- 1Department of Neurosurgery, Kantonsspital Aarau, Aarau, Switzerland
- 2Faculty of Medicine, University of Bern, Bern, Switzerland
- 3Department of Gynecology and Obstetrics, Kantonsspital Lucerne, Lucerne, Switzerland
- 4Department of Anaesthesiology and Pain Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 5Department of Neurosurgery, University Hospital of Basel, Basel, Switzerland
- 6Department of Neurosurgery, Medical Center, University of Freiburg, Freiburg, Germany
- 7Department of Endocrinology, Diabetes and Metabolism, University Hospital of Basel, Basel, Switzerland
Objectives: Prolactinomas represent the most common type of secreting pituitary adenomas, yet are rarely encountered in adolescent-onset (AO; i.e. <18 years) or elderly-onset (EO; i.e. ≥65 years) cohorts. As a result, it is not clear whether long-term strategies should be focused differently at both age extremes when comparing their therapeutic outcomes. We aimed at investigating long-term endocrinological outcomes, looking for differences between the two cohorts and evaluating the dependence on continued dopamine agonist (DA) therapy.
Methods: Retrospective cross-sectional comparative study analyzing prolactinoma patients with a follow-up of ≥4 years. Clinical, radiological and biochemical characteristics were assessed at diagnosis and last follow-up. Longitudinal endocrinological outcomes between groups of extreme ages (i.e. AO and EO) and middle age (i.e. ≥18 years to 65 years) were compared. Independent risk factors for long-term dependence on DAs were calculated.
Results: Follow-up at ≥4 years was recorded for 108 prolactinoma patients; 10 patients with AO and 10 patients with EO. Compared to AO patients, EO patients were predominantly men (p = 0.003), and presented with significantly higher prolactin (PRL) levels (p = 0.05) and higher body mass index (p = 0.03). We noted a significant positive correlation between patients' PRL values and their age (r = 0.5, p = 0.03) or BMI (r = 0.6, p = 0.03). After a median follow-up of 115 months, remission was noted in 87 (83%) patients; 9 (90%) in AO patients, and 7 (70%) in EO patients (p = 0.58). Continuation of DAs was required in 4 patients (40%) with AO and 7 patients (70%) with EO (p = 0.37). Patients with elderly-onset were an independent predictor of long-term dependence on DAs (HR 2.8, 95% CI 1.1-7.2, p = 0.03).
Conclusions: Long-term control of hyperprolactinemia and hypogonadism does not differ between members of the AO and EO cohorts, and can be attained by the majority of patients. However, adjuvant DAs are often required, independent of the age of onset. Considering the clinical significance of persistent DA therapy for the control of hyperprolactinemia in many patients at both extremes of age, long-term monitoring may become recommended, in particular in patients with elderly-onset.
Introduction
While prolactinomas account for 32% to 66% of all pituitary adenomas (1), they are rarely encountered in adolescent (AO) or elderly (EO) patients (2–6). Although the incidence of pituitary adenomas increases with age (7, 8), data on prolactinoma patients who are elderly at onset are infrequently reported, probably because endocrinological and neurological symptoms get misinterpreted as age-related disturbances (9). On the other hand, the prevalence of dopamine-agonist (DA) treated hyperprolactinemia has a preponderance in women with a peak at 25–34 years (10), and a much later peak in men (11). However, many symptoms of pituitary adenoma reported in adulthood were already evident during adolescence, suggesting that the true prevalence of AO's is higher than initially believed (2). As a result, it is not clear whether long-term strategies should be focused differently at both age extremes when comparing the therapeutic long-term outcomes.
We aimed at investigating long-term endocrinological outcomes, including differences between the adolescent and elderly cohorts and assessment of the dependence on continued DA therapy.
Methods
Study design
We analyzed the medical data of a prospectively maintained database, including all consecutive prolactinoma patients with middle age onset (i.e., ≥18 years to 65 years), and age limits defined as AO (≤18 years), EO (≥65 years) treated from January 1997 to December 2015. The minimum follow-up period permitting documentation of longitudinal changes was set at ≥4 years. Patients' demographics and endocrinological characteristics at baseline and last follow-up were analyzed. The Human Research Ethics Committee of Bern (Cantonal ethical commission KEK Bern, Bern, Switzerland) approved the study (KEK n° 10-10-2006 and 8-11-2006).
Clinical and biochemical assessment
Diagnosis was based on clinical and biochemical assessment, including a standard protocol for pituitary magnetic resonance imaging (MRI; see below). All patients fulfilled the diagnostic criteria of a prolactin (PRL)-secreting pituitary adenoma [i.e., elevated PRL levels without evidence of pituitary stalk compression, primary hypothyroidism or drug-induced hyperprolactinaemia, and positive pituitary magnetic resonance imaging (MRI) scan] (12, 13). Prolactin (PRL) levels, including the immunoradiometric PRL assay with serum dilution in order to overcome the high-dose PRL hook effect (14), were measured. The upper limits of PRL levels for diagnosis were set at 20 µg/L(15). Partial hypopituitarism was defined as impaired secretion of one or more pituitary hormones. Secondary adrenal insufficiency was characterized by the presence of low serum cortisol (<50 nmol/L) levels, or normal cortisol but inadequate responses to the adrenocorticotropin (ACTH) stimulation test or insulin tolerance test. The diagnosis of secondary hypothyroidism was made based on a finding of low-normal thyroid-stimulating hormone (TSH) levels and a low free thyroxin (FT4) level. A gonadotropin deficiency or central hypogonadism was considered in the case of low-normal levels of gonadotropins in parallel with low estradiol/testosterone levels.
Assessment of BMI
A standard body mass index (BMI) was calculated for all patients (16). A BMI of 21-25 kg/m2 was defined as normal, BMI 26–30 kg/m2 as overweight, BMI 31–35 kg/m2 as obese, and BMI > 35 kg/m2 as severely obese.
Radiological assessment
MRI was performed on a 1.5- or 3-Tesla system including a Proton/T2-weighted whole-brain study with unenhanced, contrast-enhanced, dynamic contrast-enhanced and post contrast-enhanced overlapping studies over the sellar region (17–20). A tumor with a diameter of 1–10 mm was defined as a microadenoma, and >10 mm as a macroadenoma. Infiltration of the cavernous sinus was noted (i.e., Knosp grade ≥1) (21, 22).
Indication for surgery
Besides local prolactinoma characteristics, such as apoplexy with visual disturbances or cystic adenomas, the indication for surgery was discussed by an interdisciplinary team, including the patient, and the decision was based on the patient's preference for surgical treatment rather than long-term DA therapy (23, 24). Pituitary surgery was performed using a transseptal, transsphenoidal microsurgical approach (i.e., transsphenoidal surgery, TSS) with standardized sellar reconstruction, as previously described (17–20).
Long-term assessment
A standardized protocol was followed for withdrawal from the use of DAs over the long term (25). If PRL levels had normalized and tumor reduction of >50% was attained, DAs were tapered 24 months after initiation of the medical therapy (26, 27). Recurrence was defined as an increase in PRL levels above the normal range (>20 µg/L) during the last follow-up period after a previous remission, irrespective of the radiological findings (28, 29).
Statistical analysis
Data were analyzed using IBM SPSS statistical software Version 24.0 (IBM Corp., New York, NY, USA) and GraphPad Prism (V7.04 software, San Diego, CA, USA). Continuous variables were examined for homogeneity of variance and are expressed as mean ± SD unless otherwise noted. Serum PRL levels are presented as median values and interquartile range (IQR, 25th to 75th percentile). Categorical variables are given as numbers and percentages. For comparisons of means between groups (i.e., patients with AO and EO), Student's t-test was used for normally distributed data, and the Mann–Whitney test for nonparametric data. The Wilcoxon signed-rank test was used to evaluate paired differences in PRL and BMI levels before and after treatment. Categorical variables were compared using Pearson's chi-square test or Fisher's exact test, as appropriate. The Spearman rank-order correlation coefficient was calculated to check for the strength of association between different variables (i.e., PRL, age, patients' BMI, DA dependency). We assessed the proportion of patients with long-term dependence on DAs and performed time-dependent multivariable regression analysis to calculate hazard ratios (HR) for potential risk factors. The variables tested were: age at diagnosis, initial PRL levels, BMI (kg/m2), hypopituitarism at diagnosis, baseline gonadotropin deficiency, prevalence of headache at diagnosis, adenoma size, and cavernous sinus invasion. The multivariable regression analysis included all dependent risk factors in the univariable regression with a p value ≤ 0.05. Baseline PRL values were log transformed before being imputed in the regression and correlation analysis analysis, as data showed a positively skewed distribution. Significance level was set at p ≤ 5%.
Results
Patients' characteristics at diagnosis
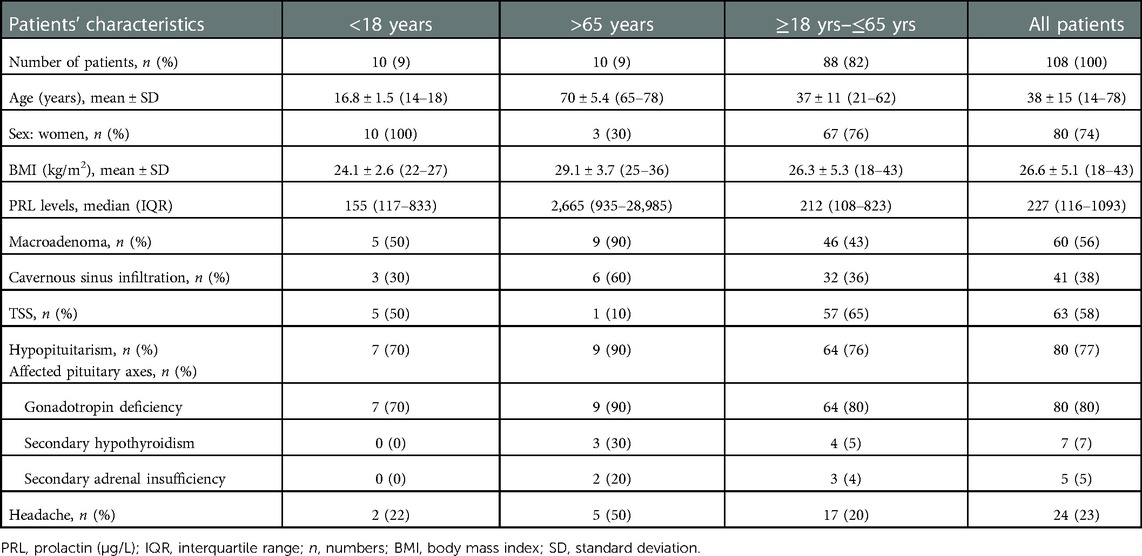
A follow-up at ≥4 years was performed for 108 patients; 10 women with AO, and 3 women and 7 men with EO. Baseline characteristics are detailed in Table 1, Table 4. At diagnosis, mean age was 16.8 years (range 14–18) in the AO cohort and 69.9 years (range 65–78) in the EO cohort. Male sex, baseline PRL levels, and patients’ BMI were significantly higher in the EO cohort. The prevalence of macroadenomas or adenomas with infiltration of the cavernous sinus (i.e., Knosp grade ≥1) was higher in the EO than the AO cohort, though the results were not statistically significant. First-line TSS was performed in 63 (58%) patients . In patients at extreme ages, we noted a significant positive correlation between patients' PRL values, and both their age (r = 0.5, p = 0.03, Figure 1A), and their BMI values (r = 0.6, p = 0.03, Figure 1B), respectively.
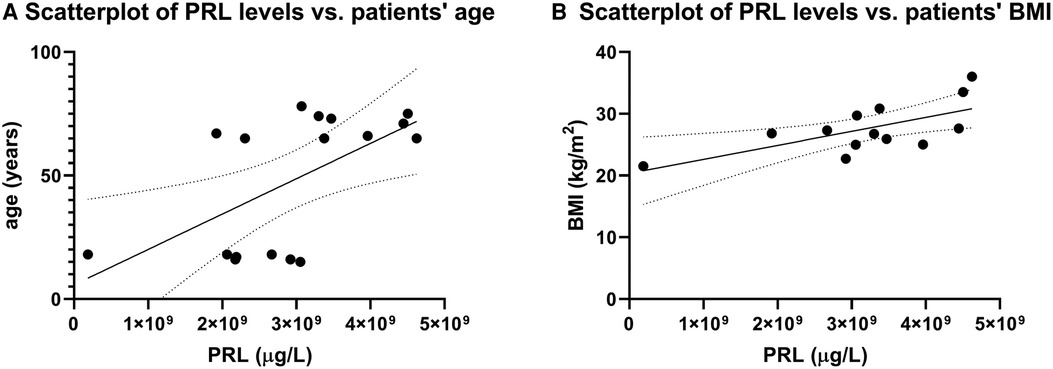
Figure 1. Correlation between BMI and PRL levels. (A) Scatterplots reveal a significant positive correlation between patients’ serum baseline PRL values and age (r = 0.5, p = 0.03). Likewise, we noted a significant positive correlation between baseline PRL values and patients’ BMI (r = 0.6, p = 0.03).
Characteristics at last follow-up
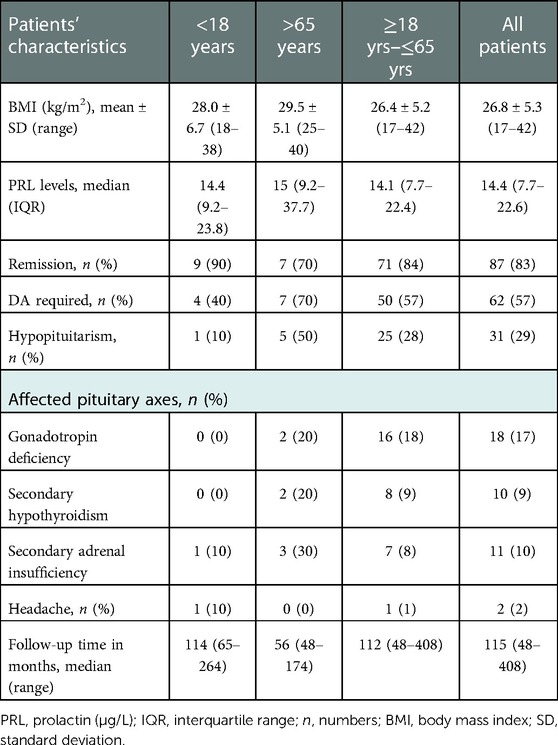
Patients' characteristics at last follow-up are detailed in Table 2, Table 5. The median follow-up period was 115 months (range, 48–408 months). PRL levels decreased from 155 µg/L (IQR 177–833 µg/L) to 14 µg/L (IQR 9–24 µg/L; p = 0.06) in the AO cohort, and from 2665 µg/l (IQR 935-28985 µg/L) to 15 µg/L (IQR 9-37 µg/L; p = 0.04; Figure 2) in the EO cohort, respectively. Serum PRL levels at last follow-up were not significantly different between the groups at extreme ages (p = 0.42).
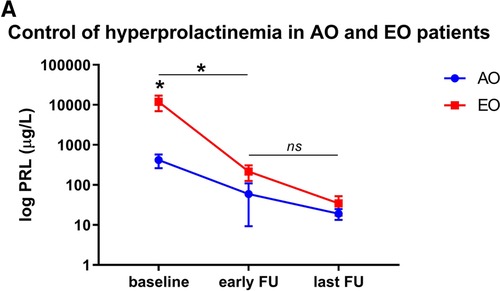
Figure 2. PRL levels in adolescent-onset (AO) patients and elderly onset (EO) patients. (A) Differences in PRL levels in both cohorts at baseline, early (i.e. 3 months) and last follow-up. Baseline PRL levels were significantly higher in patients in the EO cohort than the AO cohort (p = 0.05), but not at early (p = 0.20) or long-term follow-up (p = 0.39). PRL levels at early follow-up significantly decreased in both cohorts compared to baseline values (p = 0.04 both in the AO and EO cohort). There is no significant difference between early and long-term PRL values (p = 0.48 for AO; p = 0.07 for EO, respectively).
There was an increase in AO patients' BMIs over the long-term, from 23.8 ± 2.6 kg/m2 to 26.9 ± 6.7 kg/m2; p = 0.46), but it was not statistically significant. Obesity (BMI > 31 kg/m2) was noted in 3 (30%) AO patients and 2 (20%) EO patients. The rate of gonadotropin deficiency significantly decreased in both cohorts at extreme ages (p = 0.003 for AO and p = 0.006 for EO). Over the long-term, there was no significant difference in the rates of gonadotropic, thyrotropic or corticotropic insufficiencies. Remission was noted in 87 (83%) patients, including 9 (90%) with AO and 7 (70%) with EO (p = 0.58). For the long-term control of hyperprolactinemia, a greater, but non-significant need for continuation of DAs was noted in 70% of EO patients compared to 40% of AO patients (p = 0.37).
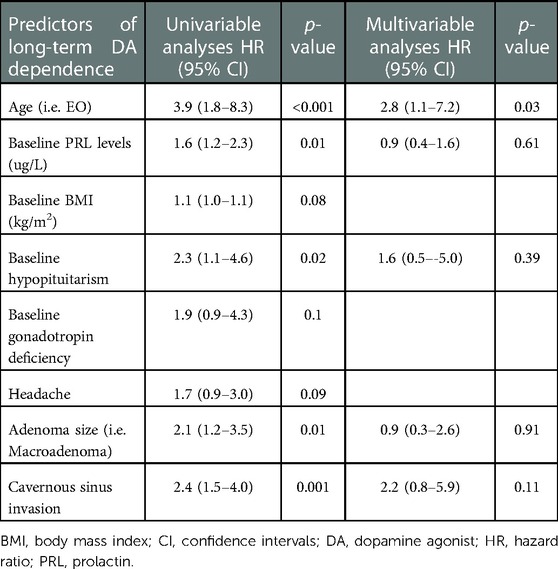
The risk factors for long-term DA dependence are summarized in Table 3. Significant risk factors in the univariable analysis included patients with EO, baseline PRL levels, hypopituitarism at diagnosis, presence of a macroprolactinoma, and cavernous sinus invasion. Multivariable Cox regression analyses revealed patients with EO as an independent risk factor for dependence on DAs (HR 2.8, 95% CI 1.1–7.2, p = 0.03).
Discussion
Our long-term data indicate that at a median follow-up of 9.5 years, control of hyperprolactinemia and hypogonadism does not differ between members of the AO and EO cohorts, and can be attained by the majority of patients. However, adjuvant DAs are often required, in particular in patients with elderly-onset.
Although prolactinomas represent the most common type of secreting pituitary adenomas, their diagnosis both in adolescents and in the elderly is rare (30). Pituitary adenomas in young patients typically present with endocrinopathies in keeping with their adenoma type, rather than resulting in local complications, meaning that macroadenomas are less frequently encountered (31). In accordance with these findings, we noted that macroprolactinomas were present in 90% of patients in the EO cohort vs. 50% of patients in the AO cohort. Likewise, the number of female patients was significantly higher in the AO group than the EO group. This corroborates a report on 41 patients ≤21 years of age, in which the disruption of the menstrual cycle was the most common symptom encountered in 85% of female patients, with the majority being diagnosed with a microprolactinoma (31).
While amenorrhea in female patients is clinically apparent and easily detected, non-specific symptoms of hypogonadism in men, such as loss of libido, are frequently not reported. Subsequently, a higher prevalence of macroprolactinomas in men than in women has been recorded (32, 33). As macroprolactinomas are associated with longer lasting hyperprolactinemia and related hypogonadism (34, 35), the significantly higher rates of baseline PRL levels in the EO cohort probably reflect on the longer disease duration in more oligosymptomatic men (36, 37). Likewise, EO was an independent predictor of long- term dependence on DAs. This is an intriguing finding. In a recent meta-analysis, the probability of persistent hyperprolactinemia was higher in younger patients (38). However, their participant mean cut-off age was set at 33.2 years, which is considerably lower than the reported age groups of the present analysis (i.e., EO), where the disease is extremely rare. As hyperprolactinaemia was showed to recur early in most macroprolactinomas (93%) following DA therapy discontinuation after 7 years of therapy (39), or in those adenomas with cavernous sinus infiltration (40), it is conceivable that the greater number of patients with a macroprolactinoma and those with cavernous sinus infiltration in the EO cohort allegedly overlooks a significant effect given the relatively small number of patients fulfilling the study inclusion criteria. Beside the 70% patients in the EO cohort with continuing DA therapy, it is striking that 40% of patients in the AO cohort were still dependent on DAs after almost 10 years too. These results corroborates with a recent review reporting of less than half of pediatric patients with prolactinomas were eligible for DA withdrawal, and less than one-fourth achieved control of hyperprolactinemia following treatment cessation (41). Both the Pituitary (42) and Endocrine Society (15) recommends tapering DAs after three and two years of treatment in the case of PRL normalization, respectively. However, in contrast to initial studies reporting that many patients treated with DAs remained in remission after drug withdrawal (27), frequent early recurrence of hyperprolactinemia following discontinuation of DAs is becoming increasingly common (43, 44). In addition, cumulative doses over the long term might contribute to potentially adverse effects, including the recently documented personality changes (45), lack of compliance, and inconvenience for patients (46, 47). As for controlling hyperprolactinemia and associated hypogonadism, both DAs and surgery can be effective in selected patients (1, 25, 36, 48–50).
With the goal of minimizing the need for continuation of DAs over the long term, upfront surgery has been proposed in highly selected patients (49, 51, 52). Although surgery might be effective with regard to non-dependency on DA therapy in the long-term, particularly in microprolactinomas (51, 52) or macroprolactinomas not infiltrating the cavernous sinus (49), results are mixed. Ongoing long-term DA therapy after surgery has been reported in 66% patients with prolactinomas (53). In addition, treatment guidelines focusing on long-term strategies becomes vague in patients at extreme ages. While the prevalence of DA treated hyperprolactinemia has a preponderance in women with a peak at 25–34 years (10), it has been suggested that children and adolescents with a prolactinoma should receive DAs as a first-line treatment (31, 42). However, normalization of hyperprolactinemia frequently requires DA over the long term (22). As a result, TSS was predominantly the treatment of choice in the AO onset compared to the EO, though not significantly. Independent of the selected first-line treatment, long-term monitoring of patients with prolactinomas thus becomes necessary, highlighting the need for a longitudinal survey in young and – considering the risk of persistent DA therapy – in old cohorts in particular.
Interestingly, we noted a significant correlation between baseline PRL levels and both patients' BMI and age, along with higher baseline PRL and BMI values in the EO compared to the AO cohort. While it is conceivable that increases in BMI are partly related to the normal age-associated increase in body weight, hyperprolactinemia and associated hypogonadism have been shown to impact patients' BMIs (54). While the underlying mechanism remains unclear (55), this association is possibly related to the longer exposure to increased PRL levels and associated hypogonadism in elderly patients and/or men with non-specific symptoms (56). Of note, BMI values over the long term were not significantly different in the two cohorts, with a non-significant increase in BMI values being noted in the AO cohort despite control of hyperprolactinemia in the majority of group members. In a study of young patients with pituitary adenomas, the highest BMI at diagnosis was measured in prolactinoma patients, with ongoing weight gain in some patients despite normalization of PRL levels (31). While several studies reported on weight loss following control of hyperprolactinemia (57), others couldn't confirm this association (58). Thus, control of hyperprolactinemia and hypogonadism remains the primary treatment target, and a weight control program may be necessary, as obesity resulting from pituitary adenomas can lead to significant morbidity and mortality (54).
Study limitations
The main limitations of our study are the small sample size, and its retrospective, single-center design. To allow for statistical comparison between the AO and EO cohorts, we calculated the BMI in all patients. Given that the lower age range in the AO cohort was 14 years, we used BMI z-scores or BMI standard deviation scores (SDS) to calculate the relative weight adjustment for a child's age and sex (59). Despite the relatively small number of patients meeting the inclusion criteria, we believe this study adds to the existing literature, presenting important changes in longitudinal outcome in patients with adolescent- and elderly-onset followed for up to 22 years. Our findings could have with direct implications for the care of patients with prolactinomas at extreme ages.
Conclusions
Long-term control of hyperprolactinemia and hypogonadism does not differ between members of the AO and EO cohorts, and can be attained by the majority of patients. Considering the clinical significance of persistent DA therapy for the control of hyperprolactinemia in many patients at both extremes of age, long-term monitoring may become recommended, in particular in patients with elderly-onset.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by The Human Research Ethics Committee of Bern (Cantonal ethical commission KEK Bern, Bern, Switzerland) approved the study (KEK n° 10-10-2006 and 8-11-2006). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
LA contributed to study conception and design, statistical analysis and interpretation, drafting of the manuscript, critical revision and final approval of the article. EC contributed to study conception and design, data interpretation, critical revision, and final approval of the article. JF contributed to the acquisition of data, and final approval of the article. AT, GAS, CM, MML, LM and JB contributed to critical revision and final approval of the article. All authors contributed to the article and approved the submitted version.
Acknowledgments
The assistance of Ms. Jeannie Wurz in editing the manuscript is greatly appreciated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. (2017) 317(5):516–24. doi: 10.1001/jama.2016.19699
2. Cannavo S, Venturino M, Curto L, De Menis E, D'Arrigo C, Tita P, et al. Clinical presentation and outcome of pituitary adenomas in teenagers. Clin Endocrinol (Oxf). (2003) 58(4):519–27. doi: 10.1046/j.1365-2265.2003.01748.x
3. De Menis E, Visentin A, Billeci D, Tramontin P, Agostini S, Marton E, et al. Pituitary adenomas in childhood and adolescence. Clinical analysis of 10 cases. J Endocrinol Invest. (2001) 24(2):92–7. doi: 10.1007/BF03343820
4. Spina A, Losa M, Mortini P. Pituitary adenomas in elderly patients: clinical and surgical outcome analysis in a large series. Endocrine. (2019) 65(3):637–45. doi: 10.1007/s12020-019-01959-0
5. Mindermann T, Wilson CB. Pediatric pituitary adenomas. Neurosurgery. (1995) 36(2):259–68. discussion 269. doi: 10.1227/00006123-199502000-00004
6. Mindermann T, Wilson CB. Age-related and gender-related occurrence of pituitary adenomas. Clin Endocrinol (Oxf). (1994) 41(3):359–64. doi: 10.1111/j.1365-2265.1994.tb02557.x
7. Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol. (2014) 117(3):379–94. doi: 10.1007/s11060-013-1354-5
8. McDowell BD, Wallace RB, Carnahan RM, Chrischilles EA, Lynch CF, Schlechte JA. Demographic differences in incidence for pituitary adenoma. Pituitary. (2011) 14(1):23–30. doi: 10.1007/s11102-010-0253-4
9. Minniti G, Esposito V, Piccirilli M, Fratticci A, Santoro A, Jaffrain-Rea ML. Diagnosis and management of pituitary tumours in the elderly: a review based on personal experience and evidence of literature. Eur J Endocrinol. (2005) 153(6):723–35. doi: 10.1530/eje.1.02030
10. Kars M, Souverein PC, Herings RM, Romijn JA, Vandenbroucke JP, de Boer A, et al. Estimated age- and sex-specific incidence and prevalence of dopamine agonist-treated hyperprolactinemia. J Clin Endocrinol Metab. (2009) 94(8):2729–34. doi: 10.1210/jc.2009-0177
11. Duskin-Bitan H, Shimon I. Prolactinomas in males: any differences? Pituitary. (2020) 23(1):52–7. doi: 10.1007/s11102-019-01009-y
12. Wright K, Lee M, Escobar N, Pacione D, Young M, Fatterpekar G, et al. Tumor volume improves preoperative differentiation of prolactinomas and nonfunctioning pituitary adenomas. Endocrine. (2021) 74(1):138–45. doi: 10.1007/s12020-021-02744-8
13. Bergsneider M, Mirsadraei L, Yong WH, Salamon N, Linetsky M, Wang MB, et al. The pituitary stalk effect: is it a passing phenomenon? J Neurooncol. (2014) 117(3):477–84. doi: 10.1007/s11060-014-1386-5
14. Karavitaki N, Thanabalasingham G, Shore HC, Trifanescu R, Ansorge O, Meston N, et al. Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin Endocrinol (Oxf). (2006) 65(4):524–9. doi: 10.1111/j.1365-2265.2006.02627.x
15. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96(2):273–88. doi: 10.1210/jc.2010-1692
16. Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. (2002) 75(6):978–85. doi: 10.1093/ajcn/75.6.978
17. Andereggen L, Gralla J, Schroth G, Mordasini P, Andres RH, Widmer HR, et al. Influence of inferior petrosal sinus drainage symmetry on detection of adenomas in Cushing's Syndrome. J Neuroradiol. (2021) 48(1):10–5. doi: 10.1016/j.neurad.2019.05.004
18. Andereggen L, Mariani L, Beck J, Andres RH, Gralla J, Luedi MM, et al. Lateral one-third gland resection in cushing patients with failed adenoma identification leads to low remission rates: long-term observations from a small, single-center cohort. Acta Neurochir (Wien). (2021) 163(11):3161–9. doi: 10.1007/s00701-021-04830-2
19. Andereggen L, Hess B, Andres R, El-Koussy M, Mariani L, Raabe A, et al. A ten-year follow-up study of treatment outcome of craniopharyngiomas. Swiss Med Wkly. (2018) 148:w14521. doi: 10.4414/smw.2018.14521
20. Andereggen L, Beck J, Gralla J, Luedi MM, Christ E. Letter to the editor from lukas andereggen: “pitfalls in performing and interpreting Inferior petrosal Sinus sampling: personal experience and literature review”. J Clin Endocrinol Metab. (2021) 106(8):e3283–4. doi: 10.1210/clinem/dgab329
21. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. (1993) 33(4):610–7; discussion 617–8. doi: 10.1227/00006123-199310000-00008
22. Micko AS, Wohrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. (2015) 122(4):803–11. doi: 10.3171/2014.12.JNS141083
23. Honegger J, Nasi-Kordhishti I, Aboutaha N, Giese S. Surgery for prolactinomas: a better choice? Pituitary. (2020) 23(1):45–51. doi: 10.1007/s11102-019-01016-z
24. Akin S, Isikay I, Soylemezoglu F, Yucel T, Gurlek A, Berker M. Reasons and results of endoscopic surgery for prolactinomas: 142 surgical cases. Acta Neurochir (Wien). (2016) 158(5):933–42. doi: 10.1007/s00701-016-2762-z
25. Andereggen L, Frey J, Christ E. Long-term IGF-1 monitoring in prolactinoma patients treated with cabergoline might not be indicated. Endocrine. (2021) 72(1):216–22. doi: 10.1007/s12020-020-02557-1
26. Wass JA. When to discontinue treatment of prolactinoma? Nat Clin Pract Endocrinol Metab. (2006) 2(6):298–9. doi: 10.1038/ncpendmet0162
27. Colao A, Di Sarno A, Cappabianca P, Di Somma C, Pivonello R, Lombardi G. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. (2003) 349(21):2023–33. doi: 10.1056/NEJMoa022657
28. Qu X, Wang M, Wang G, Han T, Mou C, Han L, et al. Surgical outcomes and prognostic factors of transsphenoidal surgery for prolactinoma in men: a single-center experience with 87 consecutive cases. Eur J Endocrinol. (2011) 164(4):499–504. doi: 10.1530/EJE-10-0961
29. Raverot G, Wierinckx A, Dantony E, Auger C, Chapas G, Villeneuve L, et al. Hypopronos. Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocrinol Metab. (2010) 95(4):1708–16. doi: 10.1210/jc.2009-1191
30. Breil T, Lorz C, Choukair D, Mittnacht J, Inta I, Klose D, et al. Clinical features and response to treatment of prolactinomas in children and adolescents: a retrospective single-centre analysis and review of the literature. Horm Res Paediatr. (2018) 89(3):157–65. doi: 10.1159/000486280
31. Steele CA, MacFarlane IA, Blair J, Cuthbertson DJ, Didi M, Mallucci C, et al. Pituitary adenomas in childhood, adolescence and young adulthood: presentation, management, endocrine and metabolic outcomes. Eur J Endocrinol. (2010) 163(4):515–22. doi: 10.1530/EJE-10-0519
32. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. (2006) 91(12):4769–75. doi: 10.1210/jc.2006-1668
33. Huber M, Luedi MM, Schubert GA, Musahl C, Tortora A, Frey J, et al. Machine learning for outcome prediction in first-line surgery of prolactinomas. Front Endocrinol (Lausanne). (2022) 13:810219. doi: 10.3389/fendo.2022.810219
34. De Rosa M, Zarrilli S, Di Sarno A, Milano N, Gaccione M, Boggia B, et al. Hyperprolactinemia in men: clinical and biochemical features and response to treatment. Endocrine. (2003) 20(1-2):75–82. doi: 10.1385/ENDO:20:1-2:75
35. Andereggen L, Frey J, Andres RH, El-Koussy M, Beck J, Seiler RW, et al. Long-Term follow-up of primary medical versus surgical treatment of prolactinomas in men: effects on hyperprolactinemia, hypogonadism, and bone health. World Neurosurg. (2017) 97:595–602. doi: 10.1016/j.wneu.2016.10.059
36. Andereggen L, Frey J, Andres RH, Luedi MM, Widmer HR, Beck J, et al. Persistent bone impairment despite long-term control of hyperprolactinemia and hypogonadism in men and women with prolactinomas. Sci Rep. (2021) 11(1):5122. doi: 10.1038/s41598-021-84606-x
37. Naliato EC, Violante AH, Caldas D, Farias ML, Bussade I, Lamounier Filho A, et al. Bone density in women with prolactinoma treated with dopamine agonists. Pituitary. (2008) 11(1):21–8. doi: 10.1007/s11102-007-0064-4
38. Andersen IB, Sorensen MGR, Dogansen SC, Cheol Ryong K, Vilar L, Feldt-Rasmussen U, et al. Withdrawal of dopamine agonist treatment in patients with hyperprolactinaemia: a systematic review and meta-analysis. Clin Endocrinol (Oxf). (2022) 97(5):519–31. doi: 10.1111/cen.14714
39. Barber TM, Kenkre J, Garnett C, Scott RV, Byrne JV, Wass JA. Recurrence of hyperprolactinaemia following discontinuation of dopamine agonist therapy in patients with prolactinoma occurs commonly especially in macroprolactinoma. Clin Endocrinol (Oxf). (2011) 75(6):819–24. doi: 10.1111/j.1365-2265.2011.04136.x
40. Kim K, Park YW, Kim D, Ahn SS, Moon JH, Kim EH, et al. Biochemical remission after cabergoline withdrawal in hyperprolactinemic patients with visible remnant pituitary adenoma. J Clin Endocrinol Metab. (2021) 106(2):e615–24. doi: 10.1210/clinem/dgaa744
41. Almutlaq N, Eugster EA, Nabhan Z, Donegan D. Outcome of dopamine agonist therapy withdrawal in children with prolactinomas. Horm Res Paediatr. (2022) 95(3):291–5. doi: 10.1159/000525226
42. Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Guidelines of the pituitary society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). (2006) 65(2):265–73. doi: 10.1111/j.1365-2265.2006.02562.x
43. Kwancharoen R, Auriemma RS, Yenokyan G, Wand GS, Colao A, Salvatori R. Second attempt to withdraw cabergoline in prolactinomas: a pilot study. Pituitary. (2014) 17(5):451–6. doi: 10.1007/s11102-013-0525-x
44. Xia MY, Lou XH, Lin SJ, Wu ZB. Optimal timing of dopamine agonist withdrawal in patients with hyperprolactinemia: a systematic review and meta-analysis. Endocrine. (2018) 59(1):50–61. doi: 10.1007/s12020-017-1444-9
45. Ioachimescu AG, Fleseriu M, Hoffman AR, Vaughan Iii TB, Katznelson L. Psychological effects of dopamine agonist treatment in patients with hyperprolactinemia and prolactin-secreting adenomas. Eur J Endocrinol. (2019) 180(1):31–40. doi: 10.1530/EJE-18-0682
46. Tampourlou M, Trifanescu R, Paluzzi A, Ahmed SK, Karavitaki N. THERAPY OF ENDOCRINE DISEASE: surgery in microprolactinomas: effectiveness and risks based on contemporary literature. Eur J Endocrinol. (2016) 175(3):R89–96. doi: 10.1530/EJE-16-0087
47. Demartini B, Ricciardi L, Ward A, Edwards MJ. Dopamine agonist withdrawal syndrome (DAWS) in a patient with a microprolactinoma. J Neurol Neurosurg Psychiatry. (2014) 85(4):471. doi: 10.1136/jnnp-2013-306043
48. Andereggen L, Christ E. Commentary: “prolactinomas: prognostic factors of early remission after transsphenoidal surgery”. Front Endocrinol (Lausanne). (2021) 12:695498. doi: 10.3389/fendo.2021.695498
49. Andereggen L, Frey J, Andres RH, Luedi MM, El-Koussy M, Widmer HR, et al. First-line surgery in prolactinomas: lessons from a long-term follow-up study in a tertiary referral center. J Endocrinol Invest. (2021) 44(12):2621–33. doi: 10.1007/s40618-021-01569-6
50. Andereggen L, Frey J, Andres RH, Luedi MM, Gralla J, Schubert GA, et al. Impact of primary medical or surgical therapy on prolactinoma patients’ BMI and metabolic profile over the long-term. J Clin Transl Endocrinol. (2021) 24:100258. doi: 10.1016/j.jcte.2021.100258
51. Mattogno PP, D'Alessandris QG, Chiloiro S, Bianchi A, Giampietro A, Pontecorvi A, et al. Reappraising the role of trans-sphenoidal surgery in prolactin-secreting pituitary tumors. Cancers (Basel). (2021) 13(13):3252:1–10. doi: 10.3390/cancers13133252.
52. Kreutzer J, Buslei R, Wallaschofski H, Hofmann B, Nimsky C, Fahlbusch R, et al. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. (2008) 158(1):11–8. doi: 10.1530/EJE-07-0248
53. Donegan D, Atkinson JL, Jentoft M, Natt N, Nippoldt TB, Erickson B, et al. Surgical outcomes of prolactinomas in recent era: results of a heterogenous group. Endocr Pract. (2017) 23(1):37–45. doi: 10.4158/EP161446.OR
54. dos Santos Silva CM, Barbosa FR, Lima GA, Warszawski L, Fontes R, Domingues RC, et al. BMI And metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity (Silver Spring). (2011) 19(4):800–5. doi: 10.1038/oby.2010.150
55. Galluzzi F, Salti R, Stagi S, La Cauza F, Chiarelli F. Reversible weight gain and prolactin levels–long-term follow-up in childhood. J Pediatr Endocrinol Metab. (2005) 18(9):921–4. doi: 10.1515/JPEM.2005.18.9.921
56. Schmid C, Goede DL, Hauser RS, Brandle M. Increased prevalence of high body mass Index in patients presenting with pituitary tumours: severe obesity in patients with macroprolactinoma. Swiss Med Wkly. (2006) 136(15-16):254–8. 2006/15/smw-1095516708311
57. Greenman Y, Tordjman K, Stern N. Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. Clin Endocrinol (Oxf). (1998) 48(5):547–53. doi: 10.1046/j.1365-2265.1998.00403.x
58. Soran H, Wilding J, MacFarlane I. Body weight and prolactinoma: a retrospective study. Int J Obes Relat Metab Disord. (2004) 28(1):183. doi: 10.1038/sj.ijo.0802492
Keywords: prolactinoma, dopamine agonists, age, surgery, long-term outcome
Citation: Andereggen L, Tortora A, Schubert GA, Musahl C, Frey J, Luedi MM, Mariani L, Beck J and Christ E (2023) Prolactinomas in adolescent and elderly patients—A comparative long-term analysis. Front. Surg. 10:967407. doi: 10.3389/fsurg.2023.967407
Received: 12 June 2022; Accepted: 16 January 2023;
Published: 6 February 2023.
Edited by:
Andrea Glezer, University of São Paulo, BrazilReviewed by:
Alberto Di Somma, Hospital Clinic of Barcelona, SpainEkaterina Pigarova, Endocrinology Research Center, Russia
© 2023 Andereggen, Tortora, Schubert, Musahl, Frey, Luedi, Mariani, Beck and Christ. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas Andereggen bHVrYXMuYW5kZXJlZ2dlbkBkYm1yLnVuaWJlLmNo
†ORCID Lukas Andereggen orcid.org/0000-0003-1764-688X
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Abbreviations BMI, body mass index; DA, dopamine agonists; IQR, interquartile range; n, numbers; PRL, prolactin (µg/L); TSS, transsphenoidal surgery; SD, standard deviation.
 Lukas Andereggen
Lukas Andereggen Angelo Tortora
Angelo Tortora Gerrit A. Schubert
Gerrit A. Schubert Christian Musahl1
Christian Musahl1 Markus M. Luedi
Markus M. Luedi Luigi Mariani
Luigi Mariani Jürgen Beck
Jürgen Beck Emanuel Christ
Emanuel Christ