- Department of Neurosurgery, Peking University Third Hospital, Beijing, China
Objective: Spinal meningeal cysts (SMCs) are currently classified into three types: extradural cysts without nerve root fibers (Type I), extradural cysts with nerve root fibers (Type II), and intradural cysts (Type III). However, the sacral terminal filar cyst is a distinct subtype with the filum terminale rather than nerve roots within the cyst. This study aimed to investigate the clinicoradiological characteristics and surgical outcomes of sacral terminal filar cysts.
Methods: A total of 32 patients with sacral terminal filar cysts were enrolled. Clinical and radiological profiles were collected. All patients were surgically treated, and preoperative and follow-up neurological functions were evaluated.
Results: Chronic lumbosacral pain and sphincter dysfunctions were the most common symptoms. On MRI, the filum terminale could be identified within the cyst in all cases, and low-lying conus medullaris was found in 23 (71.9%) cases. The filum terminale was dissociated and cut off in all cases, and the cyst wall was completely resected in 23 (71.9%) cases. After a median follow-up period of 26.5 ± 15.5 months, the pain and sphincter dysfunctions were significantly improved (both P < 0.0001). The cyst recurrence was noted in only 1 (3.1%) case.
Conclusions: Sacral terminal filar cysts are rare, representing a distinct variant of SMCs. Typical MRI features, including filum terminale within the cyst and low-lying conus medullaris, may suggest the diagnosis. Although the optimal surgical strategy remains unclear, we recommend a combination of resection of the cyst wall and dissociation of the filum terminale. The clinical outcomes can be favorable.
Introduction
Spinal meningeal cysts (SMCs) refer to extradural cystic lesions communicating with the subarachnoid space via a focal dural defect (1, 2). SMCs of the sacral region, also known as sacral cysts, are relatively common findings in patients being evaluated for low back radicular pain. Sacral cysts are generally considered to be congenital lesions (3). The definite incidence of SMCs remains unclear; nevertheless, according to previous reports, the prevalence of sacral cysts can reach up to 17% in patients undergoing myelography for the investigation of sciatica (4). The most widely accepted hypothesis regarding the pathogenesis of SMCs is that cerebrospinal fluid herniates through a weak part of the spinal dura, and this herniation often forms a one-way valve. Cerebrospinal fluid accumulates into the cyst and fails to flow out, enlarging the cyst gradually and causing spinal cord or nerve root compression symptoms. Nabors and colleagues proposed a classification, in which SMCs are divided into three categories: Type I, extradural SMCs without spinal nerve root fibers; Type II, extradural SMCs with spinal nerve root fibers; and Type III, intradural SMCs (5). Additionally, Type I is further divided into two subgroups: Type IA, extradural meningeal cyst or extradural arachnoid cyst; and Type IB, meningeal cysts in the sacral canal (mostly located in S1-S3). Type II is also known as Tarlov's perineural cyst or a spinal nerve root diverticulum, and Type III actually refers to a spinal intradural arachnoid cyst (5). However, SMCs occurring in the filum terminale is a distinct subtype, as this extremely rare entity has filum terminale rather than nerve root within the cyst, and we named it as sacral terminal filar cyst (6). Due to the relative rarity, the diagnosis and treatment of this special subtype have not yet been fully understood. This study aimed to clarify the clinicoradiological characteristics, surgical strategies, and outcomes of sacral terminal filar cysts.
Materials and methods
Patients
This retrospective study has been approved by the Institutional Review Board and Ethics Committee of Peking University Third Hospital. A total of 32 patients with sacral terminal filar cyst were enrolled, including 13 males and 19 females. The average age was 36.1 ± 11.7 years, ranging from 16 to 64 years. The duration of symptoms prior to surgery was 30.2 ± 25.9 months, ranging from 10 days to 10.5 years.
Inclusion criteria included: (1) A definitive diagnosis of sacral terminal filar cyst based on spinal MRI; (2) symptomatic sacral cyst manifesting as pain, lower-extremity weakness, and/or sphincter disturbance; (3) the sacral cyst was surgically treated, and filum terminale was found within the cyst intraoperatively; and (4) complete follow-up data including clinical symptoms, physical examinations, and radiological imaging. Exclusion criteria were as follows: (1) accompanied other type SMCs; (2) concomitant other spinal diseases; or (3) a previous history of operation on the SMCs.
Clinicoradiological evaluation
The pain intensity was evaluated as per the Visual Analogue Scale (VAS). Sphincter dysfunctions were assessed using the Japanese Orthopaedic Association (JOA) scoring system: 0 point represents urodialysis or urinary incontinent; 1 point, sense of retention, difficulty with micurature, prolonged urination, or dysuria; 2 points, delayed urination, or frequent urination; 3 points represent normal functions (7). The JOA score for the lumbar spine was used to evaluate neurological status. The JOA improvement index and improvement rate were calculated according to the following formula: improvement index = postoperative JOA score—preoperative JOA score; improvement rate = [(postoperative JOA score—preoperative JOA score)/(29—preoperative JOA score)] × 100%. Perioperative and follow-up spinal MRIs were available for all patients, which were used to evaluate the size of the cyst, the level of the conus medullaris, the recovery of the tethered cord, and postoperative recurrence of cysts.
Surgical strategies
Combined intravenous and inhalation anesthesia, as well as intraoperative neurophysiological monitoring, was routinely performed. The patient was placed in a prone position with the lumbosacral portion at the highest level. A laminotomy was performed in the posterior wall of the sacral canal, following which the cyst was exposed. Next, the adhesion between the cyst and the inner wall of the spinal canal was dissected under a microscope. Then, the cyst was incised dorsally and unfolded, and the filum terminale was found passing through the orificium in the caudal end of the dural sac. From the orificium, cerebrospinal fluid constantly flowed out. To expose the filum terminale internum in the dural sac and to identify the orificium where the filum terminale internum breaks through the dural sac, a posterior midline incision of the dorsal dura was made. Subsequently, the caudal portion of the filum terminale internum was cut off, and a small part of the surrounding dural sac was resected. The cyst wall and the filum terminale externum were also dissociated and resected (Figure 1). During this procedure, the adhesion and the tethered cord were thoroughly released till the cauda equina was eased completely. At last, the caudal end of the dural sac was appropriately dissociated and tightly sutured, and the terminal cistern was reconstructed. Main surgical procedure video is given in Supplementary Video 1.

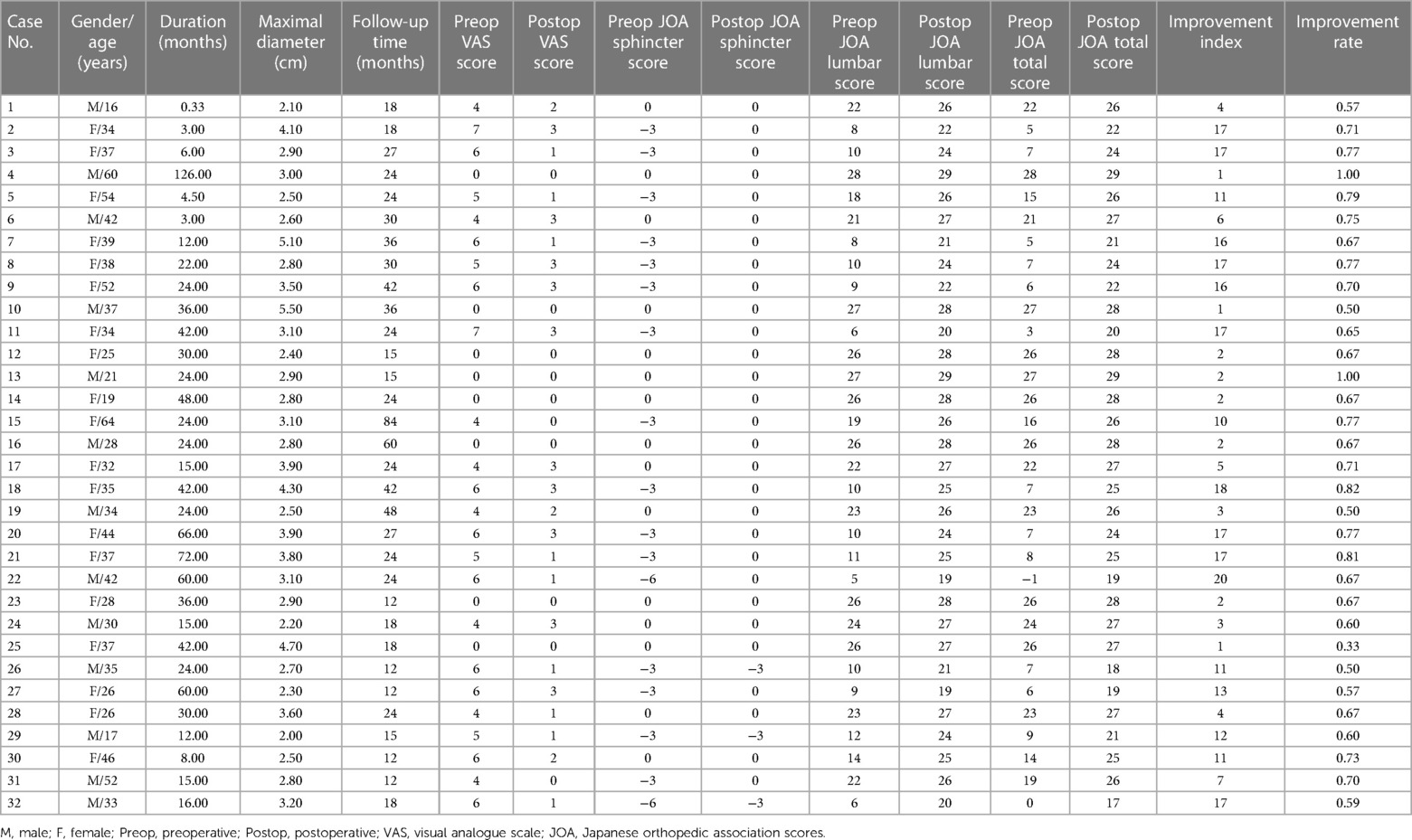
Figure 1. Surgical procedures and intraoperative findings. (A) After incision of the cyst and dorsal wall of the dural sac, a thickened filum terminale with fatty infiltration was noted (black arrow), and the cerebrospinal fluid flows into the cyst through the orificium (white arrow). (B) The caudal end of the intradural filum terminale was cut off by bipolar electrocoagulation (black arrows indicating the broken ends of the filum terminale). (C) The cyst wall (black arrow) was dissociated from the surrounding adhesion bluntly. (D) The dissociated cyst wall was resected with the filum terminale externum (white arrow). (E) The caudal end of the dural sac was dissociated and tightly sutured (black arrow). (F) The dural mater was tightly closed, and the terminal cistern was reconstructed (black arrow).
Statistical analysis
GraphPad Prism 9.3.1 (GraphPad, San Diego, CA, USA) was used for statistical analyses. The normality of data was examined using the Kolmogorov–Smirnov test. Continuous variables were presented as “mean ± standard deviation (SD)” when normally distributed or “medians (interquartile ranges, IQR)” when non-normally distributed. Categorical variables were presented as percentages. Statistical comparisons were performed using Mann–Whitney U-test or Student t-test for continuous variables, as appropriate. The threshold for significance was set as a P value less than 0.05.
Results
Clinical manifestations
Among the 32 patients, 24 (75.0%) presented with chronic lumbosacral or perineal pain, among whom 13 had radiating pain spreading along the affected nerve root. Additionally, other onset symptoms included numbness in the lower extremities (15/32; 46.9%), weakness in the lower extremities (18/32; 56.3%), and sphincter dysfunctions (17/32; 53.1%).
Physical examinations revealed that 11 (34.4%) patients had decreased acupuncture sensation at the saddle area, 18 (56.3%) patients had decreased muscle strength in the gastrocnemius, 3 (9.4%) patients had muscle atrophy, and 25 (78.1%) patients had weakened Achilles tendon reflex. No pathological reflection of Babinski's sign was induced. The VAS scores ranged from 0 to 7 points (mean 3.9 ± 2.5 points). The mean JOA score for sphincter dysfunctions was −1.8 ± 1.8 points (range, −6–0 points). The mean JOA score for the lumbar neurological functions was 17.0 ± 7.9 points (range, 5–28 points).
Radiological features
Spinal MRI demonstrated an isolated cystic lesion in the sacral canal in all cases. The locations included: L5-S2 levels in 4 cases, S1-S3 levels in 11 cases, S2-S3 levels in 9 cases, S2-S4 levels in 6 cases, and S1-S4 levels in 2 cases. The cystic lesions showed hypointensity on T1-weighted imaging and hyperintensity on T2-weighted imaging; no enhancement was observed after the administration of contrast medium. The filum terminale was identified within the cyst in all (100%) cases, which was thickened with fatty infiltration, and low-lying conus medullaris was found in 23 (71.9%) cases (Figure 2).
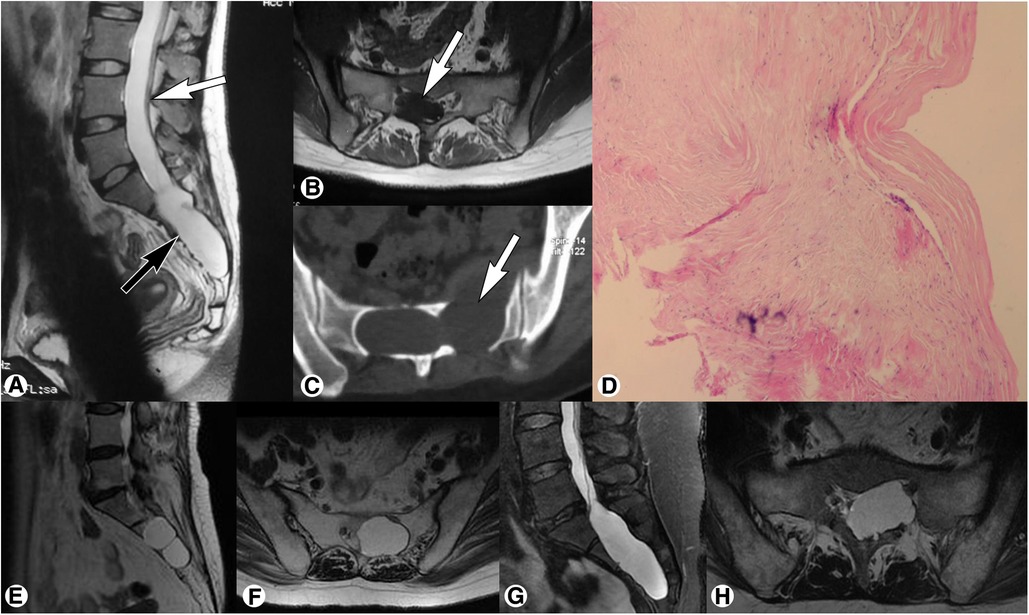
Figure 2. Preoperative magnetic resonance imaging of sacral terminal filar cyst. (A) Spinal sagittal T2-weighted imaging showed a sacral cyst (black arrow) accompanied by low-lying conus medullaris at the inferior margin of the L4 vertebral body (white arrow). (B) Axial T1-weighted imaging demonstrated a thickened filum terminale with fatty infiltration (white arrow) within the cyst. (C) Axial computed tomography showed local bone destruction (white arrow) at the anterior and posterior walls of the sacral canal. (D) Histopathological examination showed fibrous connective tissue with a cyst wall-like structure, which was consistent with the diagnosis of meningeal cysts. (E,F) Representative magnetic resonance imaging of Nabors Type IB meningeal cyst showed an extradural cyst without spinal nerve root fibers (E, sagittal T2-weighted; F, axial T2-weighted). (G,H) Representative magnetic resonance imaging of Nabors Type II meningeal cyst showed an extradural cyst with spinal nerve root fibers (G, sagittal T2-weighted; H, axial T2-weighted).
Perioperative findings
The operations lasted for 1.5–3 h (mean 1.6 ± 0.3 h). Intraoperative blood loss ranged from 30 ml to 250 ml (mean 76.3 ± 43.8 ml). The filum terminale was dissociated and cut off in all (100%) cases, and the cyst wall was completely resected in 23 (71.9%) cases and subtotally resected in 9 (28.1%) cases. The postoperative course was uneventful in all cases, and there were no significant motor complications or sphincter deterioration. Only 8 patients reported mild perianal numbness, which was gradually recovered within 3 months.
All resected specimens were subjected to pathological examinations. The cyst wall showed fibrous connective tissue with a cyst wall-like structure, and a small part of it was lined with squamous epithelium, which was consistent with the diagnosis of meningeal cysts. The resected filum terminale was thickened with fibrous tissue and adipose hyperplasia.
Clinical outcomes
The follow-up period ranged from 12 months to 7 years (mean, 26.5 ± 15.5 months). The pain, sensorimotor disturbances, and sphincter dysfunctions were all significantly improved. The postoperative mean VAS score was significantly lower than that before surgery (1.4 ± 1.2 vs. 3.9 ± 2.5; t = 7.673; P < 0.0001). The postoperative mean JOA score for sphincter dysfunctions was −0.28 ± 0.89, which was significantly better than the preoperative score (t = 4.980; P < 0.0001). The postoperative mean JOA score for lumbar neurological functions was 24.9 ± 3.0, which was significantly higher than the preoperative score (t = 8.506; P < 0.0001). The JOA improvement index was 9.4 ± 6.6 (range 1.0–20.0), and the improvement rate was 68% ± 13% (range 33%–100%). The detailed neurological function assessment results are summarized in Table 1. According to the follow-up MRI, the cyst recurrence was only noted in one (3.1%) case. The spinal cord tethering was completely relieved in all cases.
Discussion
The most common type of SMCs is Tarlov cyst (Nabors Type II) with nerve roots (2, 8), followed by sacral cyst (Nabors Type IB) without any structure inside (9). In this study, we reported a distinct subtype of sacral cysts with filum terminale inside. Sun et al. considered it as a special variant of Nabors Type I cyst (9). However, in the current study, we found that clinical symptoms, imaging features, and surgical outcomes of sacral terminal filar cysts were quite unique and different from those of conventional Nabors Type I cysts. Therefore, we recommend that it should be classified separately as a distinct subtype of sacral cysts: sacral terminal filar cyst (6).
The etiology and pathogenesis of sacral terminal filar cysts remain unclear. We found that the neck of cysts was mostly located at the site where the filum terminale breaks through the dural sac, and thus we speculated that the focal weakness of the dura mater may be the anatomical basis for the occurrence of sacral terminal filar cysts. Generally, the filum terminale internum transitions into the filum terminale externum at the caudal end of the dural sac, accordingly the focal dura mater in this site is similar to the nerve root sleeve. Therefore, we consider that sacral terminal filar cysts may share a similar pathogenetic mechanism with sacral Tarlov cysts.
Clinically, sacral terminal filar cysts are relatively rare. With the enlargement of the sacral terminal filar cyst and progressively increasing intracystic pressure, the cyst may compress the surrounding structures and causes pain in the perineum (10, 11). Additionally, sacral terminal filar cysts are often associated with tethered spinal cord symptoms such as chronic lower back pain, lower-extremity weakness and muscle atrophy, and dysfunctions of the bladder and bowel. Accompanying tethered spinal cord can help clinicians to distinguish sacral terminal filar cysts from other sacral cyst subtypes (6, 12).
MRI is the preferred imaging modality for the diagnosis of sacral cysts (13, 14). The radiological features of sacral terminal filar cysts are similar to those of other sacral cyst subtypes, showing cystic lesions without contrast enhancement (14, 15). Noteworthily, the filum terminale can be visible within the sacral terminal filar cyst, and low-lying conus medullaris may be present (6, 16). The filum terminale can be easily identified due to fatty infiltration, manifesting as linear fat strip signals. The filum terminale without fatty infiltration may be confused with nerve roots (17). However, the filum terminale is generally thicker and can be traced from the end of the spinal cord proceeding gradually downward to the cyst continuously.
Sacral nerve root irritation and tethered spinal cord symptoms are indications of surgical treatment of sacral terminal filar cysts (18). The surgical goals and strategies for treating sacral terminal filar cysts are distinct from those for other sacral cyst subtypes (2, 19). Considering simply cutting off the filum terminale externum cannot relieve the spinal cord tethering sufficiently, we chose to cut off the filum terminale internum in the dural sac. Moreover, simply ligating the cyst neck at the orificium fistulae without removing the filum terminale would not completely seal the cerebrospinal fluid leakage. Intraoperatively, we cut off the filum terminale subdurally and resected a small part of the surrounding dural sac. To locate the orificium fistulae, we need to incise the dural sac along the dorsal midline, and the weak part of the dura mater was exposed where the filum terminale break through the dural sac. Then, we dissociated the filum terminale internum, and the tethered spinal cord was completely relieved (20). Additionally, the caudal end of the dural sac was appropriately dissociated and tightly sutured, and the terminal cistern was reconstructed.
Surgical outcomes of sacral terminal filar cysts are satisfactory. Neurological deficits were all recovered, and cyst recurrence was only noted in one case after a mean follow-up period of 26.5 ± 15.5 months. Therefore, we propose that surgical treatment combining resection of the cyst wall and releasing the tethered spinal cord by dissociation of the filum terminale is a safe and efficient approach leading to a favorable prognosis. Furthermore, reconstruction of the terminal cistern is a crucial procedure for eliminating cerebrospinal fluid leakage and reducing the probability of cyst recurrence.
Conclusions
Sacral terminal filar cysts represent a distinct subtype of SMCs, with typical MRI characteristics including filum terminale within the cyst and low-lying conus medullaris. A combination of resection of the cyst wall and dissociation of the filum terminale is a safe and effective surgical approach, and the clinical outcomes are favorable.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Institutional Review Board and Ethics Committee of Peking University Third Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. TY: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. JZ: Data curation, Formal analysis, Methodology, Writing – review & editing. YS: Data curation, Formal analysis, Methodology, Writing – review & editing. CW: Data curation, Investigation, Methodology, Writing – review & editing. CM: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. BL: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. JX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the National Natural Science Foundation of China (81901202 to CY), Beijing Natural Science Foundation (7222217 to CY), the Capital Health Research and Development of Special (2022-4-40918 to CY), AO Spine Research Start-up Grant (AOS-Startup-21-016 to CY), and Clinical Medicine Plus X-Young Scholars Project, Peking University, the Fundamental Research Funds for the Central Universities (PKU2021LCXQ007 to CY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1272580/full#supplementary-material
References
1. Jian Q, Song G, Liu Z, Duan W, Guan J, Jian F, et al. Location distribution of fistulas and surgical strategies for spinal extradural meningeal cysts: a retrospective analysis of 30 cases at a single center. Neurospine. (2022) 19(1):188–201. doi: 10.14245/ns.2142526.263
2. Elsawaf A, Awad TE, Fesal SS. Surgical excision of symptomatic sacral perineurial tarlov cyst: case series and review of the literature. Eur Spine J. (2016) 25(11):3385–92. doi: 10.1007/s00586-016-4584-3
3. Fogel GR, Cunningham PY 3rd, Esses SI. Surgical evaluation and management of symptomatic lumbosacral meningeal cysts.. Am J Orthop (Belle Mead NJ). (2004) 33(6):278–82.15239354
4. Larsen JL, Smith D, Fossan G. Arachnoidal diverticula and cystlike dilatations of the nerve-root sheaths in lumbar myelography. Acta Radiol Diagn (Stockh). (1980) 21(2A):141–5. doi: 10.1177/028418518002102a02
5. Nabors MW, Pait TG, Byrd EB, Karim NO, Davis DO, Kobrine AI, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. (1988) 68(3):366–77. doi: 10.3171/jns.1988.68.3.0366
6. Xie J, Wang Z, Chen X. Diagnosis and surgical treatment of sacral spinal meningeal cysts of fila terminale complicated with tethered spinal cord syndrome. Chin J Clin Neurosurgery. (2015) 20(11):651–3.
7. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. (1985) 67(4):609–15. doi: 10.1302/0301-620X.67B4.4030860
8. Liu B, Wang ZY, Lin GZ, Zhang J. Radiculoplasty with reconstruction using 3d-printed artificial dura mater for the treatment of symptomatic sacral canal cysts two case reports. Medicine (Baltimore). (2018) 97(49):e13289. 1097/MD.0000000000013289 30544388
9. Sun JJ, Wang ZY, Liu B, Li ZD, Wu HB, Yen RY, et al. Neck transfixion for sacral extradural spinal meningeal cysts without spinal nerve root fibers. Eur Spine J. (2016) 25(6):1945–52. doi: 10.1007/s00586-014-3471-z
10. Sun J, Wang Z, Li Z, Wu H, Yen R, Zheng M, et al. Reconstruction of nerve root sheaths for sacral extradural spinal meningeal cysts with spinal nerve root fibers. Sci China Life Sci. (2013) 56(11):1007–13. doi: 10.1007/s11427-013-4536-7
11. Van de Kelft E, Van Vyve M. Sacral meningeal cysts and perineal pain. Lancet (London, England). (1993) 341(8843):500–1. doi: 10.1016/0140-6736(93)90260-n
12. Selcuki M, Mete M, Barutcuoglu M, Duransoy YK, Umur AS, Selcuki D. Tethered cord syndrome in adults: experience of 56 patients. Turk Neurosurg. (2015) 25(6):922–9. doi: 10.5137/1019-5149.JTN.11700-14.1
13. Sugawara T, Higashiyama N, Tamura S, Endo T, Shimizu H. Novel wrapping surgery for symptomatic sacral perineural cysts. J Neurosurg Spine. (2021) 36(2):185–92. doi: 10.3171/2021.5.SPINE21179
14. Davis SW, Levy LM, LeBihan DJ, Rajan S, Schellinger D. Sacral meningeal cysts: evaluation with mr imaging. Radiology. (1993) 187(2):445–8. doi: 10.1148/radiology.187.2.8475288
15. Acosta FL J, Quinones-Hinojosa A, Schmidt MH, Weinstein PR. Diagnosis and management of sacral tarlov cysts. Case report and review of the literature. Neurosurg Focus. (2003) 15(2):E15. doi: 10.3171/foc.2003.15.2.15
16. Feigenbaum F, Hale S. Association between symptomatic giant sacral meningeal diverticulum and spinal cord tethering with thickened lipomatous Filum. Spine. (2011) 36(18):E1230–E2. doi: 10.1097/BRS.0b013e31820e4720
17. Howells M, Hamby T, Honeycutt J, Donahue DJ. Detethering of mri-demonstrated tethered cord syndrome. Pediatr Neurosurg. (2022) 57(2):85–92. doi: 10.1159/000522135
18. Kunz U, Mauer UM, Waldbaur H. Lumbosacral extradural arachnoid cysts: diagnostic and indication for surgery. Eur Spine J. (1999) 8(3):218–22. doi: 10.1007/s005860050161
19. Cabrilo I, Zaidman N, Casey AT. Midline sacral meningeal cyst decompression and repair. Acta Neurochir (Wien). (2021) 163(10):2777–81. doi: 10.1007/s00701-021-04948-3
Keywords: spinal meningeal cyst, sacral terminal filar cyst, filum terminale, MRI, surgery, outcome
Citation: Lin G, Yang C, Yu T, Zhang J, Si Y, Wu C, Ma C, Liu B, Yang J and Xie J (2023) Sacral terminal filar cyst: a distinct variant of spinal meningeal cyst and midterm clinical outcome following combination resection surgery. Front. Surg. 10:1272580. doi: 10.3389/fsurg.2023.1272580
Received: 15 August 2023; Accepted: 25 October 2023;
Published: 3 November 2023.
Edited by:
Enbo Wang, China Medical University, ChinaReviewed by:
Yuxi Su, Children’s Hospital of Chongqing Medical University, ChinaJie Wen, Hunan Provincial People’s Hospital, China
Xiong-tao Li, Wuhan Children’s Hospital, China
© 2023 Lin, Yang, Yu, Zhang, Si, Wu, Ma, Liu, Yang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Yang eWFuZ2pieXN5QGJqbXUuZWR1LmNu Jingcheng Xie dHNlamNAYmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Guozhong Lin†
Guozhong Lin† Chenlong Yang
Chenlong Yang Jun Yang
Jun Yang Jingcheng Xie
Jingcheng Xie