
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 07 November 2023
Sec. Visceral Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1293270
This article is part of the Research Topic Clinical Applications of Major Magnetosurgery Techniques View all 4 articles
Background: The magnetic sphincter augmentation (MSA) procedure is an effective treatment for gastroesophageal reflux disease (GERD). Adverse events requiring MSA device removal are rare, but the true prevalence and incidence may be underestimated.
Methods: Retrospective study on a prospectively collected database. Patients who underwent MSA procedure between March 2007 and September 2021 in two tertiary-care referral centers for esophageal surgery were included. The trend of MSA explant, the changes in the sizing technique and crura repair over the years, the technique of explant, and the clinical outcomes of the revisional procedure were reviewed.
Results: Out of 397 consecutive patients, 50 (12.4%) underwent MSA removal, with a median time to explant of 39.5 [IQR = 53.7] months. Main symptoms leading to removal were dysphagia (43.2%), heartburn (25%), and epigastric pain (13.6%). Erosion occurred in 2.5% of patients. Smaller (12- and 13-bead) devices were the ones most frequently explanted. The majority of the explants were performed laparoscopically with endoscopic assistance. There was no perioperative morbidity, and the median length of stay was 2.8 ± 1.4 days. After 2014, changes in sizing technique and crura repair resulted in a decreased incidence of explants from 23% to 5% (p < 0.0001). Multivariate analysis confirmed the protective role of added bead units [HR 0.06 (95% CI = 0.001–0.220); p < 0.000].
Conclusion: Oversizing and full mediastinal dissection with posterior hiatoplasty may improve the outcomes of the MSA procedure and possibly reduce removal rates.
The magnetic sphincter augmentation (MSA) device, first introduced in 2007 and approved by FDA in 2012, has gained increasing acceptance as a valid therapeutic option for the management of gastroesophageal reflux disease (GERD) (1–3). MSA has been shown to be effective with relief of reflux symptoms, discontinuation of daily proton pump inhibitors (PPI), and reduction of esophageal acid exposure. Compared to the traditional fundoplication, MSA seems associated with less gas bloat symptoms and maintained ability to belch and vomit (4–6). Other potential advantages of MSA are procedural standardization and preservation of esophago-gastric anatomy (7–12).
However, concerns about MSA-related complications, such as persistent dysphagia and full-thickness erosion, have led to criticism mainly based on historical and anecdotal evidence with the Angelchick prosthesis (13). Previous studies reported MSA device removal rates ranging from 1.1% to 6.7% (14–17). While the use of the MSA is increasing worldwide, studies focusing on device failure, explant techniques, and clinical outcomes of revisional surgery remain essential at this stage of MSA adoption in surgical practice. We aimed to analyze the long-term safety profile of MSA and to identify factors predictive for removal.
A retrospective, observational two-center cohort study was designed and approved by the local Institutional Review Board of two tertiary care University hospitals (IRCCS Policlinico San Donato and IRCCS Ospedale Galeazzi-Sant’Ambrogio). The prospectively collected dataset was queried to identify adult patients who underwent MSA device implant and/or removal from March 2007 and September 2021 with 12-month minimum follow-up. Patients with abnormal esophageal acid exposure and persistent GERD symptoms despite maximal PPI therapy for at least 6 months were eligible for MSA implant. Patients with severe esophageal dysmotility, previous esophageal surgery, known allergy to titanium/nickel, or eating disorders were excluded. Patients were divided into two groups, MSA-R (removed) and MSA-P (preserved), and were compared in terms of demographics, clinical features, procedural characteristics, and postoperative outcomes.
Age, sex, body mass index (BMI), comorbidity, symptoms, duration disease, and duration of PPI use were recorded. Severity of symptoms was measured by means of the Gastroesophageal Reflux Disease Health-Related Quality of Life (GERD-HRQL) scale (18). Preoperative data included upper gastrointestinal endoscopic findings (Hill grade, size of hiatal hernia, Barrett's esophagus, esophagitis), swallow study (hiatal hernia, esophageal dysmotility), conventional or high-resolution esophageal manometry (motility pattern, distal esophageal amplitude, lower esophageal sphincter basal and residual tone, total/intra-abdominal LES length), and 24-hour pH- or pH-impedance monitoring (percent pH <4, total number of reflux episodes).
Collected operative data included year of surgery, MSA device size, number of added bead units after sizing, type of mediastinal dissection (no dissection ND; minimal dissection MD; full dissection FD), and number of stitches required to approximate the crural defect. Operative time, and intra- and postoperative complications graded according by the Clavien-Dindo (C-D) classification were collected (19). Safety of the procedure was defined as no occurrence of major intraoperative or early and long-term complications (C-D ≥3). Patients who underwent explant of the device in other hospitals were interviewed by telephone.
The laparoscopic technique for MSA implant evolved over the study period. Since 2014, the original metal sizer ring was replaced by a new sizer, equipped with a soft tip for a dynamic measurement of the esophageal circumference. To determine the proper size of the device to be implanted, care was taken to repeat sizing at least twice and to rotate the shaft at each size for visual inspection of esophageal compression during closure of the tip. A variable number of 1 to 3 beads were added from the point of release of the sizing tool. Since January 2018, full mediastinal dissection was routinely performed in all patients.
The surgical technique of laparoscopic MSA explant consisted of the following steps: (1) identification of the fibrous capsule covering the device; (2) incision of the capsule with electrocautery to grasp the anterior beads; (3) unlocking the device or cutting the wire between adjacent beads; (4) progressive retrieval of the entire device from the retroesophageal tunnel; (5) device extraction from the abdominal cavity through a 12 mm port site (Figure 1). Intraoperative endoscopic assistance was used to identify the gastroesophageal junction and to check the integrity of the esophageal mucosa after removal. In some patients, partial or complete retrieval of the MSA device eroded into the esophageal lumen could be performed endoscopically after unlocking the device or cutting the wire connecting the beads. A concurrent cruroplasty with or without partial fundoplication was performed in selected patients. The choice of fundoplication was based on patients’ preference, persistent GERD symptoms, presence of hiatal hernia, and persistently abnormal esophageal acid exposure on 24-hour pH monitoring.
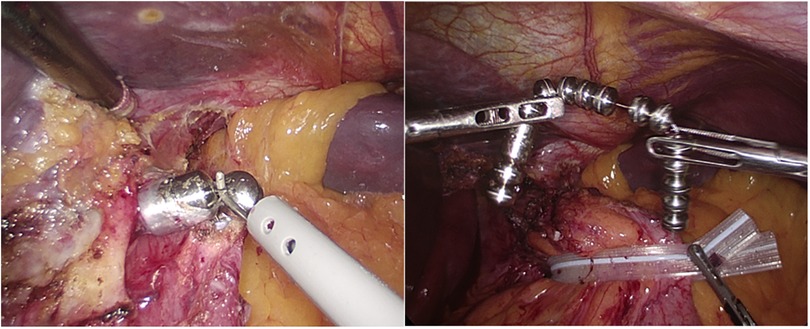
Figure 1. Incision of the fibrotic capsule overlying the MSA device. After cutting the wire connecting the beads, the device is removed and extracted though a 10 mm port.
The post-operative follow-up consisted of barium swallow study and/or upper-GI endoscopy within one year after surgery. Clinical evaluation was performed yearly thereafter. All patients completed the GERD-HRQL questionnaire at each follow-up time point. Selected patients underwent esophageal manometry and/or 24-hour pH monitoring.
All procedures were conducted in accordance with the ethical standards and with the Helsinki Declaration. Appropriate informed consent was collected from all patients.
Categoric variables are presented as frequency and percentages while continuous variables are presented as means ± SD for normal distributions or median and interquartile range for non-normal distributions. Event was defined MSA removal. Time to event was definite as the time from surgery to removal. Patients without events at the last follow-up were entered in the survival analysis. The study compared Removal vs. No-Removal patients and within the Removal group, patients with erosion and dysphagia. Median follow-up time was calculated according to the Kaplan–Meier method. The Mann–Whitney U-test was used to compare continuous variables, whereas the Fisher's exact test or Pearson's χ2 test was used to compare proportions for categorical variables. Multivariate logistic regression analysis was performed to identify independent predictors for MSA removal. Multicollinearity testing was performed to identify the correlation between these variables. The accuracy of the test was calculated using the area under the curve with a 95% confidence interval (CI). Variables with p values less than 0.05 were considered significant. All analyses were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC).
During the study period, 397 patients underwent MSA device implant for refractory GERD. The MSA device explant rate was 12.6%, and the median time from index surgery to device removal was 39.5 [IQR = 53.7] months. Dysphagia (43.2%), heartburn (25%), epigastric pain (13.6%), and erosion (2.5%) were the main reasons for device removal. Two patients were explanted due to the need of 3-T MRI studies. In one patient presenting with de-novo epigastric pain, the chest x-ray showed an unlocked device.
No significant differences were found between patients with and without dysphagia in terms of preoperative symptoms, radiological, endoscopic, manometric findings, and operative technique (type of hiatal dissection, number of crura stitches, and number of added beads). The median time to explant was similar in patients with and without dysphagia (41 vs. 39.5 months; p = 0.99). MSA-related erosion occurred in 2.5% of cases, more commonly in patients with smaller (12 and 13 beads) devices. No significant differences were found between patients with and without erosion in terms of preoperative symptoms, radiological/endoscopic/manometric findings, and operative variables.
All patients underwent laparoscopic MSA device removal with endoscopic assistance. There were no conversions to an open procedure. Eight patients consented only for device removal and refused any additional antireflux repair. Two patients underwent a combined single-stage endo-laparoscopic procedure. A 36-month pregnant patient with MSA erosion underwent a two-stage procedure, partial endoscopic removal first and subsequent laparoscopic removal after delivery. A totally endoscopic explant was successfully completed in one patient. Most explanted MSA devices were the 12- and 13-bead. The overall morbidity rate of the revisional procedure was 6% (Tables 1, 2). Toupet fundoplication was performed in patients with recurrent heartburn; in patients that gave consent, an anterior fundoplication was fashioned after MSA explant.
The highest rates of explants were recorded between the years 2009 and 2014 (Figure 2). Since 2014, upon modification of the surgical procedure, the incidence of explants significantly decreased from 23% to 5% (p < 0.0001). The removal rate according to the number of added beads was also significantly lower in patients with at least 2 added units (56.8% vs. 18%; p < 0.0001). At the 60-month follow-up, with a total of 196 patients observed, the cumulative risk of explant was 9.9%. The Kaplan Meier curve shows the trend of device removal after MSA implantation (Figure 3).
Preoperative hiatus hernia, greater percent of acid exposure in the supine position, no or minimal hiatal dissection, use of non-iterative sizer ring, and use of smaller devices were significantly more prevalent in the removal group at univariate analysis (Table 3). Hiatal hernia at the time of diagnosis (OR 7.03, 95% CI = 2.93–16.89), the number of added units (OR 3.25, 95% CI = 1.36–7.80), and the type of hiatal dissection (OR 2.65 (95% CI = 1.38–5.10) were risk factors for MSA explant. Use of a larger device was associated with a reduced risk for removal (OR 0.30, 95% CI = 0.12–0.72). In the logistic regression analysis, hiatal hernia at time of diagnosis was an independent predictor of MSA device explant (OR 18.1, 95% CI = 8.80–37.26; p < 0.0001), while oversizing with added bead units appeared to be a protective factor (OR 0.06, 95% CI = 0.001–0.22; p < 0.0001) (Table 4).
In our study, the rate of MSA removal was 12.6%, with a median time to explant of 39.5 months. The main reasons leading to device removal were dysphagia and persistent heartburn/epigastric pain. Device oversizing and full hiatoplasty improved the long-term success rate of the MSA procedure.
Removal of the MSA device is reported in 1.1% to 6.7% of the patients (14–17), with a median time to explant ranging from 3 to 24 months. Reasons for the higher removal rate in our series are twofold. First, previous published studies (9–11) are based on FDA centralized data and self- reporting MAUDE database, therefore complications may be underreported. Second, our study has a long median follow-up and includes a large number of patients implanted during the early years of experience. The main reasons for MSA removal reported in previous series were dysphagia and persistent GERD symptoms (15, 16). Although MSA-related dysphagia was thoroughly investigated in our study, we were unable to find predictors of postoperative dysphagia. Despite the lack of statistical significance, post-operative dysphagia requiring MSA removal occurred more frequently with smaller device sizes. This is similar to a recent retrospective series of 268 patients with a median follow-up of 23 months showing that MSA device size <13 was the only factor associated with postoperative dysphagia (20). Ayazi et al. (21), in a cohort study of 380 patients, found that risk factors for postoperative dysphagia were preoperative dysphagia and less than 80% peristaltic contractions on preoperative manometry. Another recent paper showed that preoperative dysphagia was the only factor significantly associated with postoperative dysphagia (22). Since dysphagia remains the most frequent side effect of MSA placement, it remains of paramount importance to establish the proper post-operative management to prevent the formation of a tight fibrous capsule (23, 24). In particular, early swallowing exercise with frequent small and solid meals appears to be crucial after MSA implant. Ayazi et al. (21) found persistent postoperative dysphagia in 15.5% of the patients, one third of whom required at least one endoscopic dilatation. It appears that early dilation (<8 weeks) worsened dysphagia, probably due to an increased inflammatory response around the device, so the authors advised steroid pulses and delayed endoscopic dilation. In our cohort, the dilation rate was 5.2%, lower than that reported in literature, and the median time to postoperative dilation was longer (13 months after MSA placement).
Mucosal erosion and device migration is the most feared complication and a relevant parameter of safety after any foreign body placement at the EGJ. The overall prevalence in our series was 2.5%, with median implant duration of 59 months. The erosion occurred with device size 12 in most cases, and this is consistent with the analysis of the manufacture database (11). The association of dysphagia/erosion with a smaller device can reasonably be explained by malfunctioning of the device due to a constrictive capsule fibrosis as a result of an exaggerated inflammatory response. All the erosion events reported in literature, as well as in our experience, were non-acute on presentation and were not associated with intrabdominal abscess or peritonitis.
We also assessed the safety of the MSA explant. All procedures were performed electively, by a single stage laparoscopic or combined endo-laparoscopic approach; when migration of beads was found, partial transoral removal of the device was occasionally performed without complications (16, 25). In a retrospective study of 425 patients with a removal rate of 5.5%, Tatum et al. (15) suggested to avoid additional intervention and to redo an MSA implant or perform fundoplication only in patient with malposition of the device or hiatal hernia. In a recent study, removal for dysphagia yielded excellent outcomes regardless of concurrent antireflux repair, whereas patients with persistent GERD had worse outcomes without antireflux repair ( 26). Our preference is to perform a posterior Toupet fundoplication in patients with hiatal hernia or persistent GERD, and an anterior fundoplication in patients with erosion.
The size of the MSA device appears critical to avoid esophageal constriction and to reduce an excessive capsular fibrosis around the device. In our series, from 2007 to 2013, measurement of esophageal circumference was performed using a metal sizing tool (1), and smaller devices were used, which are no longer available. Since 2014, the new standard was to repeat sizing at least twice, rely on visual cues, and oversize by 1 to 3 beads from the point of release of the sizing tool to reduce the risk of mismatch between the device and the esophageal lumen size. These modifications led to the use of larger devices and decrease in the rate of both dysphagia and erosions. More recently, a novel technology of intraoperative impedance planimetry assessed by Functional Lumen Imaging Probe (FLIP) was used for real-time evaluation of esophago-gastric junction distensibility to calibrate the surgical procedure and optimize outcomes. However, more data and normative FLIP values are needed to define the role of this technology in selecting the appropriate device size (27, 28).
In our study, on multivariate analysis, the addition of beads resulted to be an independent protective factor, while the presence of hiatal hernia with GERD was associated with an increased risk of device removal. Interestingly, a few retrospective studies demonstrated favorable short- and medium-term results for patients with >3 cm hiatal hernia (29–32). Irribarra et al. (33) and Tatum et al. (34) found that concurrent hiatal dissection with restoration of esophageal length and crura repair during the MSA procedure were more likely to normalize postoperative DeMeester score and acid exposure, reduce the rate of recurrence/progression of hiatal hernia, and reduce revisional surgery rates compared to minimal hiatal dissection. According to the hypothesis of the sphincter-like action of the diaphragmatic crura (35), the concept of full hiatoplasty was progressively incorporated into the MSA procedure. Inadequate mediastinal dissection and suboptimal crura repair may explain some failures of the MSA procedure. In fact, comparison of the subgroup of patients with hiatal hernia in our series seems to corroborate this analysis. Smaller hernia size (<3 cm) and minimal dissection were associated to worse outcomes. Therefore, patients with larger hernias are likely to have received adequate mediastinal dissection, compared to patients with smaller hernias in whom a less invasive hiatal repair was chosen. These results are not confirmed in our multivariate analysis model probably due to the small sample size, the low rate of removal events, and the retrospective study design.
This study has several limitations. Its retrospective design does not allow for patient stratification based on pre-established clinical and preoperative characteristics. Patients’ selection criteria for MSA changed during the study period, with the extension of the indications to patients who would have previously been considered ineligible for MSA. On the other hand, preoperative assessment has also evolved during the study period. Finally, the relatively small sample size of explanted patients did not allow to identify additional predictive factors with adequate statistical power to reach robust conclusions.
Technical advances in the MSA procedure, including formal mediastinal dissection, posterior crura repair, and use of a larger device, have improved clinical outcomes and reduced removal rates over the years. Explant of the device performed in a tertiary center by experienced surgeons has proven safe and effective through either a laparoscopic or endoscopic approach. Nevertheless, more data are needed to improve patient selection, long-term safety, and outcomes of the MSA procedure.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by IRCCS Policlinico San Donato Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CF: Formal analysis, Investigation, Methodology, Writing – original draft. AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. DB: Supervision, Writing – review & editing. LB: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Data curation, Investigation, Methodology, Project administration, Validation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bonavina L, Saino GI, Bona D, Lipham J, Ganz RA, Dunn D, et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg. (2008) 12(12):2133–40. doi: 10.1007/s11605-008-0698-1
2. Bonavina L, DeMeester T, Fockens P, Dunn D, Saino G, Bona D, et al. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg. (2010) 252(5):857–62. doi: 10.1097/SLA.0b013e3181fd879b
3. Lipham JC, DeMeester TR, Ganz RA, Bonavina L, Saino G, Dunn DH, et al. The LINX® reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc. (2012) 26(10):2944–9. doi: 10.1007/s00464-012-2289-1
4. Saino G, Bonavina L, Lipham JC, Dunn D, Ganz RA. Magnetic sphincter augmentation for gastroesophageal reflux at 5 years: final results of a pilot study show long-term acid reduction and symptom improvement. J Laparoendosc Adv Surg Tech A. (2015) 25(10):787–92. doi: 10.1089/lap.2015.0394
5. Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, DeVault KR, et al. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol. (2016) 14(5):671–7. doi: 10.1016/j.cgh.2015.05.028
6. Ferrari D, Asti E, Lazzari V, Siboni S, Bernardi D, Bonavina L. Six to 12-year outcomes of magnetic sphincter augmentation for gastroesophageal reflux disease. Sci Rep. (2020) 10(1):13753. doi: 10.1038/s41598-020-70742-3
7. Aiolfi A, Asti E, Bernardi D, Bonitta G, Rausa E, Siboni S, et al. Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: systematic review and meta-analysis. Int J Surg. (2018) 52:82–8. doi: 10.1016/j.ijsu.2018.02.041
8. Guidozzi N, Wiggins T, Ahmed AR, Hanna GB, Markar SR. Laparoscopic magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: systematic review and pooled analysis. Dis Esophagus. (2019) 32(9):doz031. doi: 10.1093/dote/doz031
9. Lipham JC, Taiganides PA, Louie BE, Ganz RA, DeMeester TR. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus. (2015) 28(4):305–11. doi: 10.1111/dote.12199
10. Smith CD, Ganz RA, Lipham JC, Bell RC, Rattner DW. Lower esophageal sphincter augmentation for gastroesophageal reflux disease: the safety of a modern implant. J Laparoendosc Adv Surg Tech A. (2017) 27(6):586–91. doi: 10.1089/lap.2017.0025
11. DeMarchi J, Schwiers M, Soberman M, Tokarski A. Evolution of a novel technology for gastroesophageal reflux disease: a safety perspective of magnetic sphincter augmentation. Dis Esophagus. (2021) 34(11):doab036. doi: 10.1093/dote/doab036
12. Ayazi S, Zheng P, Zaidi AH, Chovanec K, Salvitti M, Newhams K, et al. Clinical outcomes and predictors of favorable result after laparoscopic magnetic sphincter augmentation: single-institution experience with more than 500 patients. J Am Coll Surg. (2020) 230(5):733–43. doi: 10.1016/j.jamcollsurg.2020.01.026
13. Pence MM, Hubbard M, Singla MB, Young PE. Esophagogastric fistula caused by an angelchik antireflux prosthesis. ACG Case Rep J. (2015) 2(4):213–5. doi: 10.14309/crj.2015.62
14. Asti E, Siboni S, Lazzari V, Bonitta G, Sironi A, Bonavina L. Removal of the magnetic sphincter augmentation device: surgical technique and results of a single-center cohort study. Ann Surg. (2017) 265(5):941–5. doi: 10.1097/SLA.0000000000001785
15. Tatum JM, Alicuben E, Bildzukewicz N, Samakar K, Houghton CC, Lipham JC. Removing the magnetic sphincter augmentation device: operative management and outcomes. Surg Endosc. (2019) 33(8):2663–9. doi: 10.1007/s00464-018-6544-y
16. Alicuben ET, Bell RCW, Jobe BA, Buckley FP 3rd, Daniel Smith C, Graybeal CJ, Lipham JC. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. (2018) 22(8):1442–7. doi: 10.1007/s11605-018-3775-0
17. Bona D, Saino G, Mini E, Lombardo F, Panizzo V, Cavalli M, et al. Magnetic sphincter augmentation device removal: surgical technique and results at medium-term follow-up. Langenbecks Arch Surg. (2021) 406(7):2545–51. doi: 10.1007/s00423-021-02294-7
18. Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. (2007) 20(2):130–4. doi: 10.1111/j.1442-2050.2007.00658.x
19. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
20. Bologheanu M, Matic A, Feka J, Asari R, Bologheanu R, Riegler FM, et al. Severe dysphagia is rare after magnetic sphincter augmentation. World J Surg. (2022) 46(9):2243–50. doi: 10.1007/s00268-022-06573-2
21. Ayazi S, Zheng P, Zaidi AH, Chovanec K, Chowdhury N, Salvitti M, et al. Magnetic sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J Gastrointest Surg. (2020) 24(1):39–49. doi: 10.1007/s11605-019-04331-9
22. Riva CG, Siboni S, Sozzi M, Lazzari V, Asti E, Bonavina L. High-resolution manometry findings after linx procedure for gastro-esophageal reflux disease. Neurogastroenterol Motil. (2020) 32(3):e13750. doi: 10.1111/nmo.13750
23. Zadeh J, Andreoni A, Treitl D, Ben-David K. Spotlight on the linx™ reflux management system for the treatment of gastroesophageal reflux disease: evidence and research. Med Devices (Auckl). (2018) 11:291–300. doi: 10.2147/MDER.S113679
24. Bielefeldt K. Adverse events after implantation of a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol. (2016) 14(10):1507–8. doi: 10.1016/j.cgh.2016.05.003
25. Yeung BPM, Fullarton G. Endoscopic removal of an eroded magnetic sphincter augmentation device. Endoscopy. (2017) 49(7):718–9. doi: 10.1055/s-0043-109236
26. Eriksson S, Schwameis K, Ayazi S, Hoppo T, Zheng P, Jobe BA. Removal of magnetic sphincter augmentation device: an assessment of etiology, clinical presentation, and management. Surg Endosc. (2023) 37(5):3769–79. doi: 10.1007/s00464-023-09878-y
27. Su B, Dunst C, Gould J, Jobe B, Severson P, Newhams K, et al. Experience-based expert consensus on the intra-operative usage of the endoflip impedance planimetry system. Surg Endosc. (2021) 35(6):2731–42. doi: 10.1007/s00464-020-07704-3
28. Froiio C, Tareq A, Riggio V, Siboni S, Bonavina L. Real-world evidence with magnetic sphincter augmentation for gastroesophageal reflux disease: a scoping review. Eur Surg. (2023) 55:8–19. doi: 10.1007/s10353-022-00789-1
29. Rona KA, Reynolds J, Schwameis K, Zehetner J, Samakar K, Oh P, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc. (2017) 31(5):2096–102. doi: 10.1007/s00464-016-5204-3
30. Rona KA, Tatum JM, Zehetner J, Schwameis K, Chow C, Samakar K, et al. Hiatal hernia recurrence following magnetic sphincter augmentation and posterior cruroplasty: intermediate-term outcomes. Surg Endosc. (2018) 32(7):3374–9. doi: 10.1007/s00464-018-6059-6
31. Buckley FP 3rd, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc. (2018) 32(4):1762–8. doi: 10.1007/s00464-017-5859-4
32. Dunn CP, Zhao J, Wang JC, Patel TA, Putnam LR, Eka A, et al. Magnetic sphincter augmentation with hiatal hernia repair: long term outcomes. Surg Endosc. (2021) 35(10):5607–12. doi: 10.1007/s00464-020-08063-9
33. Irribarra MM, Blitz S, Wilshire CL, Jackson AS, Farivar AS, Aye RW, et al. Does treatment of the hiatus influence the outcomes of magnetic sphincter augmentation for chronic GERD? J Gastrointest Surg. (2019) 23(6):1104–12. doi: 10.1007/s11605-019-04180-6
34. Tatum JM, Alicuben E, Bildzukewicz N, Samakar K, Houghton CC, Lipham JC. Minimal versus obligatory dissection of the diaphragmatic hiatus during magnetic sphincter augmentation surgery. Surg Endosc. (2019) 33(3):782–8. doi: 10.1007/s00464-018-6343-5
Keywords: magnetic sphincter augmentation device, LINX, erosion, dysphagia, MSA removal, gastroesophageal reflux disease (GERD)
Citation: Froiio C, Aiolfi A, Bona D and Bonavina L (2023) Safety profile of magnetic sphincter augmentation for gastroesophageal reflux disease. Front. Surg. 10:1293270. doi: 10.3389/fsurg.2023.1293270
Received: 12 September 2023; Accepted: 23 October 2023;
Published: 7 November 2023.
Edited by:
Xiaopeng Yan, The First Affiliated Hospital of Xi'an Jiaotong University, ChinaReviewed by:
Marialuisa Lugaresi, University of Bologna, Italy© 2023 Froiio, Aiolfi, Bona and Bonavina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Bonavina bHVpZ2kuYm9uYXZpbmFAdW5pbWkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.