
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 21 November 2023
Sec. Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1253432
 Xiaoliang Yin1
Xiaoliang Yin1 Jia Zhang1
Jia Zhang1 Qianquan Ma1
Qianquan Ma1 Suhua Chen1
Suhua Chen1 Chao Wu1
Chao Wu1 Chenlong Yang1
Chenlong Yang1 Yu Si1
Yu Si1 Haihui Jiang1
Haihui Jiang1 Wei Guo2
Wei Guo2 Ying Liu2
Ying Liu2 Huishu Yuan2
Huishu Yuan2 Jun Yang1*
Jun Yang1* Jianjun Sun1,3*
Jianjun Sun1,3*
Introduction: Sacral laminoplasty with titanium mesh and titanium screws can reduce symptomatic sacral extradural spinal meningeal cysts (SESMCs) recurrence and operation complications. However, due to a defect or thinning of the sacrum, the screws cannot be securely anchored and there are also problems with permanent metal implantation for titanium mesh and screws. We propose that sacral laminoplasty with absorbable clamps can provide rigid fixation even for a thinned or defected sacrum without leaving permanent metal implants.
Methods: In the direct microsurgical treatment of symptomatic SESMCs, we performed one-stage sacral laminoplasty with autologous sacral lamina reimplantation fixed by absorbable fixation clamps. Retrospectively, we analyzed intraoperative handling, planarity of the sacral lamina, and stability of the fixation based on clinical and radiological data.
Results: Between November 2021 to October 2022, we performed sacral laminoplasty with the absorbable craniofix system in 28 consecutive patients with SESMCs. The size of the sacral lamina flaps ranged from 756 to 1,052 mm2 (average 906.21 ± 84.04 mm2). We applied a minimum of two (in four cases) and up to four (in four cases) Craniofix clamps in the operation, with three (in 20 cases) being the most common (82.14%, 20/28) and convenient to handle. Excellent sacral canal reconstruction could be confirmed intraoperatively by the surgeons and postoperatively by CT scans. No intraoperative complications occurred.
Conclusions: One-stage sacral laminoplasty with absorbable fixation clamps is technically feasible, and applying 3 of these can achieve a stable fixation effect and are easy to operate. Restoring the normal structure of the sacral canal could reduce complications and improve surgical efficacy.
Sacral extradural spinal meningeal cysts (SESMCs) are extradural meningeal cysts located in the sacral canal (1, 2). The most accepted theory is the ball-valve mechanism, which is attributed to congenital dysplasia or acquired trauma, inflammation and other factors, arachnoidal defect, and a one-way valve that communicates with the subarachnoid space formed (3, 4). Driven by pulsatile and hydrodynamic forces, cerebrospinal fluid flows in one direction, resulting in the formation and continuous expansion of SESMCs (3, 5). The enlargement of the SESMCs can exert a significant mass effect due to their elevated internal pressure, resulting in compression of the surrounding neural tissue and gradual damage to the adjacent bone (6–8).
In the past, the sacral lamina was not considered a crucial structure, and sacral laminoplasty was rarely been performed. However, recent evidence has shown that laminoplasty may reduce postoperative CSF leakage rates and mitigate epidural fibrosis (9–11). Chen et al. reported that sacral laminoplasty using titanium mesh and titanium screws effectively lowered the recurrence of SESMCs and minimized complications related to cerebrospinal fluid leakage (9). Nevertheless, in cases involving severe erosion of the dorsal sacral wall, resulting in a substantial defect or thinning of the sacral canal, securely anchoring screws can be problematic. Additionally, metal implants may introduce artifacts in MRI images.
Craniofix® Absorbable (FF017, Aesculap, AG, Tuttlingen, Germany) (Figure 1) is a type of fixation clamp constructed from absorbable polyester, widely utilized for the fixation of the cranial flap after craniotomy (12, 13). We performed sacral laminoplasty with absorbable fixation clamps in direct microsurgical treatment of SESMCs and obtained satisfactory fixation effect and good clinical efficacy, even in cases involving bone defects. To the best of our knowledge, this is the first reported clinical application of the absorbable Craniofix technique for sacral laminoplasty.
Between November 2021 to October 2022, we conducted 28 direct microsurgical treatments for SESMCs. We carried out a clinical assessment to determine the feasibility and safety of sacral laminoplasty using absorbable Craniofix (Figure 2). Approval for the study was obtained from the Research Ethics Board of Peking University Third Hospital. All subjects provided written informed consent for surgical procedures. All surgeons possessed extensive experience in performing absorbable Craniofix procedures for cranioplasty before transitioning to sacral laminoplasty.
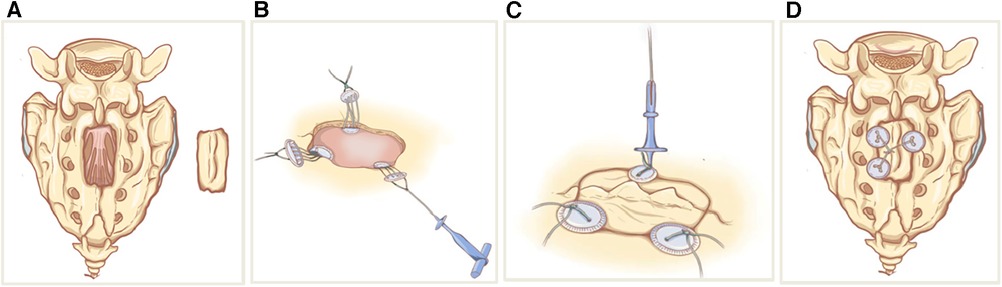
Figure 2. Schematic sketches showing the principle of sacral laminoplasty with absorbable fixation clamps. (A) The bilateral laminae sacrum was incised by ultrasonic bone scalpel(UBS) to form an integral spinous process-lamina complex bone flap. (B) The bottom clamps of the absorbable craniofix position inferior to the edge of the sacral bone window and place the upper clamp outside. (C) The spinous process-lamina complex bone flap is inserted and the upper clamps are drawn down by pulling the prepared sutures in the manner of a chain block. (D) The prepared sutures on the opposite sides are crossed and fixed with knots so the sacral lamina is rigidly refixed with carniofixes.
Patients were included in the study if they met the following criteria: (1) MRI results confirming the presence of SESMCs, (2) neurological symptoms attributed to SESMCs, (3)the presence of symptoms lasting for a period of at least 6 months, and (4)severe pain refractory to medical treatment. Preoperative CT scans of the sacral canal were conducted to evaluate the integrity of the sacral lamina on scale 1 (intact) (Figure 3A) to scale 2(eroded thinning) (Figure 3B) and scale 3 (full-thickness defect) (Figure 3C). Patients were excluded from the study if their symptoms could not be distinguished from lumbar spinal stenosis or lumbar intervertebral disc herniation. All patients included in this study provided informed consent and signed an acceptance letter.
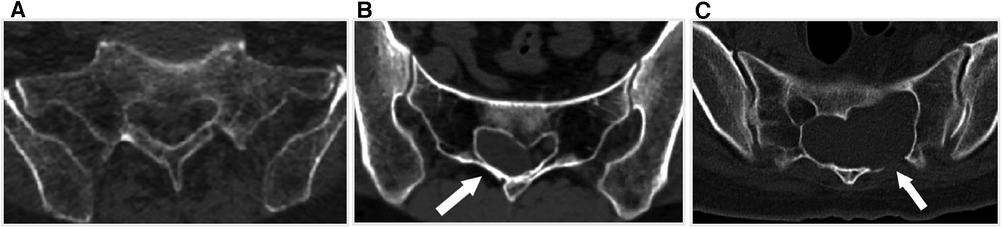
Figure 3. The integrity scale of the sacral lamina. (A) The sacral lamina is intact. (B) The sacral lamina is eroded and thinning (white arrow). (C) The sacral lamina is partial full-thickness defect (white arrow).
A comprehensive description of the microsurgical procedures has been previously documented in our published articles (14, 15). A brief summary of the procedure is as follows: Under general anesthesia, patients were positioned in the prone waist-bridge posture with the head facing downward. Real-time monitoring of somatosensory evoked potential (SEP) and motor evoked potential (MEP) was conducted. According to the location of the cyst on the preoperative MRI scan (Figure 4A), a corresponding skin incision was made in the posteromedial line and the paraspinal muscles were dissected along the midline to expose the sacral spinous process and bilateral fused lamina. The bilateral laminae were meticulously opened using an ultrasonic bone scalpel (UBS) (BoneScalpel, Misonix, Farmingdale, New York), removing the entire spinous process-lamina complex(Figure 4B) with care to preserve the integrity of the cyst wall. Preoperative MRI findings were confirmed under a microscope. Cysts with nerve root passage underwent nerve root sheath reconstruction, and cysts without nerve roots received neck transfixion.
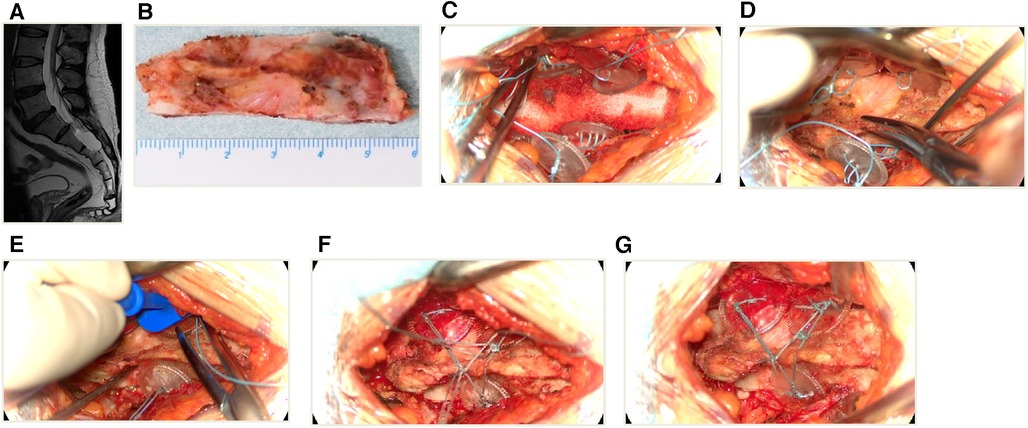
Figure 4. An illustrative case of sacral laminoplasty with absorbable fixation clamps. (A) The preoperative MRI T2-weighted image of a SESMC case. (B) The bilateral laminae were opened with UBS and the entire spinous process-lamina complex was removed. (C) Placement of three absorbable Craniofixes with one on the left and two on the right side of the sacral lamina bone window. (D) The sacral lamina bone flap was inserted. (E) The upper clamp was drawn down by pulling the prepared suture in the manner of chain block plates. (F) The prepared sutures on the opposite sides are crossed and fixed with knots. (G) The excess sutures were cut off and the sacral lamina was fixed with excellent planarity and stability.
Following the completion of the aforementioned procedure, the reconstruction was executed as follows. We usually use a minimum of two and a maximum of four absorbable Craniofixes on the left and right sides of the sacral lamina. First, we sequentially insert half of the bottom clamp inferior to the edge of the sacral bone window and place the upper clamp outside(Figure 4C), thereafter the sacral lamina bone flap is inserted (Figure 4D). Once the sacral lamina bone flap securely fits the bone window, the upper clamp is positioned against the lower clamp and tightened by pulling the prepared suture in the manner of a chain block until the sacral lamina bone flap is clamped firmly (Figure 4E). Since the traction torque is predefined by the disruption of the handle at a predetermined breaking point, the handle is broken by increasing the pulling force. Following this, three knots are tied using the prepared sutures to lock the absorbable Craniofix (Figure 4F). Finally, the prepared sutures on the opposite side are intertwined, secured with knots, and subsequently trimmed (Figure 4G), before the final step of wound closure.
The intraoperative records encompassed several key aspects, including the count and placement of absorbable Craniofixes, an assessment of the planarity and stability of the bone flap, and the time required for sacral laminoplasty. The intraoperative planarity scale ranged from scale 1 to scale 3 (as detailed in Table 1). Both the subjective rating of the stability of the bone flap on a scale from 1 (properly fixed) to 5 (loose) and the subjective rating of the handling of the clamps on a scale from 1 (very good) to 5(complicated) was evaluated by the surgeons (Table 2).
Postoperatively, all patients remained in a prone position for several days. Wound healing was assessed and categorized as either progressing well, experiencing delayed healing, or requiring debridement and suturing. Sacral canal CT scans were obtained on average 8 (6–9) days after the operation, from which we evaluated the planarity of the bone flap and calculated the size (Figures 5A,B). Additionally, MRI scans were performed 2 weeks post-surgery (Figures 5C,D), and follow-up MRI scans were performed 6 months after surgery (Figures 5E,F). Postoperative radiological evaluation of the sacral canal was performed by a neuroradiologist who remained blinded to the patient's intraoperative diagnosis. Results were classified into three categories: complete cyst resolution, residual cyst, or disappearance of cysts with effusion into the canal cavity.
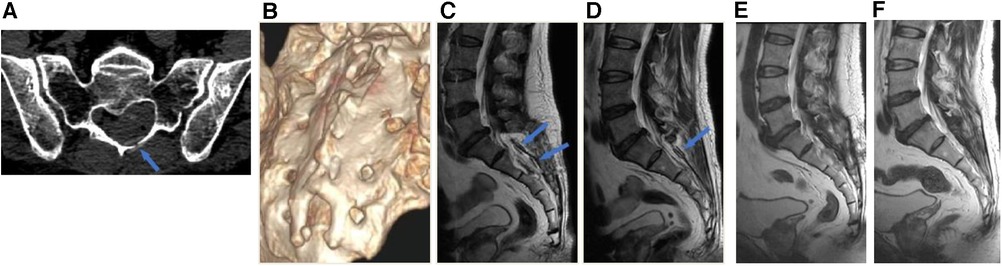
Figure 5. Postoperative radiological assessments. (A) The absorbable clamp (arrow) can be seen at the left side of the laminotomy on the postoperative axial CT image. (B) Postoperative 3D-CT showed sacral laminoplasty with excellent planarity and stability. (C) Postoperative 2-week MRI T2-weighted image showed 2 absorbable carniofixes at the right side of sacral laminectomy (blue arrows). (D) Postoperative 2-week MRI T2-weighted image showed 1 absorbable carniofix at the left side of sacral laminectomy (blue arrow). (E and F) Postoperative 6-month MRI revealed the bone structure of the sacral canal posterior wall remained stable, in comparison to the MRI at 2 weeks post-surgery, there was a significant improvement in the reduction of soft tissue swelling in the para-vertebral region.
From November 2021 to October 2022, 28 patients ranging from 26 to 75 years of age (mean 42.18 ± 15.49 years) with SESMCs underwent direct microsurgery and the absorbable Craniofix system was used for sacral canal closure. Of all the patients, 67.86% (19/28) were female, and 32.14% (9/28) were male. The lesions were located at S1–S5, where S2 was the most common site. Notably 92.86% (26/28) of the cysts involved more than one vertebral segment, as detailed in Table 3. Preoperative CT scans showed sacrum involvement in a total of 18 (64.29%, 18/28)patients, including 10 (35.71%, 10/28) patients with eroded thinning (scale 2) and 8 (28.57%, 8/28) patients with full-thickness defect (scale 3) respectively.
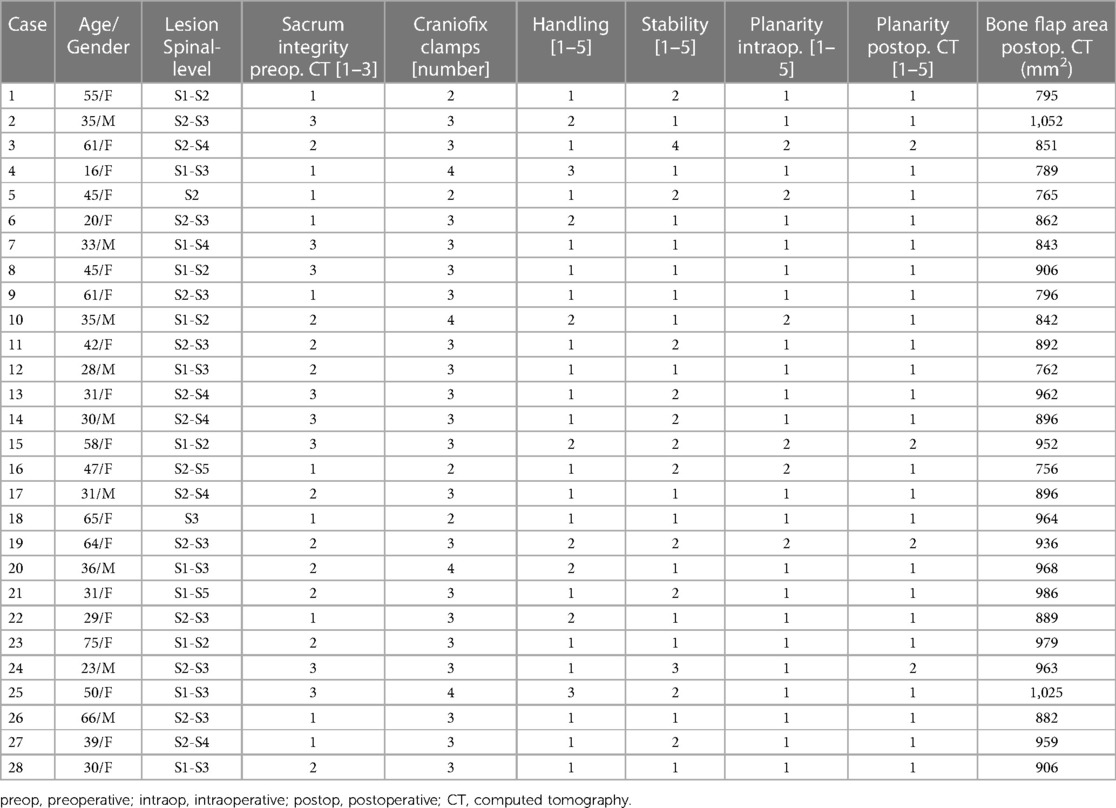
Table 3. Consecutive cases of SESMCs treated with sacral laminoplasty with autologous sacral laminar reimplantation fixed by absorbable fixation clamp.
We did not observe intraoperative complications, nor did we need to change the method in any patient since the absorbable Craniofix clamp can be successfully applied to sacral canaloplasty with good fixation even for partial full-thickness sacral defects. We applied a minimum of two (in four cases) and up to four (in four cases) Craniofix clamps in the operation, with three (in 20 cases) being the most common (82.14%, 20/28). The procedure time for sacral laminoplasty ranged from 12 min to 16 min (average 14.16 ± 1.26 min). The size of the sacral lamina flaps ranged from 756 to 1,052 mm2 (average 906.21 ± 84.04 mm2±) (Table 1). The average value of handling the clamps, subjectively evaluated by the surgeon, was 1.42 ± 0.73 (Table 2). The average stability scale of the bone flap, subjectively evaluated by the surgeon, was 1.57 ± 0.73 (Table 3). The average planarity scale was 1.21 ± 0.32 intraoperatively evaluated by the surgeon and 1.14 ± 0.35 postoperatively evaluated by CT images. The postoperative period was uneventful and the wounds healed well and postoperative MRI scans on 2 weeks showed total resection of cysts without pseudocysts and effusions in all cases (as illustrated in Figures 5C,D). At the 6-month postoperative follow-up, MRI scans revealed secure and stable bone structures of the sacral canal posterior wall, with no signs of abnormal reactions or inflammation around the fixation material. Furthermore, in comparison to the MRI scans at 2 weeks, there was a noticeable improvement in the reduction of soft tissue swelling in the para-vertebral region. The sacral canal cyst did not reoccur, and there were no complications such as subcutaneous fluid accumulation or pseudocysts (as illustrated in Figures 5E,F).
Direct microsurgical treatment of SESMCs has demonstrated efficacy in previous studies by us and other authors (16–19)and is therefore recommended. Techniques for nerve root and terminal cistern reconstruction, including overlapping nerve roots with the cyst wall, redundant cyst wall excision, and strengthening of the reconstructed nerve sheath with an artificial dura mater, have been discussed in depth in our previous studies (14, 15). This study mainly focuses on the reconstruction of the sacral canal bone structure.
In the past, the sacral lamina was not considered an essential structure, and sacral laminoplasty was rarely performed (9). However, when the sacral canal is filled with muscle or fat without dorsal wall reconstruction, it can lead to the formation of numerous epidural scars within the sacral lamina defect area. This may result in nerve adhesion and secondary spinal stenosis, and even in the recurrence of nerve compression symptoms or aggravation of the original symptoms (20). Recent evidence suggests that laminoplasty can reduce postoperative CSF leakage rates and diminish epidural fibrosis. Smith et al. (11). reported sacral laminoplasty by reimplantation of the removed lamina fixed by titanium mini-plates to treat 18 symptomatic sacral perineural cyst patients. No CSF leakage and no radiographic recurrence at 12 months follow-up was found. Chen et al. (9). performed sacral laminoplasty with a titanium mesh to cover the sacral laminal defect. The titanium mesh was shaped according to the size and contour of the sacral laminal defect and fixed to the sacrum with screws (length 3–5 mm). During follow-ups, ranging from 13 to 37 months, all six patients experienced no recurrence of dural ectasia or pseudomeningocele and were free from local symptoms. In our study of the 28 consecutive cases, the additional reconstruction of the sacral canal posterior wall took approximately 12–16 min, which did not significantly extend the overall surgical duration. Nevertheless, this supplementary step improved the surgical results significantly. The postoperative period was uneventful, and an MRI scan showed the complete resection of SESMCs without cerebrospinal fluid leakage, pseudocyst formation, or effusion in all cases.
Based on our previous institutional experience (21), we found that sacral laminoplasty using titanium plates or titanium mesh achieved satisfactory outcomes for small cysts with intact sacral canal bones. However, for large cysts, the sacral canal bone is often eroded, thinned, or defective, making it challenging to achieve reliable fixation. The mechanism of Craniofix relies on the firm clamping of the sacral lamina between the two clamps and it is not affected by the thickness of the sacral lamina. Therefore, sacral laminoplasty with absorbable Craniofix fixation is feasible no matter whether there are intact, eroded, or partial full-thickness defect bones. Our results confirmed that all patients can obtain good spinal canal reconstruction and no case needed a change to the surgical method. Especially for those with bone defects, similar to the fixation in the burr hole area of the skull, the application of the cranial clip can also partially cover the sacral bone defect area. This effectively reconstructed the posterior wall of the sacral canal, removed the sacral canal cyst and subcutaneous cyst, and closed the fistula between them, significantly reducing the recurrence rate.
Different from cranial bone flap fixation, the position of the sacral lamina is deep and the operating space is relatively narrow. Based on our experiences with the absorbable Craniofixes, they were convenient to handle when we used up to two or three implants. The use of four Craniofixes tends to entwine with each other, making the operation inconvenient. Lemcke et al. (12). reported that three Craniofixes ensured sufficient stability for free bone flaps up to approximately 3,000 mm2. The size of the sacral bone flaps we fixed ranged from 756 to 1,025 mm2. Therefore, in most cases (20/28) we applied three implants, which is not only convenient for operation but also achieves a firm fixing effect. The stability and planarity were adequate in all of our patients evaluated intraoperatively by the surgeons and postoperatively by the radiologists.
Although our study successfully investigates the use of absorbable Craniofixes in sacral lamina fixation, this study has some limitations. First, this was a retrospective single-center study, which may limit the generalizability of the findings. Additionally, the sample size was relatively small, which could affect the statistical power of the result. Finally, as the technical feasibility and safety are from our consecutive cases, the lack of a control group is also a limitation of our study. Therefore, prospective controlled studies are needed to evaluate the exact effect of sacral laminoplasty with absorbable Craniofixes in direct microsurgical treatment of symptomatic sacral extradural spinal meningeal cysts.
One-stage sacral laminoplasty with absorbable fixation clamps is technically feasible and safe. The use of three such clamps can achieve a stable fixation effect and is easy to perform. By restoring the normal structure of the sacral canal, this procedure can reduce complications and improve surgical efficacy. However, due to the small sample size of consecutive cases, a final conclusion needs the accumulation of cases and be confirmed by prospective controlled studies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Research Ethics Board of Peking University Third Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
XY: Investigation, Methodology, Writing – original draft. JZ: Writing – original draft. QM: Conceptualization, Investigation, Writing – review & editing. SC: Data curation, Investigation, Writing – review & editing. CW: Conceptualization, Methodology, Writing – review & editing. CY: Data curation, Investigation, Writing – review & editing. YS: Data curation, Investigation, Writing – review & editing. HJ: Investigation, Writing – review & editing. WG: Methodology, Visualization, Writing – review & editing. YL: Methodology, Visualization, Writing – review & editing. HY: Methodology, Visualization, Writing – review & editing. JY: Funding acquisition, Supervision, Writing – review & editing. JS: Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the National Natural Science Foundation of China grant (NSFC, 42293324; A74496-07), the Innovation & Transfer Fund of Peking University Third Hospital (Y74496-05), and the Proof of Concept Program of Zhongguancun Science City and Peking University Third Hospital (A74496-08).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Apel K, Sgouros S. Extradural spinal arachnoid cysts associated with spina bifida occulta. Acta Neurochir. (2006) 148(2):221–6. doi: 10.1007/s00701-005-0697-x
2. Sato K, Nagata K, Sugita Y. Spinal extradural meningeal cyst: correct radiological and histopathological diagnosis. Neurosurg Focus. (2002) 13(4):ecp1. doi: 10.3171/foc.2002.13.4.8
3. Yang AI, Rinehart CD, McShane BJ, Hitti FL, Welch WC. Growth of lumbosacral perineural (Tarlov) cysts: a natural history analysis. Neurosurgery. (2020) 86(1):88–92. doi: 10.1093/neuros/nyy586
4. Yoshioka F, Shimokawa S, Masuoka J, Inoue K, Ogata A, Abe T. Elimination of the check-valve mechanism of the sacral Tarlov cyst using a rotation flap technique in a pediatric patient: technical note. Childs Nerv Syst. (2021) 37(5):1741–5. doi: 10.1007/s00381-020-05029-z
5. Yang AI, McShane BJ, Welch WC. Growth of a sacral perineural (Tarlov) cyst: clinical images. World Neurosurg. (2018) 119:400–1. doi: 10.1016/j.wneu.2018.07.279
6. Klepinowski T, Orbik W, Sagan L. Global incidence of spinal perineural Tarlov’s cysts and their morphological characteristics: a meta-analysis of 13,266 subjects. Surg Radiol Anat. (2021) 43(6):855–63. doi: 10.1007/s00276-020-02644-y
7. Puffer RC, Gates MJ, Copeland W 3rd, Krauss WE, Fogelson J. Tarlov cyst causing sacral insufficiency fracture. Oper Neurosurg. 2017;13(3):E4–7. doi: 10.1093/ons/opw025
8. Urquiaga JF, Bagdady K, Zhang JK, Mercier PJ, Mattei TA. Complex surgical reconstruction for spinopelvic instability caused by a giant Tarlov cyst eroding the sacrum: a case report. N Am Spine Soc J. (2023) 14:100212. doi: 10.1016/j.xnsj.2023.100212
9. Chen YN, Yang SH, Chou SC, Kuo MF. The role of sacral laminoplasty in the management of spina bifida and sacral cystic lesions: case series. Neurosurg Focus. (2019) 47(4):E20. doi: 10.3171/2019.7.FOCUS19414
10. Weigel R, Polemikos M, Uksul N, Krauss JK. Tarlov cysts: long-term follow-up after microsurgical inverted plication and sacroplasty. Eur Spine J. (2016) 25(11):3403–10. doi: 10.1007/s00586-016-4744-5
11. Smith ZA, Li Z, Raphael D, Khoo LT. Sacral laminoplasty and cystic fenestration in the treatment of symptomatic sacral perineural (Tarlov) cysts: technical case report. Surg Neurol Int. (2011) 2:129. doi: 10.4103/2152-7806.85469
12. Lemcke J, Meier U, Al-Zain F. The clinical application of a new absorbable fixation clamp in craniotomy closure. A technical note after first experiences with 29 patients. Acta Neurochir. (2009) 151(10):1231–4. doi: 10.1007/s00701-009-0361-y
13. Matsukawa H, Miyama M, Miyazaki T, Uemori G, Kinoshita Y, Sakakibara F, et al. Impacts of pressure bonding fixation on a bone flap depression and resorption in patients with craniotomy. J Clin Neurosci. (2017) 41:162–7. doi: 10.1016/j.jocn.2017.02.026
14. Sun JJ, Wang ZY, Liu B, Li ZD, Wu HB, Yen RY, et al. Neck transfixion for sacral extradural spinal meningeal cysts without spinal nerve root fibers. Eur Spine J. (2016) 25(6):1945–52. doi: 10.1007/s00586-014-3471-z
15. Ma Q, Wu C, Zhang J, Yin X, Yang C, Si Y, et al. Arachnoidal diverticula of sacral extradural meningeal cyst: a novel definition and case series. World Neurosurg. (2022) 163:e106–12. doi: 10.1016/j.wneu.2022.03.052
16. Sugawara T, Higashiyama N, Tamura S, Endo T, Shimizu H. Novel wrapping surgery for symptomatic sacral perineural cysts. J Neurosurg Spine. (2021) 36(2):185–192. doi: 10.3171/2021.5.SPINE21179
17. Medani K, Lawandy S, Schrot R, Binongo JN, Kim KD, Panchal RR. Surgical management of symptomatic Tarlov cysts: cyst fenestration and nerve root imbrication-a single institutional experience. J Spine Surg. (2019) 5(4):496–503. doi: 10.21037/jss.2019.11.11
18. Elsawaf A, Awad TE, Fesal SS. Surgical excision of symptomatic sacral perineurial Tarlov cyst: case series and review of the literature. Eur Spine J. (2016) 25(11):3385–92. doi: 10.1007/s00586-016-4584-3
19. Burke JF, Thawani JP, Berger I, Nayak NR, Stephen JH, Farkas T, et al. Microsurgical treatment of sacral perineural (Tarlov) cysts: case series and review of the literature. J Neurosurg Spine. (2016) 24(5):700–7. doi: 10.3171/2015.9.SPINE153
20. Huang Q, Li J, Zhou Q, Li H, Yang X, Peng L, et al. Management of symptomatic sacral perineural cysts: a new surgical method. World Neurosurg. (2022) 167:e978–89. doi: 10.1016/j.wneu.2022.08.125
Keywords: sacral extradural spinal meningeal cysts, microsurgical treatment, sacral laminoplasty, sacral lamina, absorbable fixation clamps
Citation: Yin X, Zhang J, Ma Q, Chen S, Wu C, Yang C, Si Y, Jiang H, Guo W, Liu Y, Yuan H, Yang J and Sun J (2023) Feasibility and safety of one-stage sacral laminoplasty with autologous sacral laminar reimplantation fixed by absorbable fixation clamps in direct microsurgical treatment of symptomatic sacral extradural spinal meningeal cysts. Front. Surg. 10:1253432. doi: 10.3389/fsurg.2023.1253432
Received: 5 August 2023; Accepted: 30 October 2023;
Published: 21 November 2023.
Edited by:
Alessandro Di Rienzo, Marche Polytechnic University, ItalyReviewed by:
Erika Carrassi, Hospital Santa Maria della Misericordia of Rovigo, Italy© 2023 Yin, Zhang, Ma, Chen, Wu, Yang, Si, Jiang, Guo, Liu, Yuan, Yang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Yang YnlzeXNqd2tAMTI2LmNvbQ== Jianjun Sun c3Vuamlhbmp1bkBiam11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.