- 1Faculty of Medicine, Al-Quds University, Jerusalem, Palestine
- 2Department of Surgery, Al-Makassed Hospital, Jerusalem, Palestine
Introduction: A splenic artery aneurysm is considered an abnormal dilatation of the splenic artery layers greater than 1 cm in diameter. First described by Beaussier in 1770, it affects 1% of the population but carries a major risk for life-threatening complications of rupture in 3%–10% of cases regardless of its congenital or acquired etiology. The presentation is highly variable, from asymptomatic incidental discovery during routine imaging to aneurysmal rupture causing acute abdomen, massive gastrointestinal bleeding, and hemorrhagic shock.
Case presentation: Herein, we present a 19-year-old male patient who presented with epigastric pain and abdominal rigidity associated with a moderate amount of free peritoneal fluid that was found to be a ruptured SAA after immediate laparoscopy, which was successfully managed with splenectomy.
Conclusion: SAAs are a rare etiology of acute abdomen and hemorrhagic shock but have a very high risk of mortality even upon immediate intervention, requiring a very high level of vigilance and a low threshold for surgical intervention in unstable patients presenting with abdominal pain.
Introduction
Although rare, with only 1% prevalence (1), a splenic artery aneurysm (SAA) is considered the third most common intra-abdominal aneurysm after the aortic and iliac artery aneurysms (2); however, due to the high risk of mortality when ruptured, it is of crucial importance to diagnose and manage it to prevent fatal outcomes, even though it occurs in only 10% of cases (1). Patients may be asymptomatic, but the rupture is catastrophic, with a mortality rate as high as 66% in aneurysms greater than 2 cm (3). SAAs can be congenital or acquired, and atherosclerosis, hypertension, trauma, and septic embolism play a role in their development. Pregnancy and multiparity are risk factors in the development of SAAs (4), and indeed, SAAs are frequent in female patients as the female-to-male ratio is 4:1 (3). Our 19-year-old male patient, who presented with sudden onset stabbing epigastric pain of 1-h duration, borderline blood pressure, and tachycardia associated with abdominal rigidity, was found to have free fluid on abdominal imaging. The patient was lucky and survived an acute abdomen and a hemorrhagic shock after nontraumatic spontaneous rupture of his previously undiagnosed SAA.
Case presentation
A 19-year-old male patient with no significant medical or surgical history presented to the emergency department complaining of a 1-h history of sudden onset severe stabbing epigastric pain that then became generalized, radiated to both shoulders and back, associated with nausea but no vomiting, increased upon movement and while in the supine position but that was not related to food. The patient said they had no history of preceding trauma, vomiting episodes, hematemesis, melena, or changes in bowel habits. He also outlined that he had not had similar previous episodes, postprandial pain, alcohol consumption, and chronic analgesia ingestion. He worked as a construction worker and had no encounters with animals or sick contacts.
Upon examination, he was mildly hypotensive with a blood pressure of 106/72 and tachycardic. Capillary refilling time was 2 s; he was conscious and alert, lying in bed motionless in pain. He was pale and sweaty. Abdominal examination revealed voluntary guarding with generalized tenderness maximum on the epigastric area, questionable costovertebral angle tenderness, and hypoactive bowel sounds, and rectal examination revealed no blood or black tarry stools. Chest and heart examination revealed no abnormalities.
Initial laboratory tests showed a hemoglobin level of 14 g/dL, a white blood cell count of 8 WBCs per microliter (8 × 109/L), a platelet count of 254 platelets per microliter, normal liver enzymes, normal kidney and liver function tests, and normal lipase and amylase levels. Abdomen x-ray showed no bowel dilatation or air-fluid levels. Chest x-ray showed no subdiaphragmatic free air. Abdomen ultrasound showed normal internal organs of the abdomen except for moderate amounts of free fluid and no signs of cholecystitis, biliary dilatation, hydronephrosis, or splenic abnormalities. He underwent contrast-enhanced abdomen and pelvic computed tomography that showed moderate amounts of free fluid around the spleen, in the pelvis, between the stomach and spleen, and in Morison's pouch (Figures 1, 2); fluid density was >40 IU, suggestive of blood. No definite bleeding source was identified, so the patient was urgently transferred to the operating room for exploratory laparoscopy as a case of acute abdomen and hemoperitoneum; embolization was not attempted due to ongoing hemorrhage with peritoneal signs on examination and due to lack of definitive diagnosis. The exploration began laparoscopically, but the perisplenic, perihepatic, and pelvic areas were filled with bloodf and the source could not be identified, so it was converted to laparotomy through a midline incision where massive hemorrhage was encountered; 2 L of fresh blood was hardly suctioned, and multiple pads were required to control bleeding. The gastrocolic ligament was dissected widely, and a huge amount of coagulated blood was evacuated, allowing the identification of a bleeding arterial source that was clamped just behind the distal pancreas near the splenic hilum that was diagnosed to be an actively bleeding ruptured hilar splenic artery aneurysm measuring almost 2.5 cm. At the pancreatic upper border, the splenic artery was dissected and fixed with nonabsorbable sutures for the proximal control of the hemorrhage, effectively decreasing its volume. Hemorrhage was completely controlled via splenectomy en bloc. Running of the bowel and other internal organ examination was also done, and the result was normal with no other abnormalities. Hemostasis was secured, and the patient received two packed red blood cell units intra-operatively. Then, he was transferred to the intensive care unit for close monitoring with no immediate or early complications.
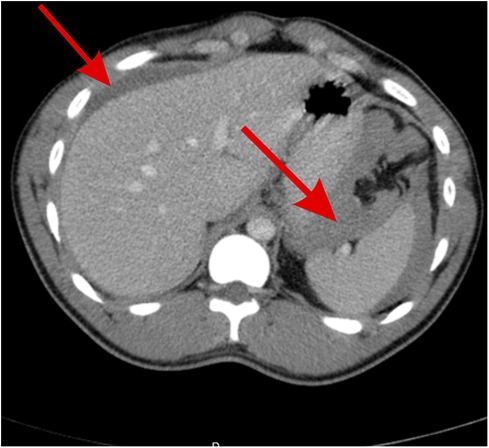
Figure 1. Abdominal CT scan showing free fluid of blood density around the spleen, between the spleen and stomach, and around the hepatic areas, indicating hemoperitoneum (red arrows).
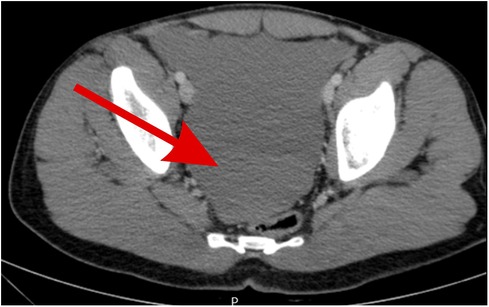
Figure 2. Pelvic CT scan showing free fluid in the pelvic area of fluid density indicating hemoperitoneum (red arrow).
Postoperatively and after resuscitation, hemoglobin decreased to 11 g/dL, but white blood cell count, platelet count, lactic acid, and C-reactive protein were normal. The patient recovered well in 4 days with no events and was discharged home in excellent condition.
At 2-week follow-up after discharge, the patient reported no complaints, was vitally stable with normal physical examination, had a hemoglobin level of 12.7 g/dL, and had taken recommended postsplenectomy vaccinations including pneumococcal, meningococcal, and Haemophilus influenzae vaccines and annual vaccination as scheduled.
Pathologic examination of the resected specimen showed a 2.5-cm true splenic artery aneurysm with normal histology, excluding secondary pathological causes of the aneurysm.
Discussion
A splenic artery aneurysm is an abnormal dilatation of the splenic artery layers greater than 1 cm in diameter (2). It was first described by Beaussier in 1770 but was never operated before 1920, 150 years since its discovery (1). In a large autopsy series that studied the incidence of SAA, it was found to range between only 0.01% and 0.2%, dramatically increasing to 10% in patients older than 60 years and in those with portal hypertension (5).
Symptomatic SAAs only occurred in 20% of cases, which presented with epigastric and left upper quadrant abdominal pain. Other nonspecific complaints included anorexia, nausea, or vomiting. The final diagnosis is nearly always obtained by abdominal imaging (6). Unfortunately, the catastrophic presentation is a spontaneous rupture of the SAA, which only occurs in 2%–10% of patients presenting complaints. The widespread availability of radiological services has reduced the rate of presenting with an acute aneurysmal rupture from 10% to approximately 3% of patients who present with sudden onset acute epigastric and right upper quadrant pain with radiation to shoulders associated with hemodynamic compromise (2). Our patient, who was unusually a male with no previous known risk factors, presented with a spontaneous nontriggered rupture of a 2-cm distal splenic artery aneurysm, fulfilling all the rare entities of this life-threatening event and surviving with no consequences.
Another rare presentation of SAA rupture is the double rupture phenomenon of the SAA that was described for the first time in 1930 also by Bockerman, in which aneurysms initially rupture into the lesser omental sac causing transient tamponade, during which patients are still stable, with mild or no symptoms. Six to ninety hours later, blood overflows into the peritoneum through the foramen of Winslow, leading to sudden hypovolemic shock and vascular collapse (7).
Another very uncommon presentation of SAA rupture causes secondary erosion of the SAA to an adjacent organ, leading to gastrointestinal hemorrhage (8).
Erosion of the aneurysm into the splenic vein also leads to arteriovenous fistula and portal hypertension and rarely results in mesenteric steal syndrome and small bowel ischemia (2).
Ultrasonography, pulsed Doppler, contrast-enhanced computed tomography scanning, magnetic resonance imaging, magnetic resonance angiography, and abdominal aortic arteriography are all radiographic modalities capable of diagnosing an asymptomatic aneurysm. Contrast-enhanced computed tomography scanning is the gold standard in detecting aneurysms and intra-abdominal bleeding. Until now, abdominal aortic angiography, however, is the most valuable modality of choice in localizing the bleeding source and assessing collateral vessels (9).
Regarding management, regardless of their dimensions, all symptomatic SAAs are believed to require treatment; patients with aneurysmal dimensions of 2 cm, pregnant or fertile patients, patients with portal hypertension, or candidates for liver transplantation patients also require management even if asymptomatic (10).
Excision, ligation, or revascularization are surgical alternatives, with or without splenectomy. Either an open or minimally invasive approach could be used. Those who are not candidates for surgery and those in elective settings are typically given the endovascular option, which includes various techniques such as coil embolization, covered stents placement, gluing, plug deployment, and endoluminal thrombin, polyvinyl alcohol, particles, or gel foam injection. However, surgical intervention remains the gold standard treatment in most centers, especially in cases of rupture (1, 10).
Conclusion
This study has described a case of a male patient who presented with acute abdomen, signs of shock, and evident free fluid on imaging that was later found to have a ruptured SAA. The rare etiology and rarer presentation in an unusual gender and age group, highlight the importance of having a very high level of awareness about SAAs as a potential cause of bleeding and a low threshold for more advanced imaging modalities for diagnosing conditions with very high mortality and an even lower threshold for intervention in which mortality rates dramatically increase with every minute of delay.
Patient's perspective
I am grateful for the opportunity to share my medical journey through this case report. It is my hope that by sharing my experience, others may gain valuable insights and knowledge that can contribute to the understanding and treatment of similar conditions. I want to express my sincere appreciation to the healthcare professionals involved in my care, whose expertise and compassion made a significant impact on my well-being. Their dedication and commitment to providing quality healthcare have been truly remarkable. Lastly, I extend my gratitude to the authors and researchers involved in the preparation of this case report for their dedication to advancing medical knowledge. Together, we can contribute to improving patient care and outcomes for future individuals facing similar medical challenges.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the patient himself for the publication of any potentially identifiable images or data included in this article.
Author contributions
YN and AB contributed to the literature review and wrote the first draft of the manuscript. MM contributed to the manuscript revision and read and approved the submitted version. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors express their sincere gratitude and appreciation to all individuals who contributed to the successful completion of this case report. First, the authors thank the patient who generously allowed them to share their medical information for educational purposes. The authors are also immensely grateful to the healthcare professionals involved in the care of the patient, whose dedication and expertise were invaluable in the diagnosis, treatment, and management of the case. The authors extend their heartfelt appreciation to the medical staff, nurses, and technicians who provided support during the patient's hospitalization. Finally, the authors thank the editorial team and reviewers for their meticulous review and insightful suggestions, which will significantly enhance the quality of this case report. The authors are truly grateful to everyone involved in this endeavor, as their contributions have been indispensable in completing this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abdulrahman A, Shabkah A, Hassanain M, Aljiffry M. Ruptured spontaneous splenic artery aneurysm: a case report and review of the literature. Int J Surg Case Rep. (2014) 5(10):754–7. doi: 10.1016/j.ijscr.2014.08.021
2. Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery – a review. Surgeon. (2010) 8(4):223–31. doi: 10.1016/j.surge.2009.11.011
3. Martin D, Teixeira Farinha H, Dattner N, Rotman S, Demartines N, Sauvain MO. Spontaneous non-traumatic splenic artery aneurysm rupture: a case report and review of the literature. Eur Rev Med Pharmacol Sci. (2018) 22(10):3147–50. doi: 10.26355/eurrev_201805_15074
4. Aydın MT, Fersahoğlu MM, Tezer S, Okuducu M, Ağca B, Memişoğlu K. Spontaneous rupture of the splenic artery aneurysm: a rare clinical presentation of acute abdomen. Ulus Travma Acil Cerrahi Derg. (2016) 22(1):106–8. doi: 10.5505/tjtes.2015.32654
5. Reardon PR, Otah E, Craig ES, Matthews BD, Reardon MJ. Laparoscopic resection of splenic artery aneurysms. Surg Endosc. (2005) 19(4):488–93. doi: 10.1007/s00464-004-8916-8
6. Matsumoto K, Ohgami M, Shirasugi N, Nohga K, Kitajima M. A first case report of the successful laparoscopic repair of a splenic artery aneurysm. Surgery. (1997) 121:462–4. doi: 10.1016/S0039-6060(97)90318-4
7. Kim JH, Chung HS, Kim JH, Park SY, Lee SB, Do BS. Splenic artery aneurysm with the double-rupture phenomenon. Clin Exp Emerg Med. (2017) 4(2):113–6. doi: 10.15441/ceem.16.193
8. Yoshikawa C, Yamato I, Nakata Y, Nakagawa T, Inoue T, Nakatani M, et al. Giant splenic artery aneurysm rupture into the stomach that was successfully managed with emergency distal pancreatectomy. Surg Case Rep. (2022) 8:148. doi: 10.1186/s40792-022-01498-3
9. Davis T, Minardi J, Knight J, Larrabee H, Schaefer G. Ruptured splenic artery aneurysm: rare cause of shock diagnosed with bedside ultrasound. West J Emerg Med. (2015) 16(5):762–5. doi: 10.5811/westjem.2015.7.25934
Keywords: splenic artery, aneurysm, spontaneous rupture, hemoperitoneum, acute abdomen, splenectomy
Citation: Njoum Y, Barqawi AD and Maree M (2023) Spontaneous rupture of a splenic artery aneurysm causing acute abdomen in a 19-year-old male patient: a case report. Front. Surg. 10:1223271. doi: 10.3389/fsurg.2023.1223271
Received: 15 May 2023; Accepted: 31 August 2023;
Published: 18 September 2023.
Edited by:
Apostolos Tassiopoulos, Stony Brook University, United StatesReviewed by:
Sinan Hatipoglu, Adiyaman University, TürkiyeAlex Giakoustidis, Aristotle University of Thessaloniki, Greece
© 2023 Njoum, Barqawi and Maree. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumna Njoum eXVtbmFhYTIxMTJAZ21haWwuY29t
 Yumna Njoum
Yumna Njoum Abdallah Deghles Barqawi2
Abdallah Deghles Barqawi2 Mohammed Maree
Mohammed Maree