
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 21 December 2023
Sec. Thoracic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1308591
Background: Postoperative pulmonary complications (PPCs) are common in gastric cancer patients after gastrectomy. The aim of our study was to investigate the perioperative risk factors and to develop a nomogram to identify patients who are at significant risk of PPCs.
Methods: The clinical data of gastric cancer patients who underwent elective gastrectomy in the First Affiliated Hospital of Nanjing Medical University from 2017 to 2021 were retrospectively collected. All patients were randomly divided into a training and a validation cohort at a ratio of 7:3. Univariate and multivariate analysis were applied to identify the independent risk factors that might predict PPCs, and a nomogram was constructed. Both discrimination and calibration abilities were estimated by the area under a receiver operating characteristic curve (AUC) and calibration curves. The clinical effectiveness of the nomogram was further quantified with the decision curve analysis (DCA).
Results: Of 2,124 included patients, one hundred and fifty patients (7.1%) developed PPCs. Binary logistic analysis showed that age > 65 years, higher total cholesterol level, longer duration of surgery, total gastrectomy, and the dose of oxycodone > 5.5 mg were independent risk factors for the occurrence of PPCs, which were contained in the nomogram. The predictive nomogram showed good discrimination and calibration [an AUC of 0.735 (95% CI: 0.687–0.783) in a training cohort and 0.781 (95% CI: 0.715–0.847) in a validation cohort]. The calibration curve and decision curve analysis showed a good agreement between nomogram predictions and actual observations.
Conclusion: We developed a nomogram model based on age, total cholesterol, extent of resection, duration of surgery, and the dose of oxycodone to predict the risk of PPCs in gastric cancer patients after elective gastrectomy.
Gastric cancer (GC) is one of the most common malignant tumors, and the third leading cause of cancer-related death throughout the world (1). It is highly prevalent in China (2). The incidence of GC increases in the elderly due to the aging process (3). Therapeutic radical gastrectomy is one of the major strategies to improve the prognosis of GC. Though the perioperative treatments, surgical skills and sophisticated anesthesiologic managements continue to be in progress, it is still difficult to avoid postoperative complications, especially after the upper-abdominal surgery. The incidence of postoperative complications is about 10%—50%, of which the incidence of postoperative pulmonary complications (PPCs) is relatively high (4, 5). Patients with severe PPCs may have to be admitted to intensive care unit (ICU) for further treatments, or even die. PPC is one of the major causes affecting the prognosis of patients and increasing medical costs (6). Therefore, seeking for an effective method to predict the complications as early as possible may reap even more benefits.
The occurrence of PPCs is related to multiple factors, including preoperative status of patients, surgical-related factors, and postoperative recovery. Chronic comorbidities, such as chronic obstructive pulmonary disease (COPD) or poor nutritional status, may increase the risk of PPCs (7, 8). Patients with GC often suffer from malnutrition, frailty, or cachexia, which has unfavorable effects on respiratory muscle mass and strength, thereby increase the risk of PPCs and other complications (9). In addition, surgical- and anesthesia- related factors, including surgery approaches, gastric resection methods, anesthesia management, and anesthetic drugs, also have an impact on the incidence of PPCs (10). The pain may arise due to the large incision after open surgery or the urinary catheters and drainage tubes after surgery, which further prevent the patients from respiratory training and delay the recovery of postoperative pulmonary function (11). Moreover, a randomized clinical trial reported that the incidence of PPCs was still as high as 38% after mechanical ventilation regardless of the ventilation strategy of low or high tidal volume with positive end expiratory pressure (PEEP) (12, 13). Analgesics are also a kind of risk factors when excessively administrated, which could lead to excessive sedation and respiratory depression (14).
Unfortunately, there are few relevant studies evaluating perioperative factors of patients to comprehensively prevent and recognize the incidence of overall PPCs after radical gastrectomy. Therefore, we conducted this study to explore the possible risk factors in the perioperative period and to establish a prediction model with a nomogram to identify patients at high risk of PPCs.
This was a retrospective, observational, single-centered cohort study. All patients undergoing gastrectomy at the department of general surgery of The First Affiliated Hospital of Nanjing Medical University from January 2017 to December 2021 were retrospectively recruited in the study.
The inclusion criteria were (1) >18 years old; (2) diagnosed with gastric cancer; and (3) undergoing elective gastrectomy. Patients with incomplete clinical records or missing data were excluded.
The study was conducted in accordance with the Declaration of Helsinki. The protocol of this study was approved by the ethical committee of the First Affiliated Hospital of Nanjing Medical University (No: 2023-SR-224). Due to the retrospective study design, informed patient consent was waived.
Clinical data were extracted from the electronic medical records, including age, gender, height, weight, body mass index (BMI), American Society of Anesthesiologists (ASA) score, chronic comorbidity [including hypertension, diabetes, cardiovascular diseases, chronic pulmonary disease (COPD), cerebrovascular accident history, renal diseases, smoking history and drinking history]. The laboratory parameters, including hemoglobin, albumin, total cholesterol, neutrophil count, lymphocyte count, and preoperative partial oxygen pressure at admission, were collected. Extent of resection, surgical approaches, duration of surgery, volume of intraoperative fluids, amount of bleeding, anesthetics, as well as analgesic managements were recorded and analyzed. Postoperative parameters including the length of postoperative hospital and discharge location were also gathered.
The nutritional status scores, including the control nutritional status score (CONUT), the geriatric nutritional risk index (GRNI), and the prognostic nutritional index (PNI) were calculated based on the relevant clinical data:
The CONUT score is calculated based on the results of serum albumin level, cholesterol level and total lymphocyte count (Supplementary Table 1) (15).
The PNI score = concentration of serum albumin (g/L) + 5 × total lymphocyte count (×109/L) (16).
The GNRI score = 1.489 × concentration of serum albumin (g/L) + 41.7 × (current weight/ideal weight), and the ideal weight = 22 × height2 (m) (17).
The PPCs in this study was a composite of the in-hospital events. The definition of PPCs was based on a previous study, which consisted of pneumonia, pleural effusion, atelectasis, pneumothorax, and respiratory failure (18).
All patients were randomly assigned to training or validation cohorts based on a ratio of 7:3 (19). Potential risk factors related to PPCs were screened out with univariate analysis. And the factors with a p value < 0.05 were further analyzed with a binary logistic regression model to identify the independent predictors of PPCs. Then the independent predictors in the training cohort were utilized to build a nomogram to visualize the results.
The accuracy of the nomogram was assessed with the area under the receiver operating characteristic (ROC) curve (AUC, or c-statistic), and calibration plots in both training and validation cohort. We made a priori decision only when an AUC c-statistic of models ≥ 0.70. The decision curve analysis (DCA) was further conducted to assess the clinical usefulness of the new models.
A large cohort study reported the incidence rate of PPCs was 15% (20). About 21 factors were considered as risk factors in this study. So, the sample size could be 2,100 patients calculated according to events per variable (EPV) rule.
Continuous variables are expressed as mean (standard deviation) or median [interquartile ranges] depending on the distribution of the data, and categorical variables are expressed as number (percentage). Differences between patients with and without PPCs were analyzed using the t-test or Mann–Whitney U test for continuous variables, and chi2 test or Fisher's exact test for categorical variables.
SPSS (version 23.0; IBM, Inc, Chicago, IL) was used to carry out the statistical analysis. Two-tail p < 0.05 was considered statistically significant. The R programming language (version R 4.3.1; R Foundation for Statistical Computing, Vienna, Austria) was used to perform a binary logistic regression analysis model for building the nomogram, ROC curves, Calibration curves, and DCA curves.
A total of 2,862 GC patients undergoing gastrectomy in the First Affiliated Hospital of Nanjing Medical University between 2017 and 2021 were identified. Seven hundred and thirty-eight patients were excluded due to incomplete or missing medical records. The demographic and clinical characteristics of 2,124 patients were eventually analyzed in this study (Figure 1).
The baseline characteristics and perioperative parameters of all patients on admission are presented in Table 1. The number of patients over 65 years accounted for 41.20% in our study. A percentage of 72.60 were male. Among patients undergoing gastrectomy for GC, PPCs developed in one hundred and fifty (7.1%) patients. A larger proportion of old people (over 65 years) was observed in the PPCs group compared with the non-PPCs group [95 (63.33%) vs. 780 (39.51%), p < 0.001]. The patients with an ASA score of 3 and 4 accounted for 23.33% in the PPCs group, while the percentage was 15.25% in the non-PPCs group (p = 0.014).
There was a higher prevalence of COPD [9 (6.00%) vs. 46 (2.33%), p = 0.013] in the patients with PPCs. The nutritional status indicators, including the level of hemoglobin (119.39 ± 24.72 vs. 125.75 ± 22.63 g/L, p = 0.001), albumin (37.28 ± 4.24 vs. 38.74 ± 4.42 g/L, p < 0.001), and total cholesterol (4.09 ± 1.04 vs. 4.44 ± 1.00 mmol/L, p < 0.001), were lower in patients suffering from PPCs. Meanwhile, the nutritional status scores (CONUT, PNI, and GNRI) were worse in the patients with PPCs (all p < 0.05) (Table 1). Patients were more likely to develop PPCs with total gastrectomy [111 (74.00%) vs. 39 (26.00%), p < 0.001], open surgery [92 (61.33%) vs. 58 (38.67%), p = 0.003] and longer duration of surgery (196.39 ± 70.61 vs. 179.05 ± 42.07 min, p = 0.003). Surprisingly, the analgesic, oxycodone, statistically increased the occurrence of PPCs. To determine the risk stratification classification for PPCs, the best cutoff value for oxycodone (5.5 mg) was calculated through the ROC curve. The dose of oxycodone > 5.5 mg significantly increased the occurrence of PPCs (p < 0.001) (Table 1). The median length of postoperative hospital stay was longer in patients with PPCs [16 (12–26) days] than those without PPCs [8 (7–10) days]. A higher probability of ICU stay was observed in patients with PPCs than those without PPCs [9 (6.00%) vs. 16 (0.81%), p < 0.001] (Table 1).
All patients were divided into a training cohort (n = 1,487) and a validation cohort (n = 637) in accordance with the random number table. Table 1 presented a detailed comparison of the predictive variables between the training and validation cohorts. There were no significant differences between the training cohort and the validation cohort (Table 1).
The univariate analysis was conducted as shown in Table 2. Univariate analysis revealed that age > 65 years, ASA score, level of hemoglobin, albumin, and total cholesterol, total gastrectomy, open surgery, duration of surgery, and the dose of oxycodone > 5.5 mg were significantly related to PPCs. The binary logistic regression analysis both in the training cohort and validation cohort showed that age > 65 years, lower level of total cholesterol, total gastrectomy, longer surgery duration, and the dose of oxycodone > 5.5 mg were independent risk factors for PPCs in patients after gastrectomy (Table 3).
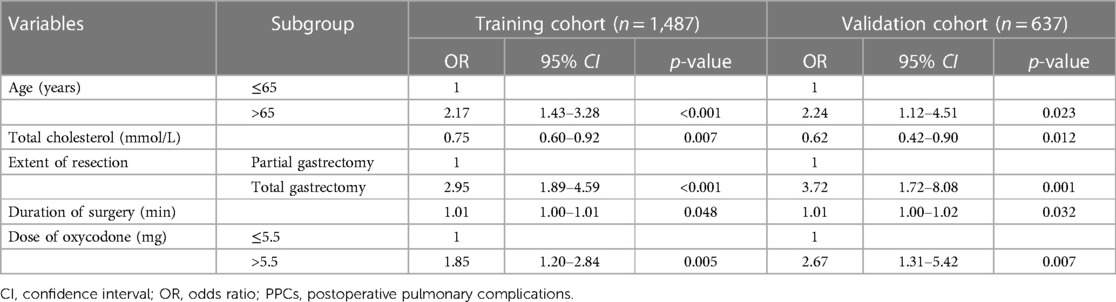
Table 3. Significant variables for prediction of PPCs in the training and validation cohort via binary logistics regression analysis.
The nomogram of predictors was presented in Figure 2, which was constructed with five independent risk factors for PPCs in the binary logistic regression analysis. The nomogram model showed good discrimination and good calibration [an AUC of 0.735 (95% CI: 0.687–0.783) in the training cohort and 0.781 (95% CI: 0.715–0.847) in the validation cohort] (Figures 3A,B). The actual observations of probability and the calibration curves were shown in Figures 3C–F, which suggested a good agreement in both training cohort and validation cohort.
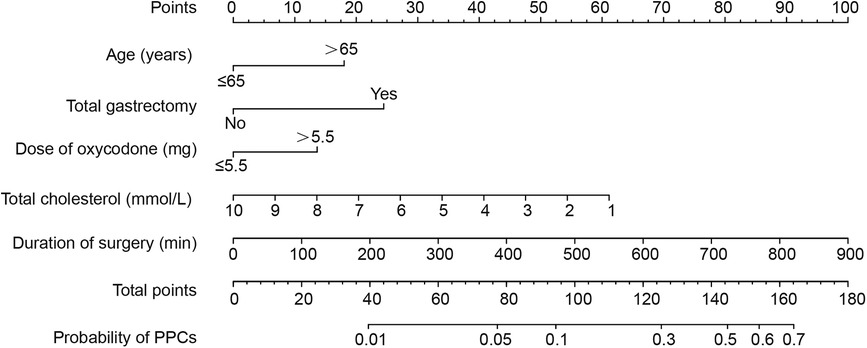
Figure 2. A nomogram for prediction of postoperative pulmonary complications in gastric cancer patients after elective gastrectomy.
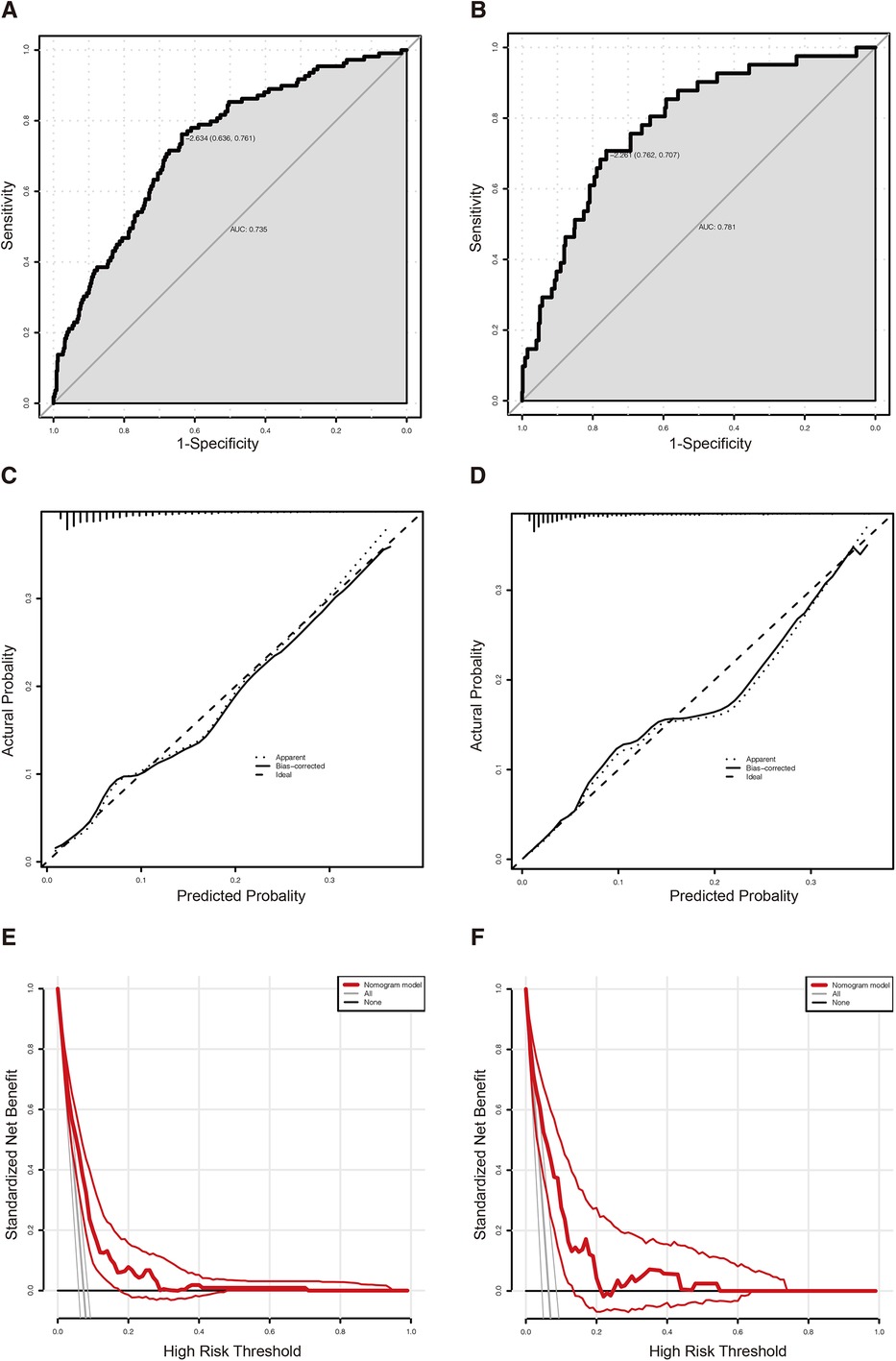
Figure 3. (A) The ROC curve of the predictive nomogram in the training cohort with an AUC value of 0.735 (95% CI: 0.687–0.783). (B) The ROC curve of the predictive nomogram in the validation cohort with an AUC value of 0.781 (95% CI: 0.715–0.847). (C) The calibration plot of the predictive nomogram in the training cohort. (D) The calibration plot of the predictive nomogram in the validation cohort. (E) The DCA curve of the predictive nomogram in the training cohort. (F) The DCA curve of the predictive nomogram in the validation cohort. ROC, receiver operating characteristic curve; DCA, decision curve analysis.
In our study, 21 perioperative parameters were taken into consideration as predictors of PPCs for gastric cancer patients after elective radical gastrectomy, and 13 factors were identified by univariate analysis. The binary logistic regression analysis showed that age > 65 years, together with lower level of total cholesterol, total gastrectomy, longer duration of surgery, and the dose of oxycodone > 5.5 mg, were good predictors of PPCs. Hence, the nomogram was constructed. The AUC was 0.735 (95% CI: 0.687–0.783) in the training cohort and 0.781 (95% CI: 0.715–0.847) in the validation cohort.
Gastric cancer is one of the most malignant tumors and radical gastrectomy is associated with considerable morbidity, specifically PPCs (21). This study revealed that PPCs not only prolonged the postoperative recovery of patients and increased the postoperative hospital stay, but also increased the possibility of admission to the ICU, which were in consistent with previous studies (22, 23). The higher medical expenses and morbidity in PPCs patients arouse the attention to identify and distinguish the patients in high risks and make integrated plans to reduce the occurrence of PPCs. To this end, we designed this retrospective cohort study to seek for the predictors and develop a nomogram model to provide the clinicians with a visual tool to identify the high-risk patients and reduce the incidence of PPCs.
The incidence of PPCs increased with the prevalence of several independent risk factors, particularly advanced age, in this study. We found that patients over 65 years old have higher odds ratio of encountering with PPCs. The results of some clinical studies are similar with our study, as PPCs after gastrectomy may be age-related (10). This observation could be explained by the normal changes with aging. Natural lung aging caused the alterations in lung function, and increased susceptibility to chronic lung diseases (24). In our study, COPD was considered as a risk factor of PPCs in the univariate analysis, but it showed no significant difference in the logistic regression. However, some previous studies reported that preoperative existing diseases should be taken into consideration when evaluating the timing of surgery (25, 26). Avoiding performing the surgery in the acute episode of COPD is prerequisite in consideration of the rapid recovery of GC patients after surgery. Moreover, with aging, the number of patients receiving surgical treatment for GC is increasing (27). Critical care units are unavoidably needed if the patient is critically ill after surgery, which is a great challenge for the medical system.
To the best of our knowledge, nutritional status is another important indicator for postoperative recovery. The level of hemoglobin, albumin, and total cholesterol are some of the nutritional indicators (28). Total cholesterol was considered to be an important element to predict PPCs in this study. Delgado-Rodríguez et al. (29) believed the level of total cholesterol was a U-shaped relationship with postoperative respiratory tract infection. While Canturk et al. (30) reported that lower total cholesterol level (≤5.18 mmol/L) might be associated with nosocomial infections in surgical patients, which came to the similar conclusion with our study. The nutritious status, evaluated with CONUT, PNI and GNRI index, were in expectation of predicting prognosis. Lee et al. (31) recommends CONUT index as a good predictor of PPCs. In this study, however, despite the result of three nutritional status ranking system showed statistical differences between the non-PPCs group and PPCs groups. Multivariate analysis cannot prove a good sensitivity and specificity of the nutritious status between the groups. Therefore, we eventually included the potential risk factors according to the results of multivariate analysis and established a nomogram model.
The surgical approach, extent of resection and reconstruction depend on the lesion location, tumor size, and lymph node metastasis are suspicious characteristics in PPCs. In our study, the incidence of PPCs in open surgery for total gastrectomy was much higher than laparoscopic surgery for partial gastrectomy. The possible reason may be that the operation area is close to the diaphragm during total gastrectomy, and it could be stimulated and lead to PPCs, such as pleural effusion and atelectasis (32). Shin et al. (33) reported that surgery process had little association with postoperative complications. However, Lee et al. (31) argued that total gastrectomy was the independent risk factor for PPCs, which was in consistence with our study. As was reported, longer duration of surgery, as well as the longer period of mechanical ventilation, may lead to PPCs (34). Unfortunately, different ventilation strategies, with low- or high-volume tide, did not reduce the incidence of PPCs. And the strategy with additional PEEP had a slight effect on avoiding PPCs. Therefore, exploring a better surgical resection method may probably shorten the duration of mechanical ventilation and surgery, and therefore decrease PPCs.
Opioid use in the perioperative period is common for perioperative pain management. Sayal et al. (35) demonstrated that patients with opioid use disorders were at increased risk for PPCs. The increasing dose of opioids might lead to worse surgical outcomes and higher lung complications (36). There was little difference in the dose of fentanyl between PPCs group and non-PPCs group in our cohort study, but the dose of oxycodone was significantly different. A single dose of intravenous oxycodone > 5.5 mg before the abdomen closure was an independent risk factor for PPCs in this study. While oxycodone was reported to regulate inflammatory cytokines and reduce more inflammatory response to alleviate postoperative pain compared with sufentanil (37). Low dose of oxycodone should be recommended after gastrectomy.
Some limitations still exist. Firstly, this is a single-center retrospective study, which may prevent the applicability of the model. A prospective study should be carried out for external evidence for this prediction model. Secondly, some studies suggested PEEP improved outcomes for patients after surgery (38). However, we did not include PEEP as a potential factor as ventilation parameters were not recorded in the anesthesia note in our hospital, and PEEP was commonly set among GC patients during the surgery.
We developed a nomogram model based on age, total cholesterol, extent of resection, duration of surgery, and the dose of oxycodone to predict the probability of PPCs in GC patients after elective gastrectomy. The model may provide convenience for the surgeons to predict the risk of patients developing PPCs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethical Committee of the First Affiliated Hospital of Nanjing Medical University (No: 2023-SR-224). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
LZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. YL: Conceptualization, Writing – original draft, Writing – review & editing. YN: Methodology, Writing – review & editing. CL: Supervision, Writing – review & editing.
This work was supported by Beijing Health Alliance Charitable Foundation, China (No: KM-20220721-01) and Natural Science Foundation of Jiangsu Province (No: SBK2021020423).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1308591/full#supplementary-material
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. (2014) 46(2):124–30. doi: 10.4143/crt.2014.46.2.124
4. Boden I, Skinner EH, Browning L, Reeve J, Anderson L, Hill C, et al. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: pragmatic, double blinded, multicentre randomised controlled trial. Br Med J. (2018) 360:j5916. doi: 10.1136/bmj.j5916
5. Prove Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, de Abreu MG, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. (2014) 384(9942):495–503. doi: 10.1016/S0140-6736(14)60416-5
6. Taylor A, DeBoard Z, Gauvin JM. Prevention of postoperative pulmonary complications. Surg Clin North Am. (2015) 95(2):237–54. doi: 10.1016/j.suc.2014.11.002
7. Yakubek GA, Curtis GL, Khlopas A, Faour M, Klika AK, Mont MA, et al. Chronic obstructive pulmonary disease is associated with short-term complications following total knee arthroplasty. J Arthroplasty. (2018) 33(8):2623–6. doi: 10.1016/j.arth.2018.03.011
8. Yang DS, Glasser J, Lemme NJ, Quinn M, Daniels AH, Antoci V Jr. Increased medical and implant-related complications in total hip arthroplasty patients with underlying chronic obstructive pulmonary disease. J Arthroplasty. (2021) 36(7S):S277–81.e2. doi: 10.1016/j.arth.2021.02.011
9. Tukanova K, Chidambaram S, Guidozzi N, Hanna G, McGregor A, Markar S. Physiotherapy regimens in esophagectomy and gastrectomy: a systematic review and meta-analysis. Ann Surg Oncol. (2022) 29(5):3148–67. doi: 10.1245/s10434-021-11122-7
10. Canet J, Mazo V. Postoperative pulmonary complications. Minerva Anestesiol. (2010) 76(2):138–43.20150855
11. Pan T, Chen XL, Liu K, Peng BQ, Zhang WH, Yan MH, et al. Nomogram to predict intensive care following gastrectomy for gastric cancer: a useful clinical tool to guide the decision-making of intensive care unit admission. Front Oncol. (2021) 11:641124. doi: 10.3389/fonc.2021.641124
12. Karalapillai D, Weinberg L, Peyton P, Ellard L, Hu R, Pearce B, et al. Effect of intraoperative low tidal volume vs conventional tidal volume on postoperative pulmonary complications in patients undergoing major surgery: a randomized clinical trial. JAMA. (2020) 324(9):848–58. doi: 10.1001/jama.2020.12866
13. Campos N, Bluth T, Hemmes S, Librero J, Pozo N, Ferrando C, et al. Intraoperative positive end-expiratory pressure and postoperative pulmonary complications: a patient-level meta-analysis of three randomised clinical trials. Br J Anaesth. (2022) 128(6):1040–51. doi: 10.1016/j.bja.2022.02.039
14. Bazurro S, Ball L, Pelosi P. Perioperative management of obese patient. Curr Opin Crit Care. (2018) 24(6):560–7. doi: 10.1097/MCC.0000000000000555
15. Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. (2018) 21(2):204–12. doi: 10.1007/s10120-017-0744-3
16. Okada S, Shimada J, Kato D, Tsunezuka H, Teramukai S, Inoue M. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg. (2017) 104(1):296–302. doi: 10.1016/j.athoracsur.2017.01.085
17. Shoji F, Matsubara T, Kozuma Y, Haratake N, Akamine T, Takamori S, et al. Preoperative geriatric nutritional risk index: a predictive and prognostic factor in patients with pathological stage I non-small cell lung cancer. Surg Oncol. (2017) 26(4):483–8. doi: 10.1016/j.suronc.2017.09.006
18. Canet J, Gallart L, Gomar C, Paluzie G, Valles J, Castillo J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. (2010) 113(6):1338–50. doi: 10.1097/ALN.0b013e3181fc6e0a
19. Yu Y, Tan Y, Xie C, Hu Q, Ouyang J, Chen Y, et al. Development and validation of a preoperative magnetic resonance imaging radiomics-based signature to predict axillary lymph node metastasis and disease-free survival in patients with early-stage breast cancer. JAMA Netw Open. (2020) 3(12):e2028086. doi: 10.1001/jamanetworkopen.2020.28086
20. Gertsen EC, Goense L, Brenkman HJF, van Hillegersberg R, Ruurda JP, Dutch Upper Gastrointestinal Cancer Audit g. Identification of the clinically most relevant postoperative complications after gastrectomy: a population-based cohort study. Gastric Cancer. (2020) 23(2):339–48. doi: 10.1007/s10120-019-00997-x
21. Liu J, Meng Z, Lv R, Zhang Y, Wang G, Xie J. Effect of intraoperative lung-protective mechanical ventilation on pulmonary oxygenation function and postoperative pulmonary complications after laparoscopic radical gastrectomy. Braz J Med Biol Res. (2019) 52(6):e8523. doi: 10.1590/1414-431(20198523
22. Sabate S, Mazo V, Canet J. Predicting postoperative pulmonary complications: implications for outcomes and costs. Curr Opin Anaesthesiol. (2014) 27(2):201–9. doi: 10.1097/ACO.0000000000000045
23. Putila E, Helminen O, Helmio M, Huhta H, Jalkanen A, Kallio R, et al. Population-based nationwide incidence of complications after gastrectomy for gastric adenocarcinoma in Finland. BJS Open. (2023) 7(5):zrad101. doi: 10.1093/bjsopen/zrad101
24. Cho SJ, Stout-Delgado HW. Aging and lung disease. Annu Rev Physiol. (2020) 82:433–59. doi: 10.1146/annurev-physiol-021119-034610
25. El-Boghdadly K, Cook TM, Goodacre T, Kua J, Blake L, Denmark S, et al. SARS-CoV-2 infection, COVID-19 and timing of elective surgery: a multidisciplinary consensus statement on behalf of the association of anaesthetists, the centre for peri-operative care, the federation of surgical specialty associations, the royal college of anaesthetists and the royal college of surgeons of England. Anaesthesia. (2021) 76(7):940–6. doi: 10.1111/anae.15464
26. Rausei S, Dionigi G, Boni L, Rovera F, Minoja G, Cuffari S, et al. Open abdomen management of intra-abdominal infections: analysis of a twenty-year experience. Surg Infect. (2014) 15(3):200–6. doi: 10.1089/sur.2012.180
27. Orsenigo E, Tomajer V, Palo SD, Carlucci M, Vignali A, Tamburini A, et al. Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer. (2007) 10(1):39–44. doi: 10.1007/s10120-006-0409-0
28. Wang S, Yuan T, Yang H, Zhou X, Cao J. Effect of complete high-caloric nutrition on the nutritional status and survival rate of amyotrophic lateral sclerosis patients after gastrostomy. Am J Transl Res. (2022) 14(11):7842–51.36505314
29. Delgado-Rodriguez M, Medina-Cuadros M, Martinez-Gallego G, Sillero-Arenas M. Total cholesterol, HDL-cholesterol, and risk of nosocomial infection: a prospective study in surgical patients. Infect Control Hosp Epidemiol. (1997) 18(1):9–18. doi: 10.1086/647494
30. Canturk NZ, Canturk Z, Okay E, Yirmibesoglu O, Eraldemir B. Risk of nosocomial infections and effects of total cholesterol, HDL cholesterol in surgical patients. Clin Nutr. (2002) 21(5):431–6. doi: 10.1054/clnu.2002.0575
31. Lee SC, Lee JG, Lee SH, Kim EY, Chang J, Kim DJ, et al. Prediction of postoperative pulmonary complications using preoperative controlling nutritional status (CONUT) score in patients with resectable non-small cell lung cancer. Sci Rep. (2020) 10(1):12385. doi: 10.1038/s41598-020-68929-9
32. Fu X, Wang Z, Wang L, Lv G, Cheng Y, Wang B, et al. Increased diaphragm echodensity correlates with postoperative pulmonary complications in patients after major abdominal surgery: a prospective observational study. BMC Pulm Med. (2022) 22(1):400. doi: 10.1186/s12890-022-02194-6
33. Shin HS, Oh SJ, Suh BJ. Factors related to morbidity in elderly gastric cancer patients undergoing gastrectomies. J Gastric Cancer. (2014) 14(3):173–9. doi: 10.5230/jgc.2014.14.3.173
34. He Y, Xu J, Shang X, Fang X, Gao C, Sun D, et al. Clinical characteristics and risk factors associated with ICU-acquired infections in sepsis: a retrospective cohort study. Front Cell Infect Microbiol. (2022) 12:962470. doi: 10.3389/fcimb.2022.962470
35. Sayal P, Bateman BT, Menendez M, Eikermann M, Ladha KS. Opioid use disorders and the risk of postoperative pulmonary complications. Anesth Analg. (2018) 127(3):767–74. doi: 10.1213/ANE.0000000000003307
36. Lo BD, Zhang GQ, Canner JK, Stem M, Taylor JP, Atallah C, et al. Preoperative opioid dose and surgical outcomes in colorectal surgery. J Am Coll Surg. (2022) 234(4):428–35. doi: 10.1097/XCS.0000000000000109
37. Lao WL, Song QL, Jiang ZM, Chen WD, Zheng XH, Chen ZH. The effect of oxycodone on post-operative pain and inflammatory cytokine release in elderly patients undergoing laparoscopic gastrectomy. Front Med. (2021) 8:700025. doi: 10.3389/fmed.2021.700025
38. Neto AS, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. (2016) 4(4):272–80. doi: 10.1016/S2213-2600(16)00057-6
Keywords: gastrectomy, risk factors, nomogram, state of nutrition, complications
Citation: Zhou L, Li Y, Ni Y and Liu C (2023) Analysis of postoperative pulmonary complications after gastrectomy for gastric cancer: development and validation of a nomogram. Front. Surg. 10:1308591. doi: 10.3389/fsurg.2023.1308591
Received: 17 October 2023; Accepted: 27 November 2023;
Published: 21 December 2023.
Edited by:
Cheng-Mao Zhou, Central People's Hospital of Zhanjiang, ChinaReviewed by:
Qing-Ren Liu, Wuxi Xishan People’s Hospital, China© 2023 Zhou, Li, Ni and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cunming Liu Y3VubWluZ2xpdUBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.