
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 22 December 2023
Sec. Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1278177
 Mikhail Harty1
Mikhail Harty1 Muhammad Waqas Saeed Baqai1
Muhammad Waqas Saeed Baqai1 Jahangir Sajjad1
Jahangir Sajjad1 Greg Fellows2
Greg Fellows2 Philip J. Clamp2
Philip J. Clamp2 Kumar Abhinav1*
Kumar Abhinav1*
Background: Most cavernous malformations (CM) usually involve the parenchyma and rarely occur in cranial nerves. Recurrence of CM associated with cranial nerves after surgical resection has not been previously reported.
Case description: This paper describes the case of an 11-year-old girl who presented with left otalgia and headache because of a left trigeminal cavernous malformation. She underwent radical resection via a left retrosigmoid approach while sparing the trigeminal nerve. Surveillance imaging at 18 months demonstrated recurrence along the length of the trigeminal nerve into Meckel's cave with significant extension into the middle cerebellar peduncle. Subsequent re-operation via an extended middle fossa approach with anterior petrosectomy enabled complete resection with division of the trigeminal nerve. Postoperatively, she had a transient left facial paresis, and right hemiparesis that resolved within 48 h.
Conclusion: This case highlights the importance of close postoperative surveillance in CM associated with cranial nerves as recurrence after nerve-sparing resection is possible. Surgical treatment due to the morphology of significant recurrence required the use of a complex skull base approach through a new corridor to achieve optimal clinical outcome.
Cavernous malformations (CM) are relatively common vascular malformation that occurs in 0.9% of the general population (1). Typically, they are found within the brain parenchyma while involvement of cranial nerves is rare (2–4). Gross et al. found that in persons less than 21 years, CM present with an average age of 10 years (5). To our knowledge we present the first case of a pediatric case with recurrent CM along the anatomical distribution of the trigeminal nerve and the surgical strategy to address the recurrence.
An 11-year-old girl presented with a 3-week history of left otalgia, headache, and an episode of syncope. Examination revealed normal neurologic findings and no focal deficits (Figure 1). Magnetic resonance imaging (MRI) of the brain showed a hemorrhagic cystic lesion in the left cerebellopontine angle (CPA) with a differential diagnosis of schwannoma vs. trigeminal CM. She initially underwent a period of surveillance during which time the lesion involuted at 1 year (Figure 2). At 2 years, repeat images showed new hemorrhage and the decision was made for surgical intervention. She underwent a left retrosigmoid craniotomy and was found to have a CM embedded within the cisternal segment of the trigeminal nerve that extended into Meckel's cave. A satisfactory resection was thought to have been achieved at surgery with preservation of the nerve fascicles and on postoperative imaging. She had an unremarkable postoperative course.
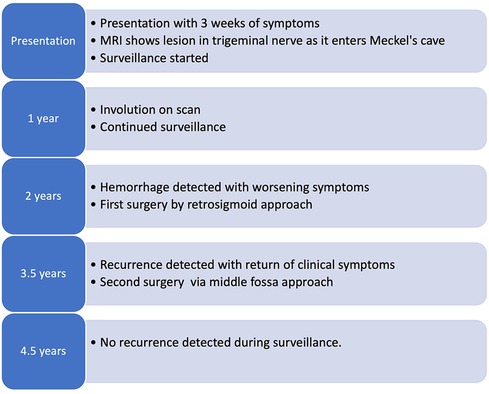
Figure 1. Schematic representation of the timeline from presentation and primary surgery through the recurrence of the lesion and the second operation.
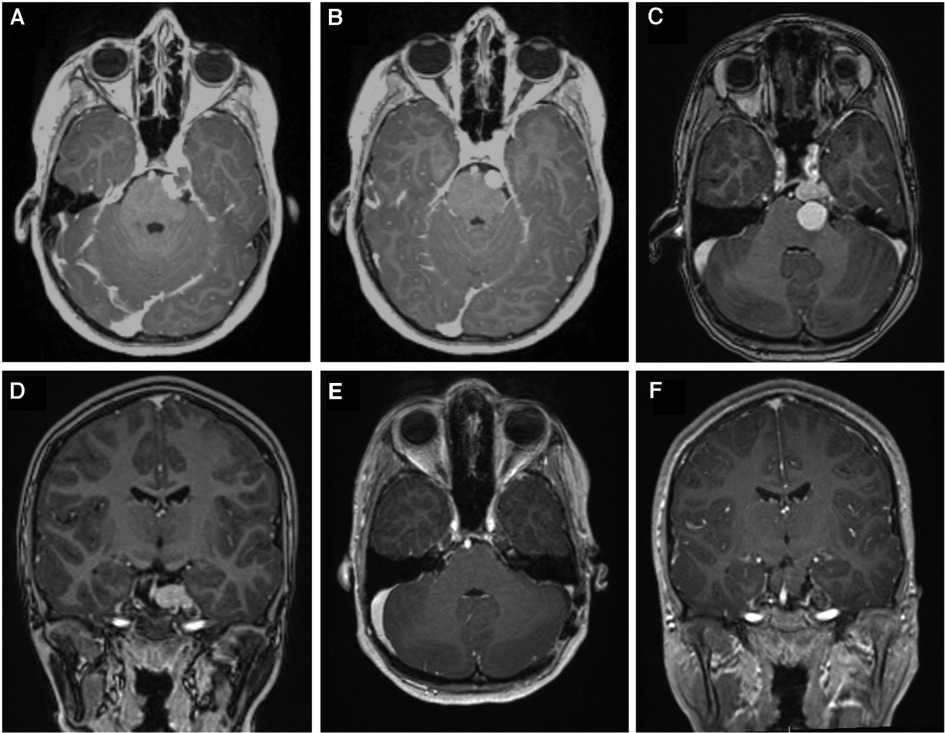
Figure 2. (A) Axial contrast enhanced T1-weighted image showing a lesion in the left trigeminal nerve extending from pontine portion into Meckel's cave. (B) shows interval image at 1 year after presentation which showed involution of the lesion. (C, D), show new hemorrhage with cystic portion invaginating the brainstem which prompted surgical intervention. (E, F) show 6-month postoperative contrast enhanced T1-weighted axial and coronal images, respectively, after the first operation via retrosigmoid approach demonstrating gross total resection.
Imaging done up to 12 months after surgery demonstrated no recurrence of the lesion. By 18 months she began to experience significant left-sided facial hypesthesia and sensory symptoms worse in the distribution of the 3rd division of the trigeminal nerve. MRI revealed considerable recurrence of the lesion along the anatomical extent of the trigeminal nerve which distorted the ventrolateral brainstem, middle cerebellar peduncle (MCP) and extended into the Meckel's cave and caudally below the trigeminal nerve toward the internal acoustic canal (Figure 3).
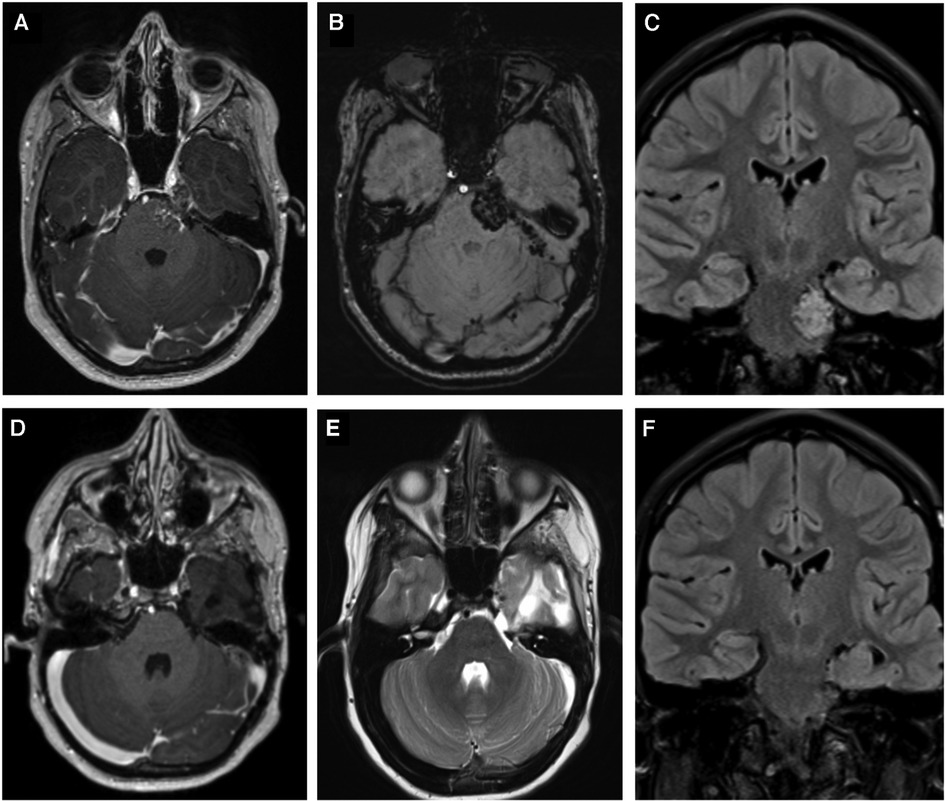
Figure 3. Shows images before and after the second operation via a subtemporal Kawase-Dolenc approach. (A) contrast enhanced T1-weighted imaging and (B) Susceptibility weighted image demonstrate the lesion filling Meckel's cave extending along the cisternal segment of the trigeminal nerve and invaginating the brain. (C) demonstrates the coronal FLAIR that shows the CM involving the brainstem and extending down to the internal acoustic meatus. (D,E) are contrast-enhanced T1, and T2-weighted images, respectively, and (F) is a coronal FLAIR that demonstrate complete resection of the recurrent lesion.
The second operation utilized an extended middle fossa (Kawase-Dolenc) approach. Intraoperative neuromonitoring was employed to aid resection. Key operative steps included a frontotemporal craniotomy with an anterior interdural approach to the mandibular nerve then posterior dural elevation to undertake anterior petrosectomy (Figure 4). The large solid exophytic CM appeared intimately related to the anatomical trajectory of the trigeminal nerve with a significant component embedded within the brainstem. The brainstem component was gently removed in piecemeal fashion while respecting the hemosiderin-stained parenchyma. The trigeminal nerve was sacrificed to achieve gross total resection with the tentorial division and mobilization to further permit the removal of the component within Meckel's cave. Postoperatively, she had a transient left facial paresis and right hemiparesis resolving within 48 h. A small pseudomeningocele resolved within four months. She had facial hypesthesia that persisted after the operative intervention.
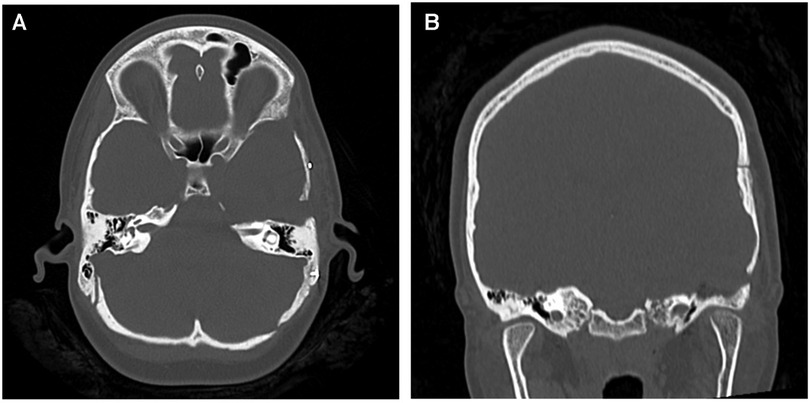
Figure 4. (A) axial and (B) coronal CT images demonstrating the extent of the anterior petrosectomy to give access to the lesion during the Kawase-Dolenc approach at the second operation.
Postoperative images showed gross total resection with no evidence of radiological recurrence at 12 months (Figure 2). Genetic testing for familial CM was negative for mutations.
Trigeminal CM are extremely rare with only 17 cases reported in literature (2, 4, 6–19). Majority of these cases presented with trigeminal neuralgia and were treated with surgical resection. Only 2 were treated with stereotactic radiosurgery and one with percutaneous balloon compression (10, 13, 19). Adachi et al. classified these lesions into 4 types according to the origin of the CM: within the Gasserian ganglion, within the cisternal segment, within the intra-axial trigeminal nerve root in the pons, and within the spinal tract of the trigeminal nerve.6The retrosigmoid approach was chosen for the first operation because it gave access to the cerebellopontine angle and is a workhorse approach for this region (4, 20). This approach facilitated removal of the hemorrhagic cystic component invaginating the brainstem while allowing extirpation of the CM. Gross total resection was thought to have been achieved intraoperatively with preservation of the trigeminal nerve fascicles and was confirmed with postoperative surveillance MRI.
The decision-making process concerning the management of the recurrence took into consideration that the lesion was high risk for clinically significant re-hemorrhage due to at least two previous documented radiological evidence of hemorrhage before the first surgery. Moreover, there was now a significant large volume symptomatic recurrence over a short period of time after prior surgical resection. Stereotactic radiosurgery was deemed to be feasible and was offered. However, due to the factors as outlined above in addition to the favorable exophytic nature of the lesion, after discussion with the family, surgery via a new operative corridor was undertaken.
At the second operation, a different anterolateral operative corridor was chosen to avoid the scarred retrosigmoid corridor and, more importantly, to resect the lesion along the axis of its recurrence while avoiding neural transgression of the surrounding middle cerebellar peduncle. In addition, the extended middle fossa approach with anterior petrosectomy enabled access to the ventrolateral pontine surface. This allowed the caudal extent of the recurrence below the trigeminal nerve to be safely resected while ensuring removal of the component within Meckel's cave after tentorial division to achieve gross total resection.
Recurrence after gross total resection of CM in the pediatric population has been rare with a reported rate of 0.7% at 1 year but none of these described recurrence of CM associated with a cranial nerve (21). Samadian et al., in a case of CM associated with the trochlear nerve recommended sectioning and reanastomosis of the nerve to achieve resection (8, 22). Liu et al. in their case report described a CM of the trigeminal nerve that was intimately related to the sensory root which was sacrificed while preserving the motor root (11). Mascarenhas et al., described resection of a CM adherent to the second and third division of the trigeminal nerve that required taking some of the rootlets of the nerve (12). It is likely in our case that the CM was located within the trigeminal nerve substance and a small remnant along the fascicles facilitated the recurrence after the first surgery while attempting preservation of the nerve. At the second operation, due to significant recurrence and because of the intimate relationship between the CM and the trigeminal nerve, the preservation of the nerve could not have been undertaken in any meaningful manner and therefore it was sacrificed during the CM resection. Attention was also paid to remove the Meckel's cave component to reduce the risk of recurrence.
Close clinical and radiological surveillance after surgical resection of trigeminal or a cranial nerve CM is needed because the clinical course is ill-defined in the pediatric population with a potentially higher risk of recurrence (9, 21). This case additionally highlights the importance of the use of complex skull base approaches when required to achieve optimal clinical and surgical outcome in patients with surgically challenging recurrent CM particularly for those that directly or indirectly involve the brainstem.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the studies involving humans because no patient identifier was used. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MH: Writing – original draft, Formal analysis, Writing – review & editing. MB: Writing – original draft, Software, Visualization. JS: Formal analysis, Visualization, Writing – review & editing. GF: Writing – review & editing. PC: Supervision, Writing – review & editing. KA: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kim DS, Park YG, Choi JU, Chung SS, Lee KC. An analysis of the natural history of cavernous malformations. Surg Neurol. (1997) 48(1): 9–17; discussion 17–18. doi: 10.1016/s0090-3019(96)00425-9
2. Goldstein HE, Solomon RA. Epidemiology of cavernous malformations. Handb Clin Neurol. (2017) 143:241–7. doi: 10.1016/b978-0-444-63640-9.00023-0
3. Deshmukh VR, Albuquerque FC, Zabramski JM, Spetzler RF. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery. (2003) 53(2): 352–7; discussion 357. doi: 10.1227/01.neu.0000073531.84342.c2
4. Deshmukh VR, Hott JS, Tabrizi P, Nakaji P, Feiz-Erfan I, Spetzler RF. Cavernous malformation of the trigeminal nerve manifesting with trigeminal neuralgia: case report. Neurosurgery. (2005) 56(3):E623.; discussion E623. doi: 10.1227/01.NEU.0000154063.05728.7E
5. Gross BA, Du R, Orbach DB, Scott RM, Smith ER. The natural history of cerebral cavernous malformations in children. J Neurosurg Pediatr. (2016) 17(2):123–8. doi: 10.3171/2015.2.Peds14541
6. Adachi K, Hasegawa M, Hayashi T, Nagahisa S, Hirose Y. A review of cavernous malformations with trigeminal neuralgia. Clin Neurol Neurosurg. (2014) 125:151–4. doi: 10.1016/j.clineuro.2014.07.025
7. De Benedittis G. SUNCT Syndrome associated with cavernous angioma of the brain stem. Cephalalgia. (1996) 16(7):503–6. doi: 10.1046/j.1468-2982.1996.1607503.x
8. Fehlings MG, Tucker WS. Cavernous hemangioma of meckel’s cave. Case report. J Neurosurg. (1988) 68(4):645–7. doi: 10.3171/jns.1988.68.4.0645
9. Frossard JT, Domingues F, Neves P, Canhedo N, de Souza JM. Cavernous malformation in the trigeminal distribution: a case report of aggressive presentation and management. World Neurosurg. (2016) 86(514):e519–522. doi: 10.1016/j.wneu.2015.10.086
10. Giacobbo Scavo C, Roperto R, Cacciotti G, Mastronardi L. Cystic progression of a cavernous malformation at the level of the trigeminal root entry zone presenting with sudden onset of trigeminal neuralgia. J Craniofac Surg. (2018) 29(8):e728–30. doi: 10.1097/scs.0000000000004501
11. Liu H, Chen C, Liu Y, Liu J, Yu X, Chen L. Trigeminal neuralgia caused by cavernoma: a case report with literature review. Front Neurol. (2022) 13:982503. doi: 10.3389/fneur.2022.982503
12. Mascarenhas L, Magalhães F, Magalhães Z, Romão H, Resende M, Resende-Pereira J, et al. Cavernous malformation of the trigeminal nerve. Neurocirugia (Astur). (2006) 17(1): 64–6; discussion 67. doi: 10.1016/s1130-1473(06)70372-4
13. Pease M, Withrow J, Ozpinar A, Lunsford LD. Gamma knife radiosurgery for trigeminal neuralgia caused by a cavernous malformation: case report and literature review. Stereotact Funct Neurosurg. (2018) 96(6):412–5. doi: 10.1159/000495476
14. Saito N, Yamakawa K, Sasaki T, Saito I, Takakura K. Intramedullary cavernous angioma with trigeminal neuralgia: a case report and review of the literature. Neurosurgery. (1989) 25(1):97–101. doi: 10.1227/00006123-198907000-00018
15. Seçkin H, Patel N, Avci E, Dempsey RJ, Başkaya MK. Removal of cavernous malformation of the meckel’s cave by extradural pterional approach using heros muscle dissection technique. Surg Neurol. (2009) 72(6): 733–6; discussion 736. doi: 10.1016/j.surneu.2009.04.007
16. Shimpo T. Trigeminal neuralgia in pontine cavernous angioma. J Neurol. (2000) 247(2):139. doi: 10.1007/pl00007795
17. Stellmann JP, Kuhn M, Töpper R. [Chronic facial pain due to a brainstem cavernoma]. Fortschr Neurol Psychiatr. (2007) 75(9):552–4. doi: 10.1055/s-2007-980088
18. Vitek L, Tettenborn B. Cavernous angioma in the brachium pontis presenting with trigeminal neuralgia: a case report. Eur Neurol. (2002) 48(4):226–8. doi: 10.1159/000066167
19. Zhang W, Jiang X, Wang Y. Percutaneous balloon compression for trigeminal neuralgia because of pontine cavernous angioma. World Neurosurg. (2020) 137:137–9. doi: 10.1016/j.wneu.2019.12.167
20. Samii M, Tatagiba M, Carvalho GA. Retrosigmoid intradural suprameatal approach to meckel’s cave and the middle fossa: surgical technique and outcome. J Neurosurg. (2000) 92(2):235–41. doi: 10.3171/jns.2000.92.2.0235
21. Prolo LM, Jin MC, Loven T, Vogel H, Edwards MSB, Steinberg GK, et al. Recurrence of cavernous malformations after surgery in childhood. J Neurosurg: Pediatr PED. (2020) 26(2):179–88. doi: 10.3171/2020.2.PEDS19543
Keywords: cranial nerve cavernoma, cavernous malformation, trigeminal nerve cavernous malformation, pediatric, cavernoma
Citation: Harty M, Baqai MWS, Sajjad J, Fellows G, Clamp PJ and Abhinav K (2023) Case Report: Recurrent pediatric cavernous malformation of the trigeminal nerve. Front. Surg. 10:1278177. doi: 10.3389/fsurg.2023.1278177
Received: 15 August 2023; Accepted: 4 December 2023;
Published: 22 December 2023.
Edited by:
Paolo Perrini, University of Pisa, ItalyReviewed by:
Prajwal Ghimire King’s College Hospital NHS Foundation Trust, United Kingdom© 2023 Harty, Baqai, Sajjad, Fellows, Clamp and Abhinav. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kumar Abhinav a3VtYXIuYWJoaW5hdkBkb2N0b3JzLm9yZy51aw==
Abbreviations CM, cavernous malformation; CN, cranial nerve; CPA, cerebello-pontine angle; MCP, middle cerebral peduncle.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.