
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 24 April 2024
Sec. Visceral Surgery
Volume 11 - 2024 | https://doi.org/10.3389/fsurg.2024.1280617
Introduction: The easy albumin-bilirubin (EZ-ALBI) score is calculated using the equation: total bilirubin (mg/dl) − 9 × albumin (g/dl), and is used to evaluate liver functional reserve. This study was designed to investigate whether the EZ-ALBI score serves as an independent risk factor for mortality and is useful for stratifying the mortality risk in adult trauma patients.
Methods: We retrospectively reviewed data from the registered trauma database of the hospital and included 3,637 adult trauma patients (1,241 deaths and 2,396 survivors) due to all trauma caused between January 1, 2009, and December 31, 2021. The patients were allocated to the two study groups based on the best EZ-ALBI cutoff point (EZ-ALBI = −28.5), which was determined based on the area under the receiver operating characteristic curve.
Results: Results revealed that the non-survivors had a significantly higher EZ-ALBI score than the survivors (−26.4 ± 6.5 vs. −31.5 ± 6.2, p < 0.001). Multivariate logistic regression analysis revealed that EZ-ALBI ≥ −28.5was an independent risk factor for mortality (odds ratio, 2.31; 95% confidence interval, 1.63–3.28; p < 0.001). Patients with an EZ-ALBI score ≥ −28.5 presented with 2.47-fold higher adjusted mortality rates than patients with an EZ-ALBI score < −28.5. A propensity score-matched pair cohort of 1,236 patients was developed to reduce baseline disparities in trauma mechanisms. The analysis showed that patients with an EZ-ALBI score ≥ −28.5 had a 4.12 times higher mortality rate compared to patients with an EZ-ALBI score < −28.5.
Conclusion: The EZ-ALBI score was a significant independent risk factor for mortality and can serve as a valuable tool for stratifying mortality risk in adult trauma patients by all trauma causes.
Because liver function is perceived as a competing issue related to patient mortality, the assessment of the liver function reserve is particularly important in the clinical setting (1). In addition to the traditional evaluation tools of the model for end-stage liver disease (MELD) score (2) or the Child-Turcotte-Pugh (CTP) classification (3), an alternative measure of liver function based solely on albumin and bilirubin, the albumin-bilirubin (ALBI) score, was proposed in international collaboration as a simple and objective method for the assessment of liver function in patients with hepatocellular carcinoma (4). Since its introduction, the ALBI score has been validated by several research groups to predict the outcome of patients with resectable or locally advanced hepatoma (5–9) as well as in those with advanced hepatoma receiving local or systemic therapy (6, 8–12). Additionally, it serves as an important biomarker for liver disease progression to reflect the possibility of hepatic failure and liver-related mortality (13–19). ALBI grade is also useful as a prognostic factor in patients with cholangiocarcinoma (20), intrahepatic cholangiocarcinoma (21), colorectal cancer with liver metastases (22), pancreatic cancer with liver metastases (23), and primary biliary cholangitis (24). Furthermore, a strong association between ALBI and mortality has been identified in many non-hepatological conditions, such as gastric cancer (25), lung cancer (16, 26, 27),esophageal cancer (28), glioma (29), medulloblastoma (30), heart failure (31, 32), acute pancreatitis (33), and aortic dissection (34).
The ALBI score is calculated using the following formula: [(log10 bilirubin (μmol/L) × 0.66) + [albumin (g/L) × −0.0852]. The complexity of the calculation of the ALBI score limits its applicability. Therefore, an easy-ALBI (EZ-ALBI) score was recently developed to replace the ALBI score based on the regression coefficients of serum albumin and bilirubin levels using a multivariate Cox proportional hazards model, and calculated by the equation: total bilirubin (mg/dl) − 9 × albumin (g/dl) (35). The EZ-ALBI score showed a high linear correlation (correlation coefficient, 0.965; p < 0.001) with the ALBI score in the entire cohort and different subgroups of patients with hepatoma (36). With easy calculation and a more user-friendly assessment, the EZ-ALBI score can evaluate liver functional reserve in patients with liver diseases receiving various treatment modalities (35–39).
While the liver plays a significant role in producing albumin, there are several other factors that can impact the albumin levels in the body, especially in the context of trauma patients, including increased capillary permeability and fluid shifts, inflammatory response, malnutrition, and impaired renal function (40–47). In addition, the level of bilirubin, a breakdown product of hemoglobin from red blood cells and primarily processed and excreted by the liver, may be influenced not only by liver function but also by hemolysis and processing of cell-free hemoglobin from the circulation (48–50). Under the hypothesis that the EZ-ALBI score may be associated with the mortality risk of trauma patients, this study aimed to investigate whether the EZ-ALBI score serves as an independent risk factor for mortality and is useful for stratifying the mortality risk of adult patients with all trauma causes. In this study, the primary outcome was the in-hospital mortality rate.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Chang Gung Memorial Hospital (protocol code 202201380B0 and date of approval 2022/09/15). The need for informed consent was waived according to the IRB regulations because of its retrospective study of design.
There were 46,808 hospitalized patients injured by all trauma causes in the Trauma Registry System of the Chang Gung Memorial Hospital between January 1, 2009, and December 31, 2021 (51–54) (Figure 1). Of the 41,131 adult patients aged ≥20 years, after excluding patients who lacked data on albumin or bilirubin (n = 36,432), those with burn injuries (n = 1,040), hanging injuries (n = 19), and patients who drowned (n = 3), 3,637 adult trauma patients were included in the study population. The major liver injuries indicated that the trauma patients had suffered an abbreviated injury scale (AIS) ≥ 3 liver injury in the abdomen. We retrieved the medical information of the study population from a registered trauma database. The data included sex, age, levels at admission of serum albumin, total bilirubin, glucose, white blood cells count, hemoglobin (Hb), hematocrit (Hct), platelets, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine, trauma regions, trauma mechanism, pre-existing comorbidities, a Glasgow Coma Scale (GCS) score, an Injury Severity Score (ISS), hospital length of stay (LOS), and in-hospital mortality. The EZ-ALBI score was calculated according to the equation: total bilirubin (mg/dl) − 9 × albumin (g/dl).
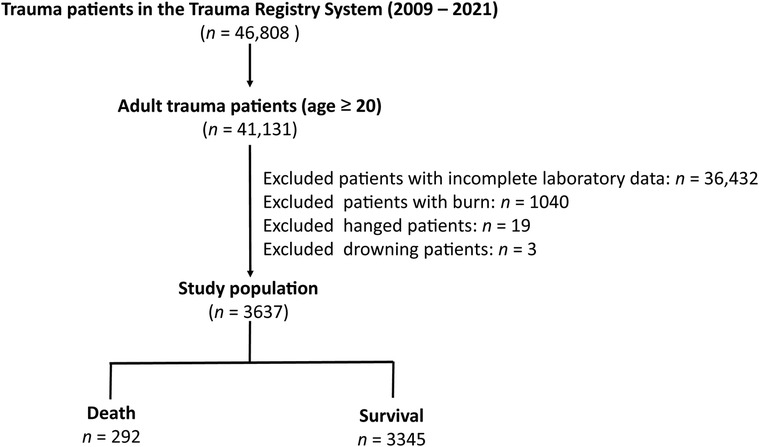
Figure 1. Flowchart illustrating the inclusion of hospitalized adult trauma patients by all trauma causes from the registered trauma database. Those patients who lacked the albumin or bilirubin data, who had burn injury, hang injuries, and drown, were excluded from the study population. The study population were assigned into death and survival two groups.
Categorical data were compared using a two-sided Fisher's exact test. Normally distributed continuous data were estimated using the Kolmogorov–Smirnov test. Non-normally distributed continuous data were analyzed using the Mann–Whitney U-test, and continuous data with a normal distribution were compared using analysis of variance with Bonferroni post hoc correction. Continuous data are expressed as the mean ± standard deviation. Non-normal distributed continuous data are presented as medians with interquartile ranges (IQR) between Q1 and Q3. Multivariate logistic regression was used to analyze the univariate predictive variables, resulting in patient mortality and identify independent risk factors for mortality. The predictive performance of EZ-ALBI for patient mortality was determined based on the area under the curve (AUC) of the receiver operating characteristic curve (ROC). Based on a value determined using sensitivity + specificity − 1, the maximal Youden index, the best cutoff point was derived from ROC. In addition to the comparison between the death and survival groups of patients, a further comparison of the patients allocated into two groups based on the best cutoff point of the EZ-ALBI value was performed with the presentation of an adjusted odds ratio (AOR) of mortality with 95% confidence intervals (CIs), calculated using logistic regression under the control of variables with significant differences in patient injury characteristics. To effectively account for any initial differences in baseline characteristics among patient groups divided by the best cutoff point of the EZ-ALBI value, particularly the impact of different trauma mechanisms, a cohort with a 1:1 propensity score matching was created using the Greedy strategy with a caliper width of 0.2 and the NCSS 10 software (NCSS Statistical program, Kaysville, Utah, USA). The statistical analyses were performed using SPSS Statistics (version 23.0; IBM Corp., Armonk, NY, USA). Statistical significance was set at p < 0.05.
A comparison between 292 deceased and 3,345 surviving patients revealed that the non-survivors comprised significantly more males and were older than the surviving patients (Table 1). Patients who died had a significantly higher EZ-ALBI score than those who survived (−26.4 ± 6.5 vs. −31.5 ± 6.2, p < 0.001). The non-survivors had a significantly lower serum albumin level than the survivors (3.1 ± 0.8 vs. 3.6 ± 0.7, p < 0.001), while there was no significant difference in total bilirubin level between these two groups of patients (1.1 ± 0.9 vs. 1.0 ± 1.3, p = 0.173). The non-survivors had a significantly different level of glucose, AST, ALT, BUN, Cr than the survivors. The non-survivors had more incidences of AIS ≥ 3 injuries to head/neck and external body regions but fewer AIS ≥ 3 injuries to extremities than the survivors. Regarding comorbidities, significantly higher rates of pre-existing comorbidities of coronary artery disease (CAD), end-stage renal disease (ESRD), and liver cirrhosis were found in patients who died than in those who survived. Patients who died presented with a significantly lower GCS (median [IQR, Q1–Q3], GCS: 7 ([3–15]) vs. 15 ([13–15]), p < 0.001) but a higher ISS (25 ([16–29]) vs. 12 ([9–20]), p < 0.001) than patients who survived. Patients who died had a significantly shorter hospitalization period than those who survived (14.2 vs. 17.7 days, p = 0.001).
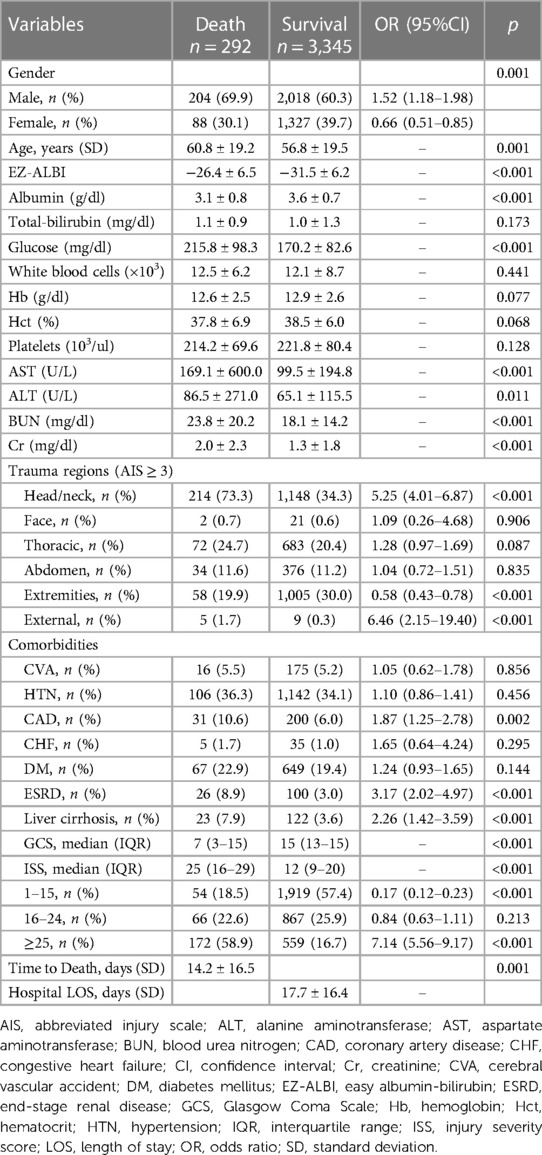
Table 1. Comparison of the injuries and patient characteristics of death and survival patients in the study population.
A comparison of the injuries and patient characteristics of the patients with and without major liver injuries revealed the patients with major liver injuries were significantly younger, had fewer incidences of HTN and DM, sustained significantly more severe injuries, and stayed longer in the hospital than those without major liver injuries (Table 2). However, these two groups of patients with or without major liver injury did not present significant differences in the level of albumin, total bilirubin, or the derived value of EZ-ALBI. The mortality and adjusted mortality corrected by age, incidences of HTN and DM, and ISS between the patients with and without major liver injuries did not present significant differences.
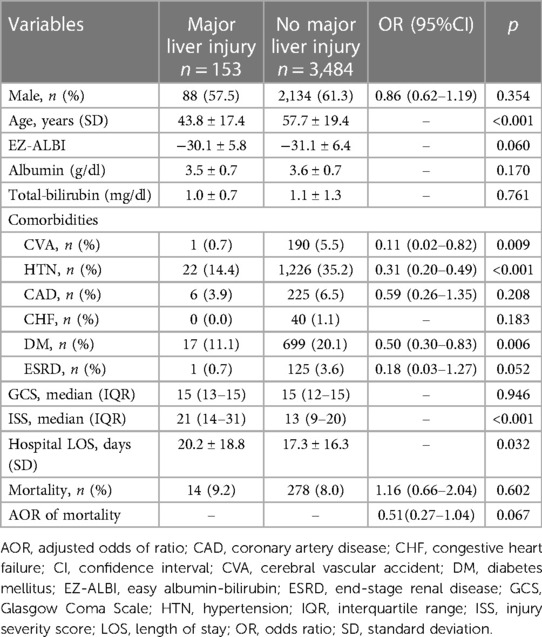
Table 2. Comparison of the injuries and patient characteristics of the trauma patients with and without major liver injury.
Based on the ROC analysis, the optimal albumin level was determined to be 3.33 g/dl, with a sensitivity of 0.600 and a specificity of 0.616 (Figure 2). The optimal EZ-ALBI score was determined to be −28.5, with a sensitivity of 0.637 and a specificity of 0.685 (Figure 2). Albumin alone and EZ-ALBI had AUCs of 0.67 and 0.72, respectively, as shown in Figure 2. The ability of EZ-ALBI alone to predict patient mortality was moderately accurate and superior to that of albumin alone (p = 0.046).
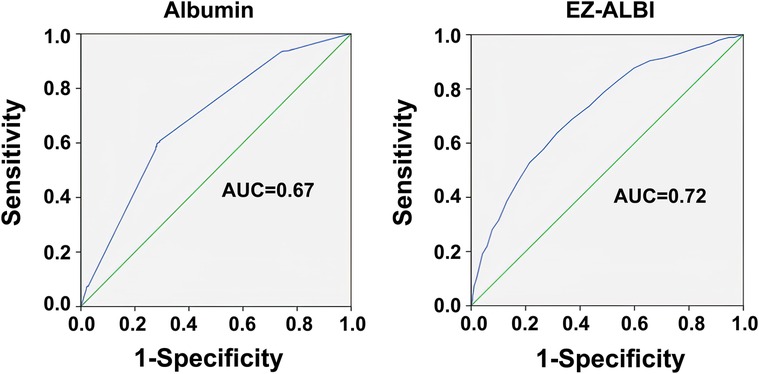
Figure 2. Receiver operating characteristic curves and area under the curve (AUC) of the EZ-ALBI score for predicting the mortality of the adult trauma patients by all trauma.
Univariate analysis revealed that sex, age, the presence of EZ-ALBI score ≥ −28.5, the level of glucose, AST, ALT, BUN, Cr, the presence of an injury of AIS ≥ 3 in the head/neck, extremities, or external, the presence of CAD, ESRD, or liver cirrhosis, the GCS score, and the ISS were significant risk factors for mortality in the study population (Table 3). Multivariate logistic regression analysis of these risk factors revealed that the presence of EZ-ALBI score ≥ −28.5 (OR, 2.31; 95% CI, 1.63–3.28; p < 0.001) was an independent risk factor for mortality. Additionally, age (OR, 1.02; 95% CI, 1.01–1.04; p < 0.001), the glucose level (OR, 1.23; 95% CI, 1.05–1.45 p = 0.011), injury to the external body region (OR, 9.48; 95% CI, 1.76–50.96 p = 0.009), liver cirrhosis (OR, 2.87; 95% CI, 1.42–5.78; p = 0.003), GCS (OR, 0.86; 95% CI, 0.83–0.90; p < 0.001), and ISS (OR, 1.05; 95% CI, 1.03–1.07; p < 0.001) were significant independent risk factors for mortality in these patients.
There was no significant difference in sex between patients with an EZ-ALBI score ≥ −28.5 and patients with an EZ-ALBI score < −28.5 (Table 4). Patients with an EZ-ALBI score ≥ −28.5 were significantly older than those with an EZ-ALBI score < −28.5 (p < 0.001). A significantly higher rate of an injury of AIS ≥ 3 in head/neck, thoracic, abdomen, and extremities body regions was found in patients with an EZ-ALBI score ≥ −28.5 compared to those with EZ-ALBI scores < −28.5. A significantly lower rate of pre-existing CVA, but no other comorbidities, was found in patients with an EZ-ALBI score ≥ −28.5 compared to those with EZ-ALBI scores < −28.5. Patients with an EZ-ALBI score ≥ −28.5 presented with a significantly lower GCS but a higher ISS than those with an EZ-ALBI score < −28.5 (GCS: 15 ([9–15]) vs. 15 ([14–15]), p < 0.001; ISS: 16 ([9–25]) vs. 9 ([8–18]), p < 0.001). Patients with an EZ-ALBI score ≥ −28.5 presented with a significantly higher mortality rate than patients with an EZ-ALBI score < −28.5 (15.1% vs. 4.4%, p < 0.001). Under the control of age, pre-existing CVA, GCS, and ISS, patients with an EZ-ALBI score ≥ −28.5 still presented with a significantly higher adjusted mortality rate than patients with an EZ-ALBI score < −28.5 (AOR, 2.47; 95% CI: 1.90–3.22, p = 0.001). Patients with an EZ-ALBI score ≥ −28.5 had significantly shorter hospitalization periods than those with an EZ-ALBI score < −28.5 (23.8 vs. 14.1 days, p = 0.001).
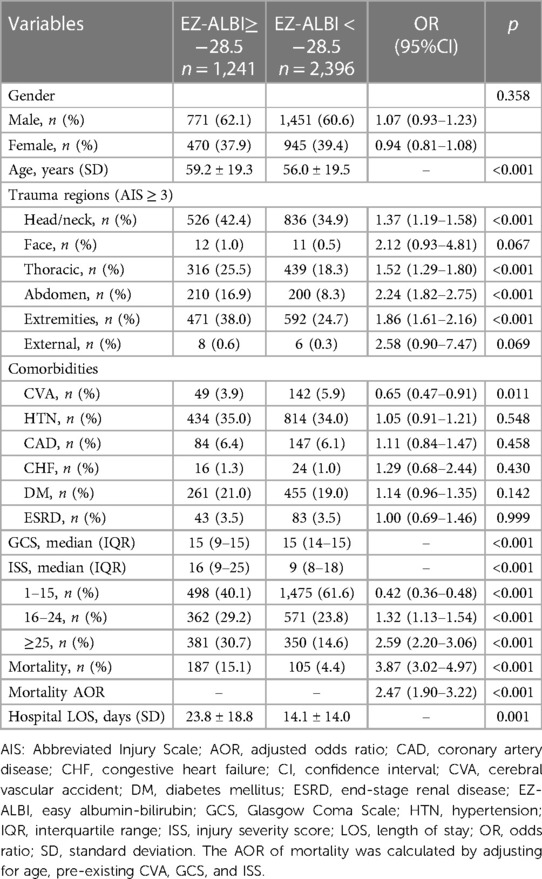
Table 4. Comparison of the injury, characteristics, and outcomes of patients with an EZ-ALBI score ≥ −28.5 vs. those with an EZ-ALBI score < −28.5.
For patients with or without EZ-ALBI scores ≥ −28.5, a propensity score-matched patient cohort of 1:1 (Table 5) was established to reduce the influence of confounding factors related to the patients’ baseline characteristics of trauma mechanisms on outcome assessments. The propensity score-matched patient populations, comprising 1,236 pairings, exhibited no statistically significant variations in terms of trauma mechanisms, including traffic accidents, fall, strike by/against objects, suicide, and electric injury. Patients with an EZ-ALBI score ≥ −28.5 presented with a significantly higher mortality (OR, 4.12; 95% CI, 2.99–5.67, p < 0.001) and longer LOS in the hospital (23.8 days vs. 14.8 days, p < 0.001) than those with an EZ-ALBI score < −28.5.
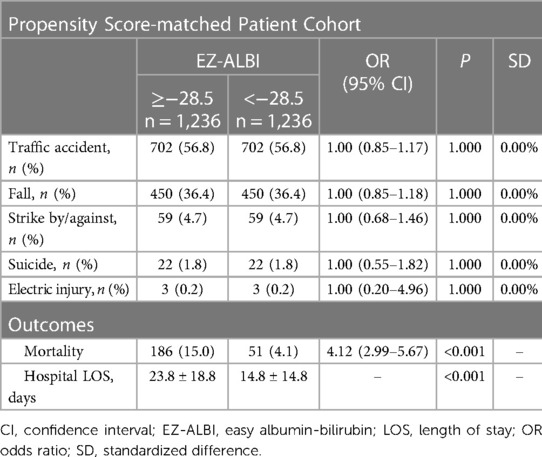
Table 5. Comparison of outcomes of propensity score-matched cohort of patients with an EZ-ALBI score ≥ −28.5 vs. those with an EZ-ALBI score < −28.5.
In this study, patients who died were significantly associated with a higher EZ-ALBI score than those who survived, and those with an EZ-ALBI score ≥ −28.5 presented with a 2.47-fold adjusted mortality rate compared to patients with an EZ-ALBI score < −28.5. The analysis in a propensity score-matched pair cohort of 1,236 patients, which was developed to reduce baseline disparities in trauma mechanisms, also showed that patients with an EZ-ALBI score ≥ −28.5 had a 4.12 times higher mortality rate compared to patients with an EZ-ALBI score < −28.5. The results revealed that the EZ-ALBI score was a significant independent risk factor for mortality in adult trauma patients due to all trauma causes and presented with a significant better predictive power for mortality than the use of albumin alone. Therefore, EZ-ALBI may serve as a valuable tool to stratify the mortality risk of adult trauma patients.
The severity of liver dysfunction is often estimated using the MELD score or CTP classification. MELD is a continuous score derived from the calculation of serum creatinine and bilirubin levels and the international normalized PT ratio (55–57). However, MELD has been widely adopted for end-stage cirrhotic patients awaiting liver transplantation (2) and is specifically designed for patients with end-stage cirrhosis (58–60). The application of the MELD score in patients with less severe liver dysfunction has been criticized (4). In addition, the CTP classification system incorporates five different factors, including serum levels of total bilirubin, albumin, and prothrombin time, and two clinical symptom indicators, ascites and hepatic encephalopathy (3). It has been argued that the variable of ascites is intercorrelated with albumin, whereas it is difficult to subjectively assess and consistently score ascites and hepatic encephalopathy among different investigators (61); and the CTP score is limited by the arbitrary determination of cutoff values of objective laboratory variables with equal weighting of five parameters (62). Moreover, a literature review revealed that there were more than 30 versions of the CTP classification, making it difficult to achieve consistent scoring (63). Hence, the potential superiority of EZ-ALBI over CTP as a prognostic indicator for death in trauma patients is an intriguing subject that warrants additional research and exploration.
The EZ-ALBI score is a combination of two indicators, total bilirubin and albumin, which include both metabolic function (total bilirubin) and synthesis function (albumin) of the liver (64). Albumin and bilirubin levels are also frequently measured as part of the assessment of liver function and general health when conducting clinical practice. Increased serum bilirubin concentrations frequently indicate variable degrees of liver failure, serving as a predictor of liver performance in many prognostic models such as the Acute Physiology and Chronic Health Evaluation (APACHE) score (65), the Sequential Organ Failure Assessment (SOFA) score (66), Simplified Acute Physiology Score (SAPS II) (67), Logistic Organ Dysfunction Score (LODS) (68), and Multiple Organ Dysfunction Score (MODS) (69). Around 40 percent of critically ill patients have elevated bilirubin levels in the blood, which is associated with increased mortality and adverse outcomes (70). In addition, the decreased level of albumin, which is synthesized in the liver, suggests dysfunction in liver synthesis and malnutrition. Hypoalbuminemia may indicate malnutrition or inflammation, both of which are common in hospitalized patients (71). Inadequate albumin levels may lead to fluid imbalances, potentially producing edema, interfering with heart function, and increasing characteristics associated with poorer outcomes in trauma patients (72–75). Furthermore, albumin levels may indicate the degree of damage and total physiological stress, acting as a predictor of complications, prolonged ICU admission, and higher mortality risk (71). However, in contrast to bilirubin, albumin levels were not generally regarded as a principal variable in the majority of intensive care unit prediction models. Notably, these two groups of patients did not present significant differences in the level of albumin, total bilirubin, or the derived value of EZ-ALBI. The mortality and adjusted mortality corrected by age, incidences of HTN and DM, and ISS between the patients with and without major liver injuries did not present significant differences. It should be recognized that the liver dysfunction is not the only way for albumin and bilirubin to be changed by trauma. Although the mechanism underlying the prognostic impact of albumin and bilirubin remains undetermined, the ALBI approach based on laboratory data avoids interobserver variation and is superior to CTP in identifying patients with distinct prognostic subgroups within CTP (65). With easy calculation and assessment, EZ-ALBI may serve as a useful marker to help identify adult trauma patients with a high mortality risk.
This study has some limitations. First, there may have been a selection bias due to the retrospective design of this study. Second, management, such as damage control, blood transfusion, resuscitation, and surgical interventions, could have led to different outcomes in the study population; Furthermore, the physiology and nutritional condition, the laboratory data presented in the emergency room, the trauma mechanisms, and the injured regions can influence the patients' survival. All of these factors may introduce bias into the relationship assessment with the mortality outcome; however, we can only assume that the outcomes of these methods were uniform across the study population. Third, this study evaluated only in-hospital mortality and not the death declared upon arrival at the emergency room or long-term mortality; therefore, a selection bias may exist regarding comparing the outcomes. In addition, the exclusion of patients due to lack of bilirubin and albumin data resulted in the exclusion of a vast majority of trauma patients and may have resulted in selection bias. Fourth, this study included trauma patients due to all trauma causes and did not specify or exclude patients with liver injury. The impact of the liver injury on the application of EZ-ALBI in patients with trauma deserves further investigation. Finally, the study population was limited to a single urban trauma center; therefore, the generalizability of the results to other regions may be limited.
This study revealed that the EZ-ALBI score was a significant independent risk factor for mortality and can serve as a valuable tool for stratifying mortality risk in adult trauma patients by all trauma causes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Chang Gung Medical Foundation Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the need for informed consent was waived according to the IRB regulations because of its retrospective study of design.
S-YH: Formal Analysis, Funding acquisition, Writing – original draft. C-SR: Writing – review & editing. C-HT: Resources, Writing – review & editing. S-EC: Data curation, Writing – review & editing. W-TS: Data curation, Writing – review & editing. C-HH: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was funded by Chang Gung Memorial Hospital, grant number CMRPG8L1511 and CMRPG8N1451 to S-YH.
We appreciate the assistance of the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for statistical analyses.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol. (2018) 68(3):563–76. doi: 10.1016/j.jhep.2017.10.020
2. Singal AK, Kamath PS. Model for end-stage liver disease. J Clin Exp Hepatol. (2013) 3(1):50–60. doi: 10.1016/j.jceh.2012.11.002
3. Cholongitas E, Senzolo M, Patch D, Shaw S, Hui C, Burroughs AK. Review article: scoring systems for assessing prognosis in critically ill adult cirrhotics. Aliment Pharmacol Ther. (2006) 24(3):453–64. doi: 10.1111/j.1365-2036.2006.02998.x
4. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. (2015) 33(6):550–8. doi: 10.1200/jco.2014.57.9151
5. Liu PH, Hsu CY, Hsia CY, Lee YH, Chiou YY, Huang YH, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD era. J Gastroenterol Hepatol. (2017) 32(4):879–86. doi: 10.1111/jgh.13608
6. Demirtas CO, D'Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. (2021) 3(5):100347. doi: 10.1016/j.jhepr.2021.100347
7. Marasco G, Alemanni LV, Colecchia A, Festi D, Bazzoli F, Mazzella G, et al. Prognostic value of the albumin-bilirubin grade for the prediction of post-hepatectomy liver failure: a systematic review and meta-analysis. J Clin Med. (2021) 10(9):2011. doi: 10.3390/jcm10092011
8. Mishra G, Majeed A, Dev A, Eslick GD, Pinato DJ, Izumoto H, et al. Clinical utility of albumin bilirubin grade as a prognostic marker in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a systematic review and meta-analysis. J Gastrointest Cancer. (2022) 54(2):420–32. doi: 10.1007/s12029-022-00832-0
9. Young LB, Tabrizian P, Sung J, Biederman D, Bishay VL, Ranade M, et al. Survival analysis using albumin-bilirubin (ALBI) grade for patients treated with drug-eluting embolic transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. (2022) 33(5):510–7.e1. doi: 10.1016/j.jvir.2022.02.005
10. Azar A, Devcic Z, Paz-Fumagalli R, Vidal LLC, McKinney JM, Frey G, et al. Albumin-bilirubin grade as a prognostic indicator for patients with non-hepatocellular primary and metastatic liver malignancy undergoing yttrium-90 radioembolization using resin microspheres. J Gastrointest Oncol. (2020) 11(4):715–23. doi: 10.21037/jgo.2020.04.01
11. Geng L, Zong R, Shi Y, Xu K. Prognostic role of preoperative albumin-bilirubin grade on patients with hepatocellular carcinoma after surgical resection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. (2020) 32(7):769–78. doi: 10.1097/meg.0000000000001618
12. Pang Q, Zhou S, Liu S, Liu H, Lu Z. Prognostic role of preoperative albumin-bilirubin score in posthepatectomy liver failure and mortality: a systematic review and meta-analysis. Updates Surg. (2022) 74(3):821–31. doi: 10.1007/s13304-021-01080-w
13. Campani C, Bamba-Funck J, Campion B, Sidali S, Blaise L, Ganne-Carrié N, et al. Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab-bevacizumab. Liver Int. (2023) 43(3):708–17. doi: 10.1111/liv.15487
14. Chen CW, Kuo CJ, Lee CW, Kuo T, Chiu CT, Lin CJ, et al. Albumin-bilirubin grade as a novel predictor of the development and short-term survival of post-banding ulcer bleeding following endoscopic variceal ligation in cirrhotic patients. Medicina (Kaunas). (2022) 58(12):1836. doi: 10.3390/medicina58121836
15. Kim TH, Kim BH, Park JW, Cho YR, Koh YH, Chun JW, et al. Proton beam therapy for treatment-naïve hepatocellular carcinoma and prognostic significance of albumin-bilirubin (ALBI) grade. Cancers (Basel). (2022) 14(18):4445. doi: 10.3390/cancers14184445
16. Shi XR, Xu XY, Zhang GL, Jiang JY, Cao DD. The prognostic role of albumin-bilirubin grade in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Eur Rev Med Pharmacol Sci. (2022) 26(20):7687–94. doi: 10.26355/eurrev_202210_30045
17. Wang R, Katz D, Lin HM, Ouyang Y, Gal J, Suresh S, et al. A retrospective study of the role of perioperative serum albumin and the albumin-bilirubin grade in predicting post-liver transplant length of stay. Semin Cardiothorac Vasc Anesth. (2023) 27(1):16–24. doi: 10.1177/10892532221141138
18. Xu FQ, Ye TW, Wang DD, Xie YM, Zhang KJ, Cheng J, et al. Association of preoperative albumin-bilirubin with surgical textbook outcomes following laparoscopic hepatectomy for hepatocellular carcinoma. Front Oncol. (2022) 12:964614. doi: 10.3389/fonc.2022.964614
19. Toyoda H, Johnson PJ. The ALBI score: from liver function in patients with HCC to a general measure of liver function. JHEP Rep. (2022) 4(10):100557. doi: 10.1016/j.jhepr.2022.100557
20. Tsilimigras DI, Hyer JM, Moris D, Sahara K, Bagante F, Guglielmi A, et al. Prognostic utility of albumin-bilirubin grade for short- and long-term outcomes following hepatic resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 706 patients. J Surg Oncol. (2019) 120(2):206–13. doi: 10.1002/jso.25486
21. Ni JY, An C, Zhang TQ, Huang ZM, Jiang XY, Huang JH. Predictive value of the albumin-bilirubin grade on long-term outcomes of CT-guided percutaneous microwave ablation in intrahepatic cholangiocarcinoma. Int J Hyperthermia. (2019) 36(1):328–36. doi: 10.1080/02656736.2019.1567834
22. Abdel-Rahman O. Prognostic value of baseline ALBI score among patients with colorectal liver metastases: a pooled analysis of two randomized trials. Clin Colorectal Cancer. (2019) 18(1):e61–8. doi: 10.1016/j.clcc.2018.09.008
23. Sakin A, Sahin S, Sakin A, Atci MM, Yasar N, Arici S, et al. Assessment of pretreatment albumin-bilirubin grade in pancreatic cancer patients with liver metastasis. J Buon Jul. (2020) 25(4):1941–6. 33099936.
24. Ito T, Ishigami M, Morooka H, Yamamoto K, Imai N, Ishizu Y, et al. The albumin-bilirubin score as a predictor of outcomes in Japanese patients with PBC: an analysis using time-dependent ROC. Sci Rep. (2020) 10(1):17812. doi: 10.1038/s41598-020-74732-3
25. Kanda M, Tanaka C, Kobayashi D, Uda H, Inaoka K, Tanaka Y, et al. Preoperative albumin-bilirubin grade predicts recurrences after radical gastrectomy in patients with pT2-4 gastric cancer. World J Surg. (2018) 42(3):773–81. doi: 10.1007/s00268-017-4234-x
26. Kinoshita F, Yamashita T, Oku Y, Kosai K, Ono Y, Wakasu S, et al. Prognostic impact of albumin-bilirubin (ALBI) grade on non-small lung cell carcinoma: a propensity-score matched analysis. Anticancer Res. (2021) 41(3):1621–8. doi: 10.21873/anticanres.14924
27. Matsukane R, Watanabe H, Hata K, Suetsugu K, Tsuji T, Egashira N, et al. Prognostic significance of pre-treatment ALBI grade in advanced non-small cell lung cancer receiving immune checkpoint therapy. Sci Rep. (2021) 11(1):15057. doi: 10.1038/s41598-021-94336-9
28. Aoyama T, Ju M, Machida D, Komori K, Tamagawa H, Tamagawa A, et al. Clinical impact of preoperative albumin-bilirubin Status in esophageal cancer patients who receive curative treatment. In Vivo. (2022) 36(3):1424–31. doi: 10.21873/invivo.12847
29. Zhang J, Xu Q, Zhang H, Zhang Y, Yang Y, Luo H, et al. High preoperative albumin-bilirubin score predicts poor survival in patients with newly diagnosed high-grade gliomas. Transl Oncol. (2021) 14(4):101038. doi: 10.1016/j.tranon.2021.101038
30. Zhu S, Cheng Z, Hu Y, Chen Z, Zhang J, Ke C, et al. Prognostic value of the systemic immune-inflammation index and prognostic nutritional Index in patients with medulloblastoma undergoing surgical resection. Front Nutr. (2021) 8:754958. doi: 10.3389/fnut.2021.754958
31. Kawata T, Ikeda A, Masuda H, Komatsu S. Association between albumin-bilirubin score at admission and in-hospital mortality in patients with acute heart failure. Int Heart J. (2021) 62(4):829–36. doi: 10.1536/ihj.21-080
32. Han S, Wang C, Tong F, Li Y, Li Z, Sun Z, et al. Prognostic impact of albumin-bilirubin score on the prediction of in-hospital mortality in patients with heart failure: a retrospective cohort study. BMJ Open. (2022) 12(1):e049325. doi: 10.1136/bmjopen-2021-049325
33. Shi L, Zhang D, Zhang J. Albumin-bilirubin score is associated with in-hospital mortality in critically ill patients with acute pancreatitis. Eur J Gastroenterol Hepatol. (2020) 32(8):963–70. doi: 10.1097/meg.0000000000001753
34. Liu J, Wu M, Xie E, Chen L, Su S, Zeng H, et al. Assessment of liver function for evaluation of short- and long-term outcomes in type B aortic dissection patients undergoing thoracic endovascular aortic repair. Front Cardiovasc Med. (2021) 8:643127. doi: 10.3389/fcvm.2021.643127
35. Kariyama K, Nouso K, Hiraoka A, Wakuta A, Oonishi A, Kuzuya T, et al. EZ-ALBI score for predicting hepatocellular carcinoma prognosis. Liver Cancer. (2020) 9(6):734–43. doi: 10.1159/000508971
36. Ho SY, Liu PH, Hsu CY, Ko CC, Huang YH, Su CW, et al. Easy albumin-bilirubin score as a new prognostic predictor in hepatocellular carcinoma. Hepatol Res. (2021) 51(11):1129–38. doi: 10.1111/hepr.13671
37. Ananchuensook P, Sriphoosanaphan S, Suksawatamnauy S, Siripon N, Pinjaroen N, Geratikornsupuk N, et al. Validation and prognostic value of EZ-ALBI score in patients with intermediate-stage hepatocellular carcinoma treated with trans-arterial chemoembolization. BMC Gastroenterol. (2022) 22(1):295. doi: 10.1186/s12876-022-02366-y
38. Ho SY, Liu PH, Hsu CY, Huang YH, Liao JI, Su CW, et al. Tumor burden score as a new prognostic surrogate in patients with hepatocellular carcinoma undergoing radiofrequency ablation: role of albumin-bilirubin (ALBI) grade vs easy ALBI grade. Expert Rev Gastroenterol Hepatol. (2022) 16(9):903–11. doi: 10.1080/17474124.2022.2117156
39. Ho SY, Yuan MH, Liu PH, Hsu CY, Huang YH, Liao JI, et al. Cryptogenic hepatocellular carcinoma: characteristics, outcome, and prognostic role of albumin-bilirubin (ALBI) grade vs easy ALBI grade. Scand J Gastroenterol. (2022) 58(1):61–9. doi: 10.1080/00365521.2022.2098052
40. Li S, Zhang J, Zheng H, Wang X, Liu Z, Sun T. Prognostic role of serum albumin, total lymphocyte count, and mini nutritional assessment on outcomes after geriatric hip fracture surgery: a meta-analysis and systematic review. J Arthroplasty. (2019) 34(6):1287–96. doi: 10.1016/j.arth.2019.02.003
41. Mizrahi EH, Fleissig Y, Arad M, Blumstein T, Adunsky A. Admission albumin levels and functional outcome of elderly hip fracture patients: is it that important? Aging Clin Exp Res. (2007) 19(4):284–9. doi: 10.1007/bf03324703
42. Aguirre Puig P, Orallo Morán MA, Pereira Matalobos D, Prieto Requeijo P. Current role of albumin in critical care [papel actual de la albúmina en cuidados críticos]. Rev Esp Anestesiol Reanim. (2014) 61(9):497–504. doi: 10.1016/j.redar.2014.04.016
43. Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: review and meta analysis. Maturitas. (2015) 81(1):17–27. doi: 10.1016/j.maturitas.2015.02.009
44. Cartotto R, Callum J. A review of the use of human albumin in burn patients. J Burn Care Res. Nov. (2012) 33(6):702–17. doi: 10.1097/BCR.0b013e31825b1cf6
45. Hülshoff A, Schricker T, Elgendy H, Hatzakorzian R, Lattermann R. Albumin synthesis in surgical patients. Nutrition. (2013) 29(5):703–7. doi: 10.1016/j.nut.2012.10.014
46. Maimin DG, Laubscher M, Maqungo S, Marais LC. Hypoalbuminaemia in orthopaedic trauma patients in a rural hospital in South Africa. Int Orthop. (2022) 46(1):37–42. doi: 10.1007/s00264-021-05022-4
47. Wiedermann CJ. Moderator effect of hypoalbuminemia in volume resuscitation and plasma expansion with intravenous albumin solution. Int J Mol Sci. (2022) 23(22):14175. doi: 10.3390/ijms232214175
48. Corrons JLV, Casafont LB, Frasnedo EF. Concise review: how do red blood cells born, live, and die? Ann Hematol. (2021) 100(10):2425–33. doi: 10.1007/s00277-021-04575-z
49. Randolph VS. Four clinical chemistry analyses for pediatric patients: glycosylated hemoglobin, free bilirubin, sweat electrolytes, neonatal thyroxine. Am J Med Technol. (1982) 48(1):15–22. 7041647.7041647
51. Hsieh CH, Hsu SY, Hsieh HY, Chen YC. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed J. (2017) 40(2):113–20. doi: 10.1016/j.bj.2016.10.005
52. Hsieh CH, Liu HT, Hsu SY, Hsieh HY, Chen YC. Motorcycle-related hospitalizations of the elderly. Biomed J. (2017) 40(2):121–8. doi: 10.1016/j.bj.2016.10.006
53. Hsieh CH, Chen YC, Hsu SY, Hsieh HY, Chien PC. Defining polytrauma by abbreviated injury scale >/=3 for a least two body regions is insufficient in terms of short-term outcome: a cross-sectional study at a level I trauma center. Biomed J. (2018) 41(5):321–7. doi: 10.1016/j.bj.2018.08.007
54. Tsai CH, Rau CS, Chou SE, Su WT, Hsu SY, Hsieh CH. Delta De ritis ratio is associated with worse mortality outcomes in adult trauma patients with moderate-to-severe traumatic brain injuries. Diagnostics (Basel). (2022) 12(12):3004. doi: 10.3390/diagnostics12123004
55. Bayona Molano MDP, Barrera Gutierrez JC, Landinez G, Mejia A, Haskal ZJ. Updates of the MELD score and impact on the liver transplant waiting list: a narrative review. J Vasc Interv Radiol. (2023) 34(3):337–43. doi: 10.1016/j.jvir.2022.12.029
56. Ge J, Kim WR, Lai JC, Kwong AJ. “Beyond MELD”—emerging strategies and technologies for improving mortality prediction, organ allocation and outcomes in liver transplantation. J Hepatol. (2022) 76(6):1318–29. doi: 10.1016/j.jhep.2022.03.003
57. Sharma P. Value of liver function tests in cirrhosis. J Clin Exp Hepatol. (2022) 12(3):948–64. doi: 10.1016/j.jceh.2021.11.004
58. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. (2005) 42(5):1208–36. doi: 10.1002/hep.20933
59. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. (2011) 53(3):1020–2. doi: 10.1002/hep.24199
60. Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. (2003) 52(1):134–9. doi: 10.1136/gut.52.1.134
61. Knox JJ. Addressing the interplay of liver disease and hepatocellular carcinoma on patient survival: the ALBI scoring model. J Clin Oncol. (2015) 33(6):529–31. doi: 10.1200/jco.2014.59.0521
62. Chan AW, Kumada T, Toyoda H, Tada T, Chong CC, Mo FK, et al. Integration of albumin-bilirubin (ALBI) score into Barcelona clinic liver cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol. (2016) 31(7):1300–6. doi: 10.1111/jgh.13291
63. Johnson PJ, Pinato DJ, Kalyuzhnyy A, Toyoda H. Breaking the child-pugh dogma in hepatocellular carcinoma. J Clin Oncol. (2022) 40(19):2078–82. doi: 10.1200/jco.21.02373
64. Ye L, Liang R, Zhang J, Chen C, Chen X, Zhang Y, et al. Postoperative albumin-bilirubin grade and albumin-bilirubin change predict the outcomes of hepatocellular carcinoma after hepatectomy. Ann Transl Med. (2019) 7(16):367. doi: 10.21037/atm.2019.06.01
65. Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. (1991) 100(6):1619–36. doi: 10.1378/chest.100.6.1619
66. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. (1996) 22(7):707–10. doi: 10.1007/bf01709751
67. Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA. (1993) 270(24):2957–63. doi: 10.1001/jama.270.24.2957
68. Heldwein MB, Badreldin AM, Doerr F, Lehmann T, Bayer O, Doenst T, et al. Logistic organ dysfunction score (LODS): a reliable postoperative risk management score also in cardiac surgical patients? J Cardiothorac Surg. (2011) 6:110. doi: 10.1186/1749-8090-6-110
69. MacGregor DA, Prielipp RC. Multiple organ dysfunction score. Crit Care Med. (1996) 24(7):1272–3. doi: 10.1097/00003246-199607000-00036
70. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
71. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43(2):181–93. doi: 10.1002/jpen.1451
72. Alderson P, Bunn F, Lefebvre C, Li WP, Li L, Roberts I, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. (2011) (10):Cd001208. doi: 10.1002/14651858.CD001208.pub3
73. Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. (2011) 2011(11):Cd001208. doi: 10.1002/14651858.CD001208.pub4
74. Frazee E, Kashani K. Fluid management for critically ill patients: a review of the current state of fluid therapy in the intensive care unit. Kidney Dis (Basel). (2016) 2(2):64–71. doi: 10.1159/000446265
Keywords: albumin-bilirubin (ALBI), easy albumin-bilirubin (EZ-ALBI), liver function, mortality, trauma
Citation: Hsu S-Y, Rau C-S, Tsai C-H, Chou S-E, Su W-T and Hsieh C-H (2024) Association of easy albumin-bilirubin score with increased mortality in adult trauma patients. Front. Surg. 11:1280617. doi: 10.3389/fsurg.2024.1280617
Received: 20 September 2023; Accepted: 9 April 2024;
Published: 24 April 2024.
Edited by:
Shang Yu Wang, Linkou Chang Gung Memorial Hospital, TaiwanReviewed by:
Chih-Ying Chien, Keelung Chang Gung Memorial Hospital, Taiwan© 2024 Hsu, Rau, Tsai, Chou, Su and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Hua Hsieh bTkzY2hpbmdodWFAZ21haWwuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.