
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg., 24 August 2023
Sec. Thoracic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1235120
This article is part of the Research TopicInsights in the Minimally Invasive Surgery for Repair of Pectus ExcavatumView all 4 articles
 R. Scott Eldredge
R. Scott Eldredge Lisa McMahon*
Lisa McMahon*
Introduction: The minimally invasive repair of pectus excavatum (PE) is a painful procedure that can result in long-term hospitalization and opioid use. To mitigate the length of stay and opioid consumption, many different analgesia strategies have been implemented. The aim of this study is to review the use and patient outcomes of intercostal nerve cryoablation (INC) during PE repair reported in the literature.
Methods: An unfunded literature search using PubMed identifying articles discussing INC during PE repair from 1946 to 1 July 2023 was performed. Articles were included if they discussed patient outcomes with INC use during PE repair. Articles were excluded if they were reviews/meta-analyses, editorials, or not available in English. Each article was reviewed for bias by analyzing the study methods, data analysis, patient selection, and patient follow-up. Articles comparing outcomes of INC were considered significant if p-value was <0.05.
Results: A total of 34 articles were included in this review that described INC use during pectus repair. Most supported a decreased hospital length of stay and opioid use with INC. Overall, INC was associated with fewer short-term and long-term complications. However, the researchers reported varied results of total hospital costs with the use of INC.
Conclusion: The review was limited by a paucity of prospective studies and low number of patients who received INC. Despite this, the present data support INC as a safe and effective analgesic strategy during the repair of PE.
Pectus excavatum (PE) is the most common chest wall deformity characterized as an inward depression of the sternum, affecting one in every 250 adults with a female predominance of 5:3 (1–3). The sternal depression is hypothesized to be secondary to inward overgrowth of the costal cartilage, which is commonly exacerbated during puberty (4, 5). PE may have a myriad of adverse effects, ranging from impaired cardiopulmonary performance during rest and exercise to poor psychosocial outcomes (2).
The current gold standard for the repair of PE is the minimally invasive repair of PE (MIRPE), or the Nuss procedure, which has smaller incisions and decreased operative time and blood loss when compared with an open chest wall reconstruction, or the Ravitch procedure. MIRPE is a very safe procedure when performed in combination with a sternal elevation and intrathoracic visualization but is associated with more pain than the Ravitch procedure (6–8). In an attempt to mitigate patients pain following MIRPE, many analgesic strategies have been proposed including the use of thoracic epidurals (TEs), intravenous patient-controlled analgesia (PCA), indwelling chest wall catheter infusion or elastomeric pain pumps (EPPs), and local or regional nerve blocks (9–13).
The use of intercostal nerve cryoablation (INC) as an analgesic adjunct during the MIRPE was first reported in 2016 by Keller et al. (14) when they found that the use of INC was associated with a decreased length of stay (LOS) and inpatient opioid consumption when compared with TE. INC is thought to have temporary neurosensory effects and takes advantage of the ability of the peripheral nerves to regenerate following injury (15–17). Since the introduction of INC during the MIPRE, many surgeons have adopted this technique. The aim of our study is to review the reported patient outcomes of those who had undergone INC during PE repair in the current literature.
A literature search was performed using “Cryoablation” or “Cryotherapy” and “Pectus Excavatum” using PubMed from 1946 to 1 July 2023. All titles and abstracts were reviewed for content and subject relevance. Articles were excluded from the review if they did not pertain to patient outcomes of PE repair with the use of INC, if the article was not available in English, if the article was a review or meta-analysis, or if the article was an opinion piece. In addition, the citations were reviewed for all included articles. If a cited article was identified that pertained to INC during PE repair, it was then included in the review.
Two reviewers screened all the articles for the inclusion and exclusion criteria; upon selection, each article was reviewed, and data were abstracted pertaining to the study methods, patient demographics, INC technique, operative duration, INC comparison group, patient LOS, inpatient and outpatient oral morphine equivalence (OME), patient-reported pain scores, hospital charges, and surgical complications. The details pertaining to INC were recorded including number of nerves and intercostal spaces cryoablated and the duration and temperature of nerve cryoablation. The operative duration was recorded as both surgical time and operating room time if reported. The patient-reported pain scores were recorded on a Likert scale from 1 to 10. All complications reported by the authors were abstracted. The data points were excluded in this review if they were not reported by the authors or if any data points were unclear.
An in-depth assessment of articles discussing the primary outcome of LOS and secondary outcomes among patients who had undergone INC vs. a control analgesic strategy was conducted. Both prospective and retrospective studies were included in this review. Comparisons of outcomes were abstracted between study groups; outcomes between groups were considered statistically significant if a p-value of <0.05 was reported. All comparisons of LOS, opioid usage, and pain scores were compiled in a table regardless of statistical significance. The patient demographics were reviewed between those who received INC and those who received a different analgesic strategy to ensure patient similarities between groups. To reduce bias, the authors of this manuscript independently reviewed each study that was identified using PubMed for the inclusion criteria.
A total of 44 articles were identified via the defined literature search (33) and article citation review (5); of these articles, 34 were included in our review. Of the 10 excluded articles, four did not pertain to INC outcomes following the MIRPE (18–21), three were opinion editorials (22–24), two were review articles (25, 26), and two were not in the English language (27). Of the articles included, the majority were single-center retrospective reviews (29/34), with one randomized control trial and four prospective reviews. A total of 47% of the articles included both pediatric and adult patients in their analysis; however, the majority of the patients were pediatric, ≤18 years old, with an average age of less than 21 years in all articles. Most articles contained fewer than 60 patients who had undergone INC, and the largest study contained 350 patients. A majority (24/34) compared patient-related outcomes between INC and a control group. The control groups included multimodal pain regimen, thoracic epidural PCA, paravertebral nerve block with and without continuous infusion, elastomeric pain pump, or unspecified analgesia strategy (Table 1).
INC was reported to be performed via an intrathoracic approach under thoracoscopic visualization in 90% of the cases. The number of intercostal nerves that were cryoablated ranged from eight to 12 between the intercostal space of T3–T8. Velayos et al. (39) reported performing INC preoperatively via a percutaneous approach. Almost all the researchers applied the cryoprobe for a single 2 min duration to each intercostal nerve, with one article reporting a single 1 min application of the cryoprobe. The temperature of the applied cryoprobe reached temperatures ranging from <−40 to −70°C. The operative times during the MIRPE with INC ranged from 60 to 153 min (Table 1).
The primary outcome discussed in the majority of the articles was hospital LOS and opioid usage (Table 2). The use of INC was associated with a significant decrease in LOS when compared with other analgesic strategies in 21 out of 22 articles (13, 14, 28–30, 32–38, 41–43, 46, 48–52, 54, 55). When comparing LOS between patients who had undergone INC vs. TE placement, Keller et al. (14) found that hospital LOS decreased from 5.8 days to 3.4 days. Other researchers have corroborated INCs effect on hospital LOS when compared with TE placement reporting a decreased LOS of 2–3.5 days (13, 28, 30, 33, 34, 41, 46, 48, 50, 52, 54). One study found no significant change in LOS when comparing INC with EPP (29); however, this study was possibly underpowered to find a statistical difference among cohorts, with only six patients receiving INC as part of their care. Alternatively, INC was found to reduce hospital LOS when compared with EPP in every other study that compared these two analgesic strategies (31, 38, 48, 53). EPP only provides analgesia while in place whereas INC provides a prolonged analgesic effect; in the prior studies, EPPs were typically in place for 48–72 h postoperatively and were discontinued prior to discharge. When INC is used in combination with a multimodal pain regimen, the researchers found that patients were able to be routinely discharged on post operative day (POD) 1 (33, 37, 40, 48, 49, 51, 56, 57). Recent publications have demonstrated the feasibility of a same-day discharge when INC is combined with a peripheral nerve block (PNB) with 65%–66% of patients being discharged on POD 0 (49, 55, 58).
Opioid usage significantly decreased with INC use during MIRPE when compared with other analgesic strategies in all studies that reported opioid consumption (13, 14, 28, 30, 31, 35, 37, 38, 41–43, 46, 48–51, 53, 54). A majority of these studies reported opioid use in terms of OME milligrams and reported the total hospital OME milligram; however, most did not account for the LOS in the non-INC cohort when reporting opioid use (14, 28, 29, 31, 35, 38, 40–43, 48, 50, 51, 54). All researchers that compared opioid OME by individual hospital days reported a significant lower amount of opioid consumption among the INC cohort than those with other analgesic strategy (37, 46, 48, 49, 53). Of all articles comparing opioid consumption between an INC and non-INC cohort, all found equivocal or lower opioid consumption among those who had INC during MIRPE. The researchers found a significant decrease in the total OME prescription at discharge and duration of opioid use post-MIRPE when INC was utilized (28, 35, 37, 38, 43, 51, 54).
The effect of INC on visual analog pain scores (VAPS) varied between investigators, with less than half (5/11) of the articles finding a significant decrease in VAPS when INC was used (Table 2) (13, 28–30, 33, 41, 44, 46, 48, 49, 53). INC was associated with significantly lower VAPS only during the initial postoperative hospitalization. At the outpatient follow-up, there was no differences found in VAPS; however, VAPS were generally low following discharge in both the INC and non-INC cohorts.
The complications associated with INC were reported in 50% of the articles reviewed. The overall complication rate was either significantly lower or no difference was found between the INC and non-INC cohorts (28, 31, 35, 38, 40, 43, 45, 50, 51, 53, 54, 59). Postoperative urinary retention was found to improve with INC with rates ranging 4%–8% compared with the 14%–34% in those who did not have INC (38, 43).
Keller et al. 2016 and Sun et al. 2021 reported higher rates of clinically significant pectus bar migration requiring reoperation in patients with INC. In these studies, bar migration occurred in 8%–12% of patients who had INC; however, neither study provided a statistical comparison of bar migration between the INC group and a control. The bar migration was hypothesized to be secondary to an increased activity in patients with INC due to an improved pain control (14, 38). However, an increased bar migration has not been supported by other studies (28, 31, 59). In fact, the largest cohort study of INC in MIRPE, containing 350 patients, reported bar migration occurring in less than 1% of patients who received INC (59).
Neurosensory outcomes following the use of INC were reported in 16% of studies (13, 32, 37, 40, 51). A complete chest wall sensory return following cryoablation was reported to occur in 76.9%–100% of patients 1 year post-INC. No difference in neuropathic pain was found between patients with INC and those with an alternative analgesic strategy (13, 51). Zobel et al. conducted a retrospective review comparing neuropathic pain between adolescent and adult patients using a validated neuropathic pain survey. They found that neuropathic pain was more common in adults (>21 years of age) (32). In children, the incidence rate of neuropathic pain was 0% at 12 months (40). While these studies demonstrate a relatively low risk for developing persistent sensory loss or chronic neuropathic pain, most studies were retrospective in nature, creating an inherent bias in their findings. No articles discussed in detail how chest wall sensory examinations were performed or validated. In addition, only one article compared sensory outcomes between INC and a control group. Graves et al. conducted a randomized control trial between INC and the use of TE during MIRPE. In this study, they reported the sensory outcomes between each cohort at different intervals postoperatively. All patients with INC (n = 10) had reported chest wall sensory loss at their 2-week postoperative exam; interestingly, 20% (2/10) of the patients without INC also had some degree of chest wall sensory loss noted 2 weeks postoperatively. A complete chest wall sensory return was noted in both the INC and non-INC cohort prior to the study completion (13). This finding suggests that some sensory loss may be attributable to surgical technique; however, this study was underpowered to truly compare sensory loss and recovery between INC and MIRPE.
The majority of articles that discuss total hospital costs and charges found that INC is associated with a decrease in cost when compared with other analgesic strategies (35, 37, 50, 51, 54). The median overall cost of MIRPE with INC ranged from $14,072 to $33,848 compared with $18,335–$40,813 MIRPE without INC. All investigators who included an itemized cost analysis found that the use of INC was associated with a greater operative room cost (35, 50, 54). One of five studies found that the use of INC was associated with higher hospital cost. Perez Holguin et al. conducted a retrospective review comparing hospital cost between TE use from 2002 to 2020 and INC use from 2017 to 2020. They found an overall increased hospital cost from $21,621 to $24,742 when INC was used compared with TE; the largest contributor to cost with INC was the intraoperative charge of $6,198 vs. $3,916 for TE. However, in their cost analysis, they failed to account for inflation and operative technique, i.e., number of pectus bars implanted, bar stabilization between groups (50). Similarly, Aiken et al. performed a cost analysis of INC compared with a standardized pain control cohort between 2016 and 2019. The total hospital costs were adjusted to 2018 dollars to standardize monetary value across each study year. They found that the total hospital cost was lower in the INC cohort, $21,924, compared with the non-INC cohort, $23,694 (Table 2) (37).
Since the introduction of INC during MIRPE, it has consistently shown to decrease hospital LOS and opioid usage among children and adolescents. In addition, INC has a favorable side effect profile with minimal associated morbidity. INC is routinely performed on the bilateral chest wall under direct visualization, using a single lung ventilatory strategy, between the intercostal nerves T3 and T8 at a temperature of −40°C to −60°C for a 2-min duration (Figure 1). The cryoprobe is allowed to actively rewarm to a temperature of −4°C prior to removal from the chest wall to avoid tissue fracture/injury. Care is taken to avoid inadvertent contact of the cryoprobe and lung tissue to avoid thermal pulmonary injury and delayed pneumothorax; in addition, the anesthesiologist continues contralateral single lung ventilation for 3 min from the last INC to avoid thermal injury from the chest wall.
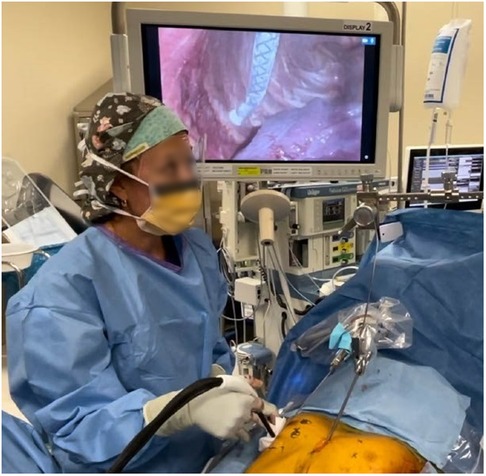
Figure 1. Intraoperative use of intercostal nerve cryoablation. The cryoprobe is inserted into the chest under direct thoracoscopic visualization. The cryoprobe is placed just inferior to the costal rig and applied for a 2 min duration at a temperature of −60°C. The probe is placed at least 4 cm from the spinal column to avoid injury to the sympathetic chain. An “ice ball” is formed at the tip of the cryoprobe during the freezing process. A lung isolation strategy is used to avoid pulmonary tissue thermal injury using a dual lumen endotracheal tube. A surgical laparotomy pad is used to protect the skin from inadvertent thermal injury as pictured.
The largest series of patients undergoing the MIRPE with INC was reported in 2022 (53). This study was a retrospective review that captured 350 patients who had undergone INC between December 2017 and August 2021. The mean age of the study cohort was 15.7 years with a Haller index of 5.4 and correction index of 35.2. The patients were divided into time-based quartiles determined by their operative dates; the patient outcomes were compared between the first and forth quartile. The authors found a decreased hospital LOS, total OME milligram, and OME milligram per day between the first and forth time-based quartiles. In addition, the patients had a relatively low morbidity with <1% having pectus bar migration and <5% requiring a 90-day readmission and a 90-day wound infection. Despite these findings, this study lacked a control arm that had not undergone INC as part of their pain strategy.
Those who do not support the use of INC during MIRPE in children and adolescents argue that while INC has been shown to decrease hospital LOS and opioid consumption, it lacks data supporting long-term safety and efficacy. They further cite that no studies have adequately compared INC with the erector spinae block, which is associated with a short hospital LOS and low opioid use and may spare adolescent patients from the possible neurosensory and neuropathic pain complications of INC (23). Future prospective studies are warranted to compare the long-term neurosensory effects of INC and to determine the incidence rate of chronic neuropathic pain.
In addition to neurosensory outcomes, there is a paucity of literature regarding the effect of INC on the psychosocial and physiologic quality of life of the patient. The repair of PE has been shown to have a significant improvement in both self-perception and physiologic status of the patient (60–62). In the current literature, only one article addressed pulmonary functions following MIRPE with INC. Lai et al. (53) demonstrated that INC did not worsen the pulmonary function of the patient, as measured by incentive spirometry, when compared with the use of an elastomeric pain pump. Research is needed to address the effects that INC has on the psychosocial and cardiopulmonary performance outcomes following MIRPE. Furthermore, an investigation of the impact that INC has on postoperative patient activity is warranted as some have reported unacceptably high rates of pectus bar migration following INC use (14, 38).
In the current literature that reviews the use of INC during the repair of PE, most studies were performed retrospectively leading to inherent bias and limitations (63). Of the studies that were conducted in a prospective manner, all were possibly underpowered without discussion of a power calculation and the largest number of patients receiving INC in any study being 35 (13, 33, 48, 49, 57, 58). Among the prospective studies, INC was only compared against TE, PCA, and intercostal nerve blocks. In addition, there has only been one randomized control trial comparing INC with any other analgesic strategy. Again, this study was limited by a small sample of five patients, who had undergone INC as part of the MIRPE (13). The paucity of appropriately powered prospective studies ultimately limits the conclusions that can be drawn with regard to the true effect that the INC has on patient outcomes. Future prospective randomized control trials are needed to compare INC with other analgesic strategies.
INC is an effective analgesic strategy following the MIPRE, with its use known to decrease hospital LOS and opioid consumption with minimal morbidity.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
RE and LM contributed to the conception and design of the study. RE wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
LM is an educational consultant for AtriCure.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1235120/full#supplementary-material
1. Biavati M, Kozlitina J, Alder AC, Foglia R, McColl RW, Peshock RM, et al. Prevalence of pectus excavatum in an adult population-based cohort estimated from radiographic indices of chest wall shape. PLoS One. (2020) 15(5):e0232575. doi: 10.1371/journal.pone.0232575
2. Obermeyer RJ, Goretsky MJ. Chest wall deformities in pediatric surgery. Surg Clin North Am. (2012) 92(3):669–84. doi: 10.1016/j.suc.2012.03.001
3. Frawley G, Frawley J, Crameri J. A review of anesthetic techniques and outcomes following minimally invasive repair of pectus excavatum (Nuss procedure). Paediatr Anaesth. (2016) 26(11):1082–90. doi: 10.1111/pan.12988
4. Fokin AA, Steuerwald NM, Ahrens WA, Allen KE. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg. (2009) 21(1):44–57. doi: 10.1053/j.semtcvs.2009.03.001
5. Brochhausen C, Turial S, Müller FKP, Schmitt VH, Coerdt W, Wihlm JM, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg. (2012) 14(6):801–6. doi: 10.1093/icvts/ivs045
6. Nuss D, Obermeyer RJ, Kelly RE. Pectus excavatum from a pediatric surgeon's perspective. Ann Cardiothorac Surg. (2016) 5(5):493–500. doi: 10.21037/acs.2016.06.04
7. Mao YZ, Tang ST, Li S. Comparison of the Nuss versus Ravitch procedure for pectus excavatum repair: an updated meta-analysis. J Pediatr Surg. (2017) 52(10):1545–52. doi: 10.1016/j.jpedsurg.2017.05.028
8. Kelly RE, Shamberger RC, Mellins RB, Mitchell KK, Lawson ML, Oldham K, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg. (2007) 205(2):205–16. doi: 10.1016/j.jamcollsurg.2007.03.027
9. Stroud AM, Tulanont DD, Coates TE, Goodney PP, Croitoru DP. Epidural analgesia versus intravenous patient-controlled analgesia following minimally invasive pectus excavatum repair: a systematic review and meta-analysis. J Pediatr Surg. (2014) 49(5):798–806. doi: 10.1016/j.jpedsurg.2014.02.072
10. St. Peter SD, Weesner KA, Weissend EE, Sharp SW, Valusek PA, Sharp RJ, et al. Epidural vs. patient-controlled analgesia for postoperative pain after pectus excavatum repair: a prospective, randomized trial. J Pediatr Surg. (2012) 47(1):148–53. doi: 10.1016/j.jpedsurg.2011.10.040
11. Burton DMH, Boretsky KR. A comparison of paravertebral nerve block catheters and thoracic epidural catheters for postoperative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr Anaesth. (2014) 24(5):516–20. doi: 10.1111/pan.12369
12. Choudhry DK, Brenn BR, Sacks K, Reichard K, Lonnqvist PA. Continuous chest wall ropivacaine infusion for analgesia in children undergoing Nuss procedure: a comparison with thoracic epidural. Paediatr Anaesth. (2016) 26(6):582–9. doi: 10.1111/pan.12904
13. Graves CE, Moyer J, Zobel MJ, Mora R, Smith D, O’Day M, et al. Intraoperative intercostal nerve cryoablation during the Nuss procedure reduces length of stay and opioid requirement: a randomized clinical trial. J Pediatr Surg. (2019) 54(11):2250–6. doi: 10.1016/j.jpedsurg.2019.02.057
14. Keller BA, Kabagambe SK, Becker JC, Chen YJ, Goodman LF, Clark-Wronski JM, et al. Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: preliminary outcomes in twenty-six cryoablation patients. J Pediatr Surg. (2016) 51(12):2033–8. doi: 10.1016/j.jpedsurg.2016.09.034
16. Gordon T. Peripheral nerve regeneration and muscle reinnervation. Int J Mol Sci. (2020) 21(22):1–24. doi: 10.3390/ijms21228652
17. Chen P, Xianhua P, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. (2015) 3:605–18. doi: 10.1007/s00401-015-1482-4
18. Shafiq M, Sethi J, Ali MS, Ghori UK, Saghaie T, Folch E. Pleural cryobiopsy: a systematic review and meta-analysis. Chest. (2020) 157(1):223–30. doi: 10.1016/j.chest.2019.09.023
19. Talsma J, Kusakavitch M, Lee D, Niederhauser C, Palmer B, Ozgediz D, et al. Forgotten branch of the intercostal nerve: implication for cryoablation nerve block for pectus excavatum repair. J Pediatr Surg. (2023) S0022-3468(23):00294–4. doi: 10.1016/j.jpedsurg.2023.05.006
20. Chen SY, Mack SJ, Stein JE, Kelley-Quon LI, Kim ES. Intercostal nerve cryoablation is associated with reduced opioid use in pediatric oncology patients. J Surg Res. (2023) 283:377–84. doi: 10.1016/j.jss.2022.11.004
21. Gologorsky R, Ewbank C, Idowu O, Kim S. Use of cryoanalgesia as a postoperative pain management for open pectus carinatum repair. Pediatr Surg Int. (2021) 37(1):179–81. doi: 10.1007/s00383-020-04768-z
22. Das B, Sadhasivam S. Response to intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair. J Pediatr Surg. (2017) 52(6):1076. doi: 10.1016/j.jpedsurg.2017.01.069
23. Chidambaran V, Garcia VF, Brown RL. Are we ready for cryoablation in children undergoing Nuss procedures? Anesth Analg. (2022) 134(4):881–4. doi: 10.1213/ANE.0000000000005857
24. McCoy N, Hollinger L. Cryoanalgesia and lung isolation: a new challenge for the Nuss procedure made easier with the EZ-Blocker™. Front Pediatr. (2021) 9:791607. doi: 10.3389/fped.2021.791607
25. Daemen JHT, de Loos ER, Vissers YLJ, Bakens MJAM, Maessen JG, Hulsewé KWE. Intercostal nerve cryoablation versus thoracic epidural for postoperative analgesia following pectus excavatum repair: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. (2020) 31(4):486–98. doi: 10.1093/icvts/ivaa151
26. Singhal NR, Jerman JD. A review of anesthetic considerations and postoperative pain control after the Nuss procedure. Semin Pediatr Surg. (2018) 27(3):156–60. doi: 10.1053/j.sempedsurg.2018.05.010
27. Pechetov AA, Lednev AN, Makov MA, Chlan TN. Intercostal nerve cryoablation in correction of pectus excavatum in adults. Khirurgiia (Sofiia). (2021) 5:14–9. doi: 10.17116/hirurgia202105114
28. Harbaugh CM, Johnson KN, Kein CE, Jarboe MD, Hirschl RB, Geiger JD, et al. Comparing outcomes with thoracic epidural and intercostal nerve cryoablation after Nuss procedure. J Surg Res. (2018) 231:217–23. doi: 10.1016/j.jss.2018.05.048
29. Morikawa N, Laferriere N, Koo S, Johnson S, Woo R, Puapong D. Cryoanalgesia in patients undergoing Nuss repair of pectus excavatum: technique modification and early results. J Laparoendosc Adv Surg Tech A. (2018) 28(9):1148–51. doi: 10.1089/lap.2017.0665
30. Sujka J, Benedict LA, Fraser JD, Aguayo P, Millspaugh DL, St Peter SD. Outcomes using cryoablation for postoperative pain control in children following minimally invasive pectus excavatum repair. J Laparoendosc Adv Surg Tech A. (2018) 28(11):1383–6. doi: 10.1089/lap.2018.0111
31. Parrado R, Lee J, McMahon LE, Clay C, Powell J, Kang P, et al. The use of cryoanalgesia in minimally invasive repair of pectus excavatum: lessons learned. J Laparoendosc Adv Surg Tech A. (2019) 29(10):1244–51. doi: 10.1089/lap.2019.0203
32. Zobel MJ, Ewbank C, Mora R, Idowu O, Kim S, Padilla BE. The incidence of neuropathic pain after intercostal cryoablation during the Nuss procedure. Pediatr Surg Int. (2020) 36(3):317–24. doi: 10.1007/s00383-019-04602-1
33. Dekonenko C, Dorman RM, Duran Y, Juang D, Aguayo P, Fraser JD, et al. Postoperative pain control modalities for pectus excavatum repair: a prospective observational study of cryoablation compared to results of a randomized trial of epidural vs. patient-controlled analgesia. J Pediatr Surg. (2020) 55(8):1444–7. doi: 10.1016/j.jpedsurg.2019.09.021
34. Pilkington M, Harbaugh CM, Hirschl RB, Geiger JD, Gadepalli SK. Use of cryoanalgesia for pain management for the modified Ravitch procedure in children. J Pediatr Surg. (2020) 55(7):1381–4. doi: 10.1016/j.jpedsurg.2019.09.016
35. Rettig RL, Rudikoff AG, Lo HYA, Shaul DB, Banzali FM, Conte AH, et al. Cryoablation is associated with shorter length of stay and reduced opioid use in pectus excavatum repair. Pediatr Surg Int. (2021) 37(1):67–75. doi: 10.1007/s00383-020-04778-x
36. Arshad SA, Hatton GE, Ferguson DM, Li LT, Austin MT, Tsao KJ. Cryoanalgesia enhances recovery from minimally invasive repair of pectus excavatum resulting in reduced length of stay: a case-matched analysis of NSQIP-pediatric patients. J Pediatr Surg. (2021) 56(7):1099–102. doi: 10.1016/j.jpedsurg.2021.03.017
37. Aiken TJ, Stahl CC, Lemaster D, Casias TW, Walker BJ, Nichol PF, et al. Intercostal nerve cryoablation is associated with lower hospital cost during minimally invasive Nuss procedure for pectus excavatum. J Pediatr Surg. (2021) 56(10):1841–5. doi: 10.1016/j.jpedsurg.2020.10.009
38. Sun RC, Mehl SC, Anbarasu CR, Portuondo JI, Espinoza AF, Whitlock R, et al. Intercostal cryoablation during Nuss procedure: a large volume single surgeon’s experience and outcomes. J Pediatr Surg. (2021) 56(12):2229–34. doi: 10.1016/j.jpedsurg.2021.03.006
39. Velayos M, Alonso M, Delgado-Miguel C, Estefanía-Fernández K, Muñoz-Serrano AJ, Santamaría MVL, et al. Percutaneous cryoanalgesia: a new strategy for pain management in pectus excavatum surgery. Eur J Pediatr Surg. (2022) 32(1):73–9. doi: 10.1055/s-0041-1740555
40. DiFiore JW, Robertson JO, Chhabada S, DeRoss AL, Hossain MS, Rincon-Cruz L, et al. Next day discharge after the Nuss procedure using intercostal nerve cryoablation, intercostal nerve blocks, and a perioperative ERAS pain protocol. J Pediatr Surg. (2022) 57(2):213–8. doi: 10.1016/j.jpedsurg.2021.10.034
41. Song SH, Moon DH, Shim YH, Jung H, Lee S. Limited cryoablation reduces hospital stay and opioid consumption compared to thoracic epidural analgesia after minimally invasive repair of pectus excavatum. Medicine (Baltimore). (2022) 101(31):E29773. doi: 10.1097/MD.0000000000029773
42. Arshad SA, Ferguson DM, Garcia EI, Hebballi NB, Buchanan AC, Tsao KJ. Cryoanalgesia is associated with decreased postoperative opioid use in minimally invasive repair of pectus excavatum. J Surg Res. (2022) 271:1–6. doi: 10.1016/j.jss.2021.10.011
43. Clark RA, Jacobson JC, Singhal A, Alder AC, Chung DH, Pandya SR. Impact of cryoablation on pectus excavatum repair in pediatric patients. J Am Coll Surg. (2022) 234(4):484–92. doi: 10.1097/XCS.0000000000000103
44. Fraser JA, Briggs KB, Svetanoff WJ, Aguayo P, Juang D, Fraser JD, et al. Short and long term outcomes of using cryoablation for postoperative pain control in patients after pectus excavatum repair. J Pediatr Surg. (2022) 57(6):1050–5. doi: 10.1016/j.jpedsurg.2022.01.051
45. Bundrant NT, Sayrs LW, Ostlie D, Lee J, Egan C, Molitor M, et al. Infectious complications of intercostal nerve cryoablation mediated by perioperative hypothermia during pediatric Nuss procedure. J Pediatr Surg. (2022) 57(6):1083–6. doi: 10.1016/j.jpedsurg.2022.01.044
46. Cockrell HC, Hrachovec J, Schnuck J, Nchinda N, Meehan J. Implementation of a cryoablation-based pain management protocol for pectus excavatum. J Pediatr Surg. (2023) 58(7):1239–45. doi: 10.1016/j.jpedsurg.2023.01.059. Available at: https://pubmed.ncbi.nlm.nih.gov/36894442/ (Accessed May 14, 2023).36894442
47. Lai K, Eldredge RS, Nguyen M, Padilla BE, McMahon LE. Initial outcomes using cryoablation in surgical management of slipping rib syndrome. J Pediatr Surg. (2023) 58(8):1430–4. doi: 10.1016/j.jpedsurg.2022.12.031. Available at: https://pubmed.ncbi.nlm.nih.gov/36737261/ (Accessed May 14, 2023).36737261
48. Downing L, Ramjist JK, Tyrrell A, Tsang M, Isaac L, Fecteau A. Development of a five point enhanced recovery protocol for pectus excavatum surgery. J Pediatr Surg. (2023) 58(5):822–7. doi: 10.1016/j.jpedsurg.2023.01.028
49. Akinboro S, John R, Reyna T, Davis R, Ayoub C, Sangster R, et al. A pilot study of multi-modal pain management for same-day discharge after minimally invasive repair of pectus excavatum (Nuss procedure) in children. Pediatr Surg Int. (2023) 39(1):159. doi: 10.1007/s00383-023-05429-7
50. Perez Holguin RA, DeAngelo N, Sinha A, Shen C, Tsai AY. Cost and outcomes of intercostal nerve cryoablation versus thoracic epidural following the Nuss procedure. J Pediatr Surg. (2023) 58(4):608–12. doi: 10.1016/j.jpedsurg.2022.12.011
51. Zeineddin S, Goldstein SD, Linton S, DeBoer C, Alayleh A, Ortiz I, et al. Effectiveness of one minute per level intercostal nerve cryoablation for postoperative analgesia after surgical correction of pectus excavatum. J Pediatr Surg. (2023) 58(1):34–40. doi: 10.1016/j.jpedsurg.2022.09.032
52. Jaroszewski DE, Bostoros P, Farina JM, Botros MM, Aly MR, Peterson M, et al. Evolution of pain control for adult pectus excavatum repair. Ann Thorac Surg. (2023) S0003-4975(23):00570–2. doi: 10.1016/j.athoracsur.2023.04.044
53. Lai K, Lee J, Notrica DM, Egan JC, McMahon LE, Molitor MS, et al. Intercostal nerve cryoablation in minimally invasive repair of pectus excavatum: effect on pulmonary function. J Laparoendosc Adv Surg Tech A. (2022) 32(12):1244–8. doi: 10.1089/lap.2022.0242
54. Rettig RL, Yang CJ, Ashfaq A, Sydorak RM. Cryoablation is associated with shorter length-of-stay and reduced opioid use after the Ravitch procedure. J Pediatr Surg. (2022) 57(7):1258–63. doi: 10.1016/j.jpedsurg.2022.02.040
55. Rettig RL, Rudikoff AG, Annie Lo HY, Lee CW, Vazquez WD, Rodriguez K, et al. Same-day discharge following the Nuss repair: a comparison. J Pediatr Surg. (2022) 57(1):135–40. doi: 10.1016/j.jpedsurg.2021.09.023
56. Cadaval Gallardo C, Martínez J, Bellía-Munzon G, Nazar M, Sanjurjo D, Toselli L, et al. Thoracoscopic cryoanalgesia: a new strategy for postoperative pain control in minimally invasive pectus excavatum repair. Cir Pediatr. (2020) 33(1):11–5. Available at: https://pubmed.ncbi.nlm.nih.gov/32166917/ (Accessed May 14, 2023).32166917
57. Torre M, Mameli L, Bonfiglio R, Guerriero V, Derosas L, Palomba L, et al. A new device for thoracoscopic cryoanalgesia in pectus excavatum repair: preliminary single center experience. Front Pediatr. (2020) 8:614097. doi: 10.3389/fped.2020.614097
58. Rettig RL, Rudikoff AG, Lo HYA, Lee CW, Vazquez WD, Rodriguez K, et al. Same day discharge for pectus excavatum—is it possible? J Pediatr Surg. (2022) 57(9):34–8. doi: 10.1016/j.jpedsurg.2021.02.007
59. Lai K, Notrica DM, McMahon LE, Kang P, Molitor MS, Egan JC, et al. Cryoablation in 350 Nuss procedures: evolution of hospital length of stay and opioid use. J Pediatr Surg. (2022) 58(8):1435–9. doi: 10.1016/j.jpedsurg.2022.10.051. Available at: https://pubmed.ncbi.nlm.nih.gov/36494205/ (Accessed May 14, 2023).36494205
60. Kuru P, Bostanci K, Ermerak NO, Bahadir AT, Afacan C, Yuksel M. Quality of life improves after minimally invasive repair of pectus excavatum. Asian Cardiovasc Thorac Ann. (2015) 23(3):302–7. doi: 10.1177/0218492314553442
61. Kuru P, Cakiroglu A, Er A, Ozbakir H, Cinel AE, Cangut B, et al. Pectus excavatum and pectus carinatum: associated conditions. Family history, and postoperative patient satisfaction. Korean J Thorac Cardiovasc Surg. (2016) 49(1):29–34. doi: 10.5090/kjtcs.2016.49.1.29
62. Lawson ML, Cash TF, Akers R, Vasser E, Burke B, Tabangin M, et al. A pilot study of the impact of surgical repair on disease-specific quality of life among patients with pectus excavatum. J Pediatr Surg. (2003) 38(6):916–8. doi: 10.1016/S0022-3468(03)00123-4
Keywords: pectus excavatum, minimally invasive repair of pectus excavatum, cryoablation, Nuss, cryoanalgesia
Citation: Eldredge RS and McMahon L (2023) Intercostal nerve cryoablation therapy for the repair of pectus excavatum: a systematic review. Front. Surg. 10:1235120. doi: 10.3389/fsurg.2023.1235120
Received: 5 June 2023; Accepted: 10 August 2023;
Published: 24 August 2023.
Edited by:
Marco Scarci, Hammersmith Hospital, United KingdomReviewed by:
Gary Raff, University of California, United States© 2023 Eldredge and McMahon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa McMahon bG1jbWFob25AcGhvZW5peGNoaWxkcmVucy5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.