N. Anastomotic leak test using indocyanine green during laparoscopic Roux-en-Y gastric bypass: a cohort study. Ann Med Surg. (2022) 84:104939. doi: 10.1016/j.amsu.2022.104939
28. Van der Meijden OA, Schijven MP. The value of haptic feedback in conventional and robot assisted minimal invasive surgery and virtual reality training: a current review. Surg Endosc. (2009) 23:1180–90. doi: 10.1007/s00464-008-0298-x
29. Buchs NC, Pugin F, Bucher P, Hagen ME, Chassot G, Koutny-Fong P, et al. Learning curve for robot-assisted Roux-en-Y gastric bypass. Surg Endosc. (2012) 26(4):1116–21. doi: 10.1007/s00464-011-2008-3
30. Wilson EB, Toder M, Snyder BE, Wilson TD, Kim K. Favorable early complications of robotic assisted gastric bypass from three high volume centers: 1,695 consecutive cases. Presented at the 29 th American Society for Metabolic & Bariatric Surgery Annual Meeting; San Diego, CA (2012).
31. Kim KC, Buffington C. Totally robotic gastric bypass: approach and technique. J Robot Surg. (2011) 5(1):47–50. doi: 10.1007/s11701-010-0242-7
Keywords: Roux-en-Y gastric bypass, bariatric surgery, robotic bariatric surgery, laparoscopic surgery, da Vinci robot®
Citation: Pavone G, Pacilli M, Gerundo A, Quazzico A, Ambrosi A and Tartaglia N (2024) Can robotic gastric bypass be considered a valid alternative to laparoscopy? Our early experience and literature review. Front. Surg. 11:1303351. doi: 10.3389/fsurg.2024.1303351
Received: 27 September 2023; Accepted: 18 January 2024;
Published: 5 February 2024.
Edited by:
Juan José Segura-Sampedro, Balearic Islands Health Research Institute (IdISBa), SpainReviewed by:
Salvatore Tramontano, University of Salerno, ItalyMaria Rosaria Valenti, University of Catania, Italy
© 2024 Pavone, Pacilli, Gerundo, Quazzico, Ambrosi and Tartaglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanna Pavone Z2lvdmFubmEucGF2b25lQHVuaWZnLml0
 Giovanna Pavone
Giovanna Pavone Mario Pacilli
Mario Pacilli Alberto Gerundo
Alberto Gerundo Antonio Ambrosi
Antonio Ambrosi Nicola Tartaglia
Nicola Tartaglia