- 1School of Medicine, University of Colorado, Aurora, CO, United States
- 2University of Rochester, Rochester, NY, United States
Background: ADHD is a condition with extensively researched increased risks of psychiatric disorders, traumatic injury, impulsivity, and delayed response times.
Objectives: To analyze the incidences of fractures in patients with ADHD on various medication regimens.
Methods: Using the TriNetX database, we created seven patient cohorts, all of age under 25, based on medication types commonly used for ADHD. The cohorts we created were: no medication use, exclusive use of a -phenidate class stimulant, exclusive use of an amphetamine class stimulant, nonexclusive use of formations of either stimulant, exclusive use of non-stimulant medications approved for ADHD, nonexclusive use, and no medications. We then examined rates while controlling for age, sex, race, and ethnicity.
Results: The comparison of ADHD to neurotypical individuals revealed an increased risk for all fracture types. For the controlled analysis, all but one cohort had significant differences in each fracture type compared to the baseline cohort of ADHD patients without any medication use. Patients in the “phenidate” cohort had an insignificant difference in risk of lower limb fractures. Patients in the “any medication,” “-etamine,” “stimulant,” and “not ADHD” groups all had significant decreased risks for all fracture types, with confidence intervals often overlapping between treatment modalities.
Conclusions: As patients experiment with different medication regimens, providers should be aware of the difference in risk of fracture by medication type. Our results highlight the need for continued research to better discern appropriate medication regimens with the goal of improving overall risk reduction and producing better outcomes for individuals with ADHD.
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is one of the most common developmental disorders, affecting up to 10% of the population (1). ADHD is a condition with extensively researched increased risks of psychiatric disorders, traumatic injury, impulsivity, and delayed response times (1). The heterogenous nature of ADHD necessitates scientific inquiry and discovery into all associated co-morbidities, environmental and social impacts, and other exacerbating factors to ensure treatment is all encompassing.
A significant yet often overlooked ADHD association is difficulty in motor coordination and tasks requiring complex coordinated movements (2). These motor difficulties are often interpreted socially as the individual being clumsy or incorrectly attributed to poor focus on surroundings/tasks due to attention-deficit and hyperactivity. They are also postulated to contribute to the statistically significant risk of accidental traumatic injuries seen in children with ADHD (3). A nationwide matched study found that the incidence of fractures in people with ADHD was 1.4 times greater than the rates seen in those without ADHD (4). An earlier study controlled for confounding factors such as socioeconomic status, and still arrived at an increased risk of 1.32 (5). Importantly, the earlier study by Chou et al. also controlled for certain conditions, such as developmental coordination disorder, that could have caused an increased fracture risk on their own (5).
In response to these well-studied increased injury risks, some recent studies have attempted to investigate whether stimulant treatment leads to protective musculoskeletal benefits. While investigating stimulants is a worthwhile endeavor, there are no studies comparing the impact of all available ADHD medications on musculoskeletal injuries. The objective of our study is to analyze the incidences of fractures in patients with ADHD on various medication regimens.
Methods
We performed a retrospective study of de-identified data from the TriNetX research database. TriNetX contains electronic medical records from nearly 60 large healthcare organizations and contains more than 90 million individual patient records. This proprietary database is available to institutions who contribute to the dataset and can be accessed by researchers at those institutions who adhere to their university policies; thus, it has been well-established for medical research, including but not limited to oral cancer (6), cardiovascular illness (7), and surgical outcomes (8), to name a few. TriNetX, LLC is compliant with the Health Insurance Portability and Accountability Act (HIPAA), the United States federal law which protects the privacy and security of healthcare data, and any additional data privacy regulations applicable to the contributing HCO (9). TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System (ISMS) to ensure the protection of the healthcare data it has access to and to meet the requirements of the HIPAA Security Rule. Any data displayed on the TriNetX Platform in aggregate form, or any patient level data provided in a data set generated by the TriNetX Platform only contains de-identified data as per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule (9).
We identified all patients under the age of 25 between November 13, 2002 and November 13, 2022, as the data were extracted on November 15, 2022. From this patient population, we separated initially into two cohorts based on presence or absence of an ADHD diagnosis (ICD: F90). The group with ADHD is the “Overall ADHD” group, and the group without ADHD was labelled the “Neurotypical” group. We then created six subgroups within the ADHD group based on medication regimen, which can be understood as three cohorts of nonexclusive medication use and three cohorts of exclusive medication use: the “no meds” cohort had never taken an FDA-approved ADHD medication (10); the “any meds” cohort had used at least one of the identified medications, and could have used any combination or switched between regimens; the “stimulants” cohort had used at least one of the -amphetamine and -phenidate class medications, and could have used any combination or switched between regimens; the “-phenidate” cohort had only exclusively used medications of the -phenidate class, such as dexmethylphenidate or methylphenidate; the “-etamine” cohort had only exclusively used medications of the -amphetamine class, such as dextroamphetamine or amphetamine; the “non-stimulant” cohort had only exclusively used medications of the FDA-approved non-stimulant ADHD medications, such as atomoxetine or guanfacine (10). Demographic information has been provided in Supplementary Table S1.
The events of interest in this study were identified as follows: “central fractures,” consisting of fractures of the skull, face, cervical vertebrae, spine, ribs, or pelvis (ICD-10: S02, S12, S22, S32); “upper limb fractures,” consisting of fractures of the shoulder, humerus, forearm, wrist, or hand (ICD-10: S42, S52, S62); “lower limb fractures,” consisting of fractures of the femur, lower leg, ankle, or foot (ICD-10: S72, S82, S92); or “any fractures,” consisting of any prior mentioned fracture type.
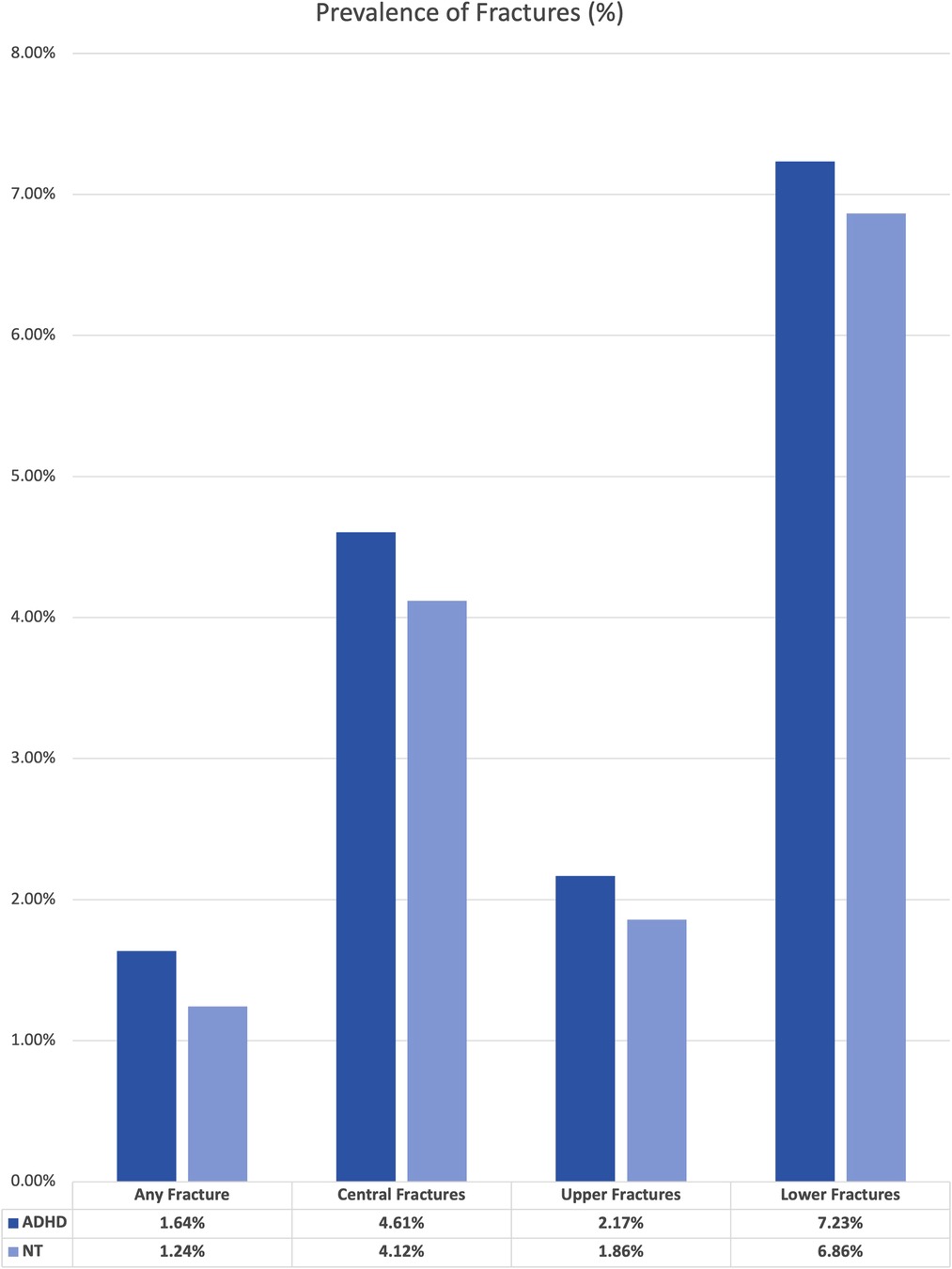
We performed two sets of analysis using the TriNetX statistical software. First, we established the overall prevalence of our events by comparing the base rate of each fracture type within the large ADHD group to the “neurotypical” or not ADHD group. The results of this set of analysis are presented in Figure 1. For this analysis, we did not control for any variables within the two cohorts.
Following this analysis, we sought to isolate the effect of ADHD medications on fracture rates. We established the “no medication” ADHD group as the baseline, as they have the neurobiology of ADHD without any medication effects. We then balanced each ADHD subgroup to this baseline cohort based on age, sex, race, and ethnicity using nearest-neighbor matching to a difference between propensity scores <0.1 (9). The identified characteristics utilized in nearest-neighbor matching, as well as pre- and post- matching t-test values for these characteristics, are presented for each sub-group in Supplementary Table S1. The outcome of this methodology was that each patient within a medication based sub-group had a peer in the “no meds” cohort who had no significant difference based on age, sex, race, or ethnicity.
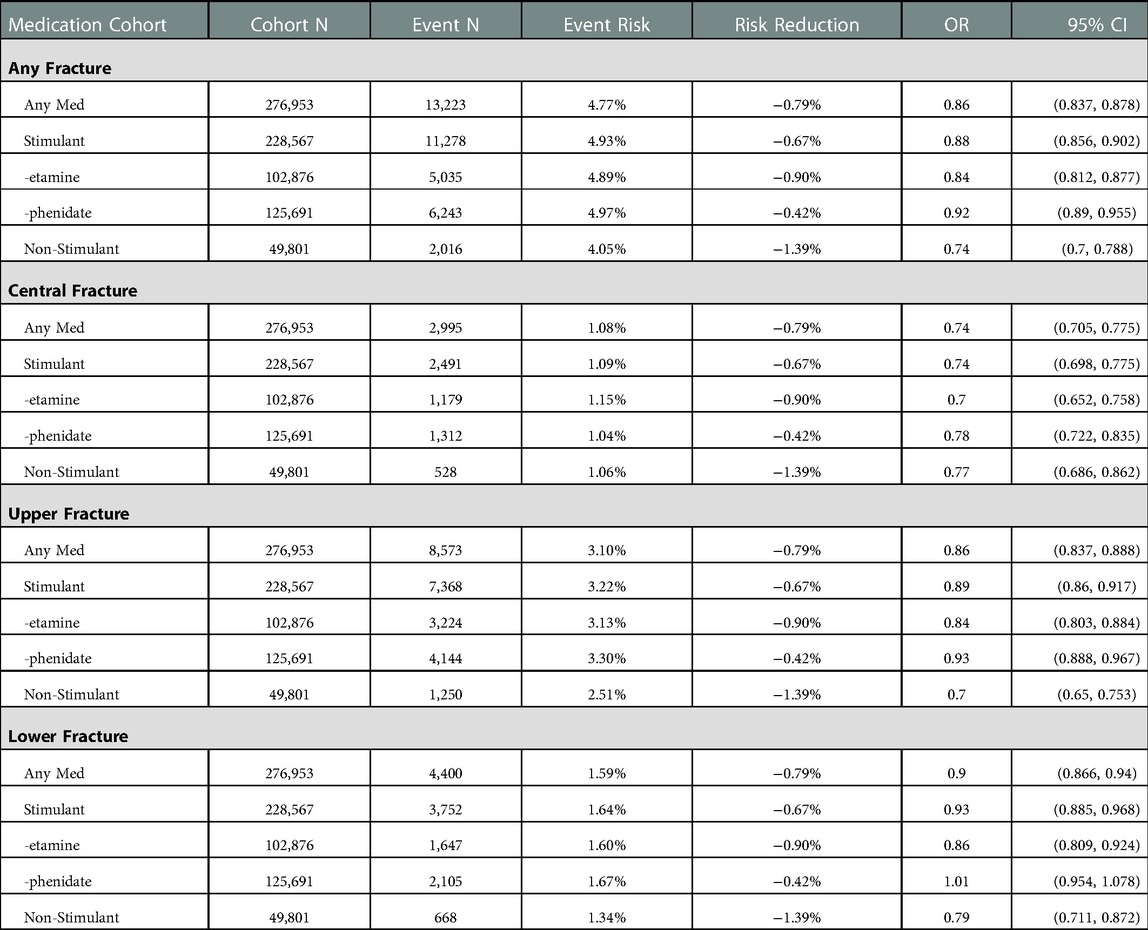
Once the cohorts were balanced, event rates were extracted from the relevant patient records, and odds ratios with 95% confidence intervals were calculated from the given incidence of each event. Risk reduction was calculated as the risk of event in the baseline group minus the risk of event in the comparison group. The baseline event rate for the “no meds” is not provided, as the individuals analyzed varied based on matching to the relevant medication sub-group. The results of this set of analysis are collected in Table 1 and presented graphically in Figure 2. Significance for this study was set at a two-tailed p-value <0.05. As this study contained only deidentified aggregate data, the Colorado Multiple Institutional Review Board (COMIRB) designated it as non-human research not in need of approval.

Figure 2. Odds ratio for fractures by medication type. Confidence bars depict a 95% CI. A value greater than 1 indicates the fracture is more likely in ADHD patients on that medication, while a value under 1 indicates the fracture is less likely.
Results
The original size of each cohort is included in Supplementary Table S1. The unmatched analysis between the “neurotypical” or “not ADHD” cohort and the “overall ADHD” cohort revealed higher rates of fractures for all types. The difference in overall prevalence ranged from 0.31% increase for upper limb fractures to 0.49% increase for central fractures.
After matching, the cohort size for each event varied from 668 to 13,223. All but one cohort had a significant difference in each fracture type, with all differences being reduced from the baseline, which was the cohort of ADHD patients without any medication use. Patients in the “phenidate” cohort had an insignificant difference in risk of lower limb fractures. Patients in the “any medication,” “-etamine,” “stimulant,” and “not ADHD” groups all had significant decreased risks for all fracture types; detailed results are in Table 1. Figure 2 presents a visual depiction of odds ratios and confidence intervals between the identified medication type and the matched portion of the “no meds” cohort.
Discussion
In our uncontrolled analysis between neurotypical individuals and all individuals with ADHD, all fracture outcomes occurred more commonly in the ADHD cohort. This replicates an important observation, and likely contributes to the higher rates of injuries in many aspects of ADHD, including traumatic and athletic injuries (3, 11). Compared to the baseline cohort of patients with ADHD who have never taken one of the identified medications, all medications cohorts had significantly reduced risks across most fracture types. The only exception is for patients in the “-phenidate” cohort, with exclusive use of stimulant medications related to methylphenidate, had increased risk for central, upper limb, and any fracture types, but had an insignificant difference in risk of lower limb fractures.
The neurobiology of ADHD seems to affect nearly all aspects of health (12). While some aspects are obvious and relatively well studied, such as mental health and social functioning (13), recent research has shown ADHD to be more impactful than previously thought. Medication regimen analyses, similar to the one performed in this study, have shown improvements for patients with ADHD on stimulant medication in a wide range of situations from transplant surgery to obstetric complications (14, 15). While an increasing amount of research is being performed on this important issue, much of it compares cohorts with ADHD to neurotypical cohorts, or individuals who likely do not have the neurobiology found in ADHD. In contrast, our study uses a baseline of individuals with ADHD and without medication treatment, which allows us to better isolate the impact of an individual medication regimen.
There are many available treatments for ADHD, and more are approved every year. For young children, the first line treatment is behavioral intervention on the part of the parents, rather than medications (16). In older children or young adults, there are a wide variety of medication options available, which can essentially be separated into stimulants and non-stimulants. Stimulant medications are those designed to act on the dopaminergic pathways in the brain, creating a neurochemical environment that is more like a neurotypical brain (17). Rather than simply addressing behavioral symptoms, stimulant medications target one of the known neurobiological differences present in ADHD. In contrast, non-stimulant medications have a weaker impact on the dopaminergic pathways and are used commonly for symptomatic management when stimulants are not appropriate (18).
Many patients, parents, and providers are concerned over the potential side effects of medication types. Within stimulants, methylphenidate is known to decrease the bone density with chronic use in both animal models and human studies (19, 20). This has been proposed as a potential cause for the increased absolute risk of fractures for patients with ADHD compared to neurotypical individuals (21). Interestingly, a large human study of chronic methylphenidate treatment has shown a decreased risk of fracture for individuals on ADHD compared to individuals with ADHD who are not on treatment (22). Our data presents a similar trend. While there is an increased absolute risk of fractures in all patients with ADHD compared to neurotypical individuals, our data showed a decreased risk for 3 out of 4 studied types of fractures in the “-phenidate” group compared to patients with ADHD who do not use medication, and an insignificant difference in lower limb fractures. If methylphenidate decreased bone density to a clinically significant extent, we would likely expect an increase compared to individuals with ADHD who have not been treated with any medication and compared to individuals who had been treated with amphetamine-type stimulants. Instead, our data suggests that bone loss due to methylphenidate is not a clinically significant factor in the increased fracture risk for patients with ADHD and confirms prior research that methylphenidate use may be protective against fractures (21).
While both stimulants and non-stimulants are known to impact behavior, stimulant medications have more frequently been the focus of research. Behavioral changes associated with stimulant medications are known to decrease the chance of fracture in patients with ADHD (21). Our data reinforces these findings, and further suggests that non-stimulants may additionally reduce the risk of fractures through a similar behavioral mechanism. It is also notable that within our study, non-stimulants had the greatest difference in absolute risk reduction of all medication types, although the confidence intervals overlapped with stimulant regimen for upper and central fractures. Given the overlapping confidence intervals, there is no statistically significant difference by treatment regimens for central fractures, potentially due to the relatively small sample size compared to other types of fractures. As some non-stimulants are also used as sedatives (18), one possibility for the difference in fractures could be a reduction in baseline activity. However, it is worth noting that non-stimulant medications are most often used for individuals with less severe symptomatology (23). An individual with less severe symptoms may have a lower fracture risk at baseline, and it is unknown how much of the effect seen in our study is related to the medication as opposed to baseline differences. As non-stimulants are not considered to contribute to loss of bone density (24), this study adds to the knowledge of the field by deepening the available evidence that compares medication types.
Our study is not without limitations. As with all studies involving deidentified, aggregate data, we were unable to trace individuals throughout time or multiple simultaneous events; for example, a patient who fractured their skull, humerus, and femur in the same incident would be present once in each event analysis, while someone who fractured their humerus and repeatedly presented to medical care for pain medication would be present in the data one time for each visit. Furthermore, we did not separate out different types of fracture such as pathological, stress, or traumatic, which may have presented different data. Additionally, we did not directly compare amphetamines and methylphenidates, although substantial differences between amphetamines and methylphenidate medications are recognized in the literature, with amphetamines often having longer effects (25). While a causal analysis is not possible in our study, our study provides a basis for further investigation and comparison between amphetamine and methylphenidate medications, with amphetamines potentially being more effective for fracture prevention. Finally, we were limited by the available data and were unable to control for severity of ADHD symptoms, although some information can be presumed based on the type of medication used.
This study offers new information that may aid providers in providing information to patients and families regarding the impact of ADHD treatment on bone health. As patients experiment with different medication regimens, providers should be aware of the difference in risk of bone fracture by medication type. Furthermore, our manuscript is the first to directly compare fracture risks in non-stimulant and stimulant medications, finding intriguing differences across medication types that cannot be understood at a causal level from the available data. However, by highlighting potential differences in fractures across medication classes, our results highlight the need for continued research to better discern appropriate medication regimens for individual patients with the goal of improving overall risk reduction and producing better outcomes for individuals with ADHD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.’
Author contributions
EWH: served as senior author, with full access to all data, initial conception of the study design and manuscript, as well as project supervision. JPS: served as first author, contributed to literature review, initial drafting, and critical revision of the manuscript. SRB: created figures and tables and contributed to critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.973266/full#supplementary-material.
References
1. Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. (2021) 128:789–818. doi: 10.1016/J.NEUBIOREV.2021.01.022
2. Mokobane M, Pillay BJ, Meyer A. Fine motor deficits and attention deficit hyperactivity disorder in primary school children. S Afr J Psychiatr. (2019) 25:247–8. doi: 10.4102/SAJPSYCHIATRY.V25I0.1232
3. Evans SI, Hale EW, Silverman MS. Mechanisms of bodily harm in emergency department youths with ADHD. Frontiers Child Adolesc Psychiatry. (2022) 1:5. doi: 10.3389/FRCHA.2022.1033822
4. Guo N-W, Lin C-L, Lin C-W, Huang M-T, Chang W-L, Lu T-H, et al. Fracture risk and correlating factors of a pediatric population with attention deficit hyperactivity disorder: a nationwide matched study. J Pediatr Orthop B. (2016) 25(4):369–74. doi: 10.1097/BPB.0000000000000243
5. Chou IC, Lin CC, Sung FC, Kao CH. Attention-deficit-hyperactivity disorder increases risk of bone fracture: a population-based cohort study. Dev Med Child Neurol. (2014) 56(11):1111–6. doi: 10.1111/DMCN.12501
6. Hoffman MJ, Hale DD, Hale EW. Patient characteristics in oral cancer staging. Front Oral Health. (2022) 3:1–4. doi: 10.3389/FROH.2022.923032
7. Singer ME, Taub IB, Kaelber DC. Risk of myocarditis from COVID-19 infection in people under age 20: a population-based analysis. medRxiv. (2022) 1:1–3. doi: 10.1101/2021.07.23.21260998
8. Singh D, Slavin BR, Holton T. Comparing surgical site occurrences in 1 versus 2-stage breast reconstruction via federated EMR network. Plast Reconstr Surg Glob Open. (2021) 9(1):3385–6. doi: 10.1097/GOX.0000000000003385
9. TriNetX. Available at: https://trinetx.com/real-world-resources/publications/trinetx-publication-guidelines/. (Accessed July 11, 2022).
11. Hale EW, Feldkamp JM, Stevens AR, Blaakman SR. Baseball injuries in adolescent athletes with ADHD. Front Sports Act Living. (2023) :4:478. doi: 10.3389/FSPOR.2022.1032558
12. Franke B, Michelini G, Asherson P, Banaschewski T, Bilbow A, Buitelaar JK, et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur Neuropsychopharmacol. (2018) 28(10):1059–88. doi: 10.1016/J.EURONEURO.2018.08.001
13. Harpin V, Mazzone L, Raynaud JP, Kahle J, Hodgkins P. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. (2016) 20(4):295–305. doi: 10.1177/1087054713486516
14. Yoon Y, Kennis M, Hale EW. Post-operative complications and ADHD. Front Child Adolesc Psychiatry. (2022) 1:4. doi: 10.3389/FRCHA.2022.1032559
15. Walsh CJ, Rosenberg SL, Hale EW. Obstetric complications in mothers with ADHD. Front Reprod Health. (2022) 4:90. doi: 10.3389/FRPH.2022.1040824
16. Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. (2022) 144(4):1–25. doi: 10.1542/PEDS.2019-2528
17. Coghill D. The benefits and limitations of stimulants in treating ADHD. Curr Top Behav Neurosci. (2022) 57:51–77. doi: 10.1007/7854_2022_331
18. Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Saint-Gerons DM, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLoS One. (2017) 12(7):1–31. doi: 10.1371/JOURNAL.PONE.0180355
19. Fu Y, Wang G, Liu J, Li M, Dong M, Zhang C, et al. Stimulant use and bone health in US children and adolescents: analysis of the NHANES data. Eur J Pediatr. (2022) 181(4):1633–42. doi: 10.1007/S00431-021-04356-W
20. Feuer AJ, Thai A, Demmer RT, Vogiatzi M. Association of stimulant medication use with bone mass in children and adolescents with attention-deficit/hyperactivity disorder. JAMA Pediatr. (2016) 170(12):1–8. doi: 10.1001/JAMAPEDIATRICS.2016.2804
21. Perry BA, Archer KR, Song Y, Ma Y, Green JK, Elefteriou F, et al. Medication therapy for attention deficit/hyperactivity disorder is associated with lower risk of fracture: a retrospective cohort study. Osteoporos Int. (2016) 27(7):2223–7. doi: 10.1007/S00198-016-3547-1
22. Chen VC-H, Yang Y-H, Liao Y-T, Kuo T-Y, Liang H-Y, Huang K-Y, et al. The association between methylphenidate treatment and the risk for fracture among young ADHD patients: a nationwide population-based study in Taiwan. PLoS One. (2017) 12(3):1–11. doi: 10.1371/JOURNAL.PONE.0173762
23. Cortese S, Adamo N, del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2018) 5(9):727–38. doi: 10.1016/S2215-0366(18)30269-4
24. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. (2018) 20(11):1–13. doi: 10.1007/S11920-018-0960-5
25. Newcorn JH, Nagy P, Childress AC, Frick G, Yan B, Pliszka S. Randomized, double-blind, placebo-controlled acute comparator trials of lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS Drugs. (2017) 31(11):999–1014. doi: 10.1007/S40263-017-0468-2
Keywords: ADHD (Attention deficit and hyperactivity disorder), fractures – bone, bone densities, pharmacology, orthopedic
Citation: Sidrak JP, Blaakman SR and Hale EW (2023) Fracture rates by medication type in attention-deficit/hyperactive disorder. Front. Surg. 10:973266. doi: 10.3389/fsurg.2023.973266
Received: 20 June 2022; Accepted: 23 January 2023;
Published: 15 February 2023.
Edited by:
Jaimo Ahn, University of Michigan, United StatesReviewed by:
Alison Sally Poulton, The University of Sydney, AustraliaFrancisco Xavier Castellanos, New York University, United States
© 2023 Sidrak, Blaakman and Hale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elijah W. Hale ZWxpamFoLmhhbGVAY3VhbnNjaHV0ei5lZHU=
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Jason P. Sidrak
Jason P. Sidrak Syler R. Blaakman2
Syler R. Blaakman2 Elijah W. Hale
Elijah W. Hale