- 1Fourth Orthopedic Department, Ganzhou Hospital of Traditional Chinese Medicine, Ganzhou, China
- 2The First Clinical Medical School, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Academic Affairs Office, Gannan Medical University, Ganzhou, China
- 4Department of Orthopaedics, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Background: Currently, there is no “gold standard” for early diagnosing PJI. The diagnosis of periprosthetic joint infection (PJI) is a challenging problem in the clinic. As we know, many serum markers have been used in the early diagnosis of PJI. The aim of this study was to validate the value of PCT in the diagnosis of PJI.
Methods: A retrospective review of 77 patients with revision arthroplasties from January 2013 to July 2020 was conducted. PJI was defined using the modified Musculoskeletal Infection Society (MSIS) criteria combined with follow-up results. Besides medical history, clinical and laboratory data was gathered. Preoperative blood was taken for serum PCT and other biomarkers measurement. Receiver operating characteristic (ROC) curves were generated to evaluate the biomarkers’ diagnostic performance and optimal cut-off value.
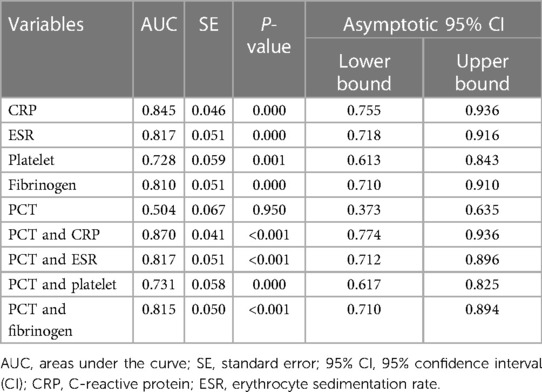
Results: Forty-one patients were identified as the PJI group (27 hips and 14 knees), while thirty-six patients were identified as the aseptic loosening (AL) group (33 hips and 3 knees). The AUCs for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), Platelets (PLT), Fibrinogen (FIB), and Procalcitonin (PCT) were 0.845 (95% CI 0.755–0.936, p < 0.001), 0.817 (95% CI 0.718–0.916, p < 0.001), 0.728 (95% CI 0.613–0.843, p < 0.001), 0.810 (95% CI 0.710–0.910, p < 0.001) and 0.504 (95% CI 0.373–0.635, p = 0.950), respectively. Higher Area under the Curve (AUC) values were obtained for the combinations of PCT and CRP (AUC = 0.870) (95% CI, 0.774–0.936), PCT and ESR (AUC = 0.817) (95% CI, 0.712–0.896), PCT and PLT (AUC = 0.731) (95% CI, 0.617–0.825), PCT and FIB (AUC = 0.815) (95% CI, 0.710–0.894). The serum PCT indicated a sensitivity of 19.51% and a specificity of 83.33% for diagnosing PJI. When the optimal cut-off value for PCT was set as 0.05 ng/ml, its positive and negative likelihood ratios were 57.1% and 47.6%, respectively.
Conclusion: In conclusion, serum PCT appeared to be no reliable biomarker in differentiating PJI from aseptic loosening before revision arthroplasties. However, PCT combined with other biomarkers further increases the diagnostic accuracy.
Introduction
Total joint replacement (TJA) is the most effective treatment for advanced arthritis, but periprosthetic joint infection (PJI) is a serious complication and a major reason for postoperative revision (1, 2). The incidence of PJI after arthroplasty was 0.7% (3). A study has shown that each patient suffered from PJI would pay at least $15,000–$30,000 (4). Therefore, early diagnosis of PJI and effective intervention are very to improve the prognosis after total joint replacement. A large number of clinical studies have shown that early diagnosis can not only preserve the prosthesis to a certain extent, but also the infection control rate can reach 70% (5). Currently, there is no “gold standard” for early diagnosing PJI. The diagnosis of periprosthetic joint infection (PJI) is a challenging problem in the clinic. This leads to the delayed diagnosis and treatment of PJI. As a result, this catastrophic complication can seriously diminish patient quality of life and increase the financial burden on families and societies (2).
Serum samples are readily available and are especially important for patients without Synovial fluid samples. As we know, many serum markers have been used to diagnose PJI early. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are recommended as essential indicators for the diagnosis of PJI by the American Academy of Orthopaedic Surgeons (AAOS) and the Musculoskeletal Infection Society (MSIS) guidelines due to their superior sensitivity and specificity (6, 7). Zhang et al. first reported that serum platelet (PLT) is a promising marker for the diagnosis of deep surgical site infection after open induction internal fixation for traumatic limb fractures (8). Klim, S.M. et al. showed that Fibrinogen (FIB) is a cost-efficient and practical marker for diagnosing PJI (9, 10). Procalcitonin (PCT) is commonly used for the diagnosis of systemic infection (11–13). However, its diagnostic value for PJI is controversial, and there is no universal threshold value (14–16). To further clarify its diagnostic value, this study evaluated the value of PCT in the diagnosis of PJI by comparing it with CRP, ESR, PLT, and FIB.
In this retrospective study, we sought to: (1) the performance of PCT in distinguishing chronic PJI and AL (Aseptic Loosening) by comparing with other inflammation indicators; (2) the value of PCT combined with CRP, ESR, PLT, or FIB for diagnosing PJI.
Methods
Study design
After approval of our hospital's institutional review board, a single-center retrospective cohort study protocol was performed in compliance with the Helsinki Declaration. We recruited patients after revision hip or knee arthroplasties from January 2013 to July 2020 in our institution to determine the diagnostic value of PCT for diagnosing PJI.
Inclusion and exclusion criteria
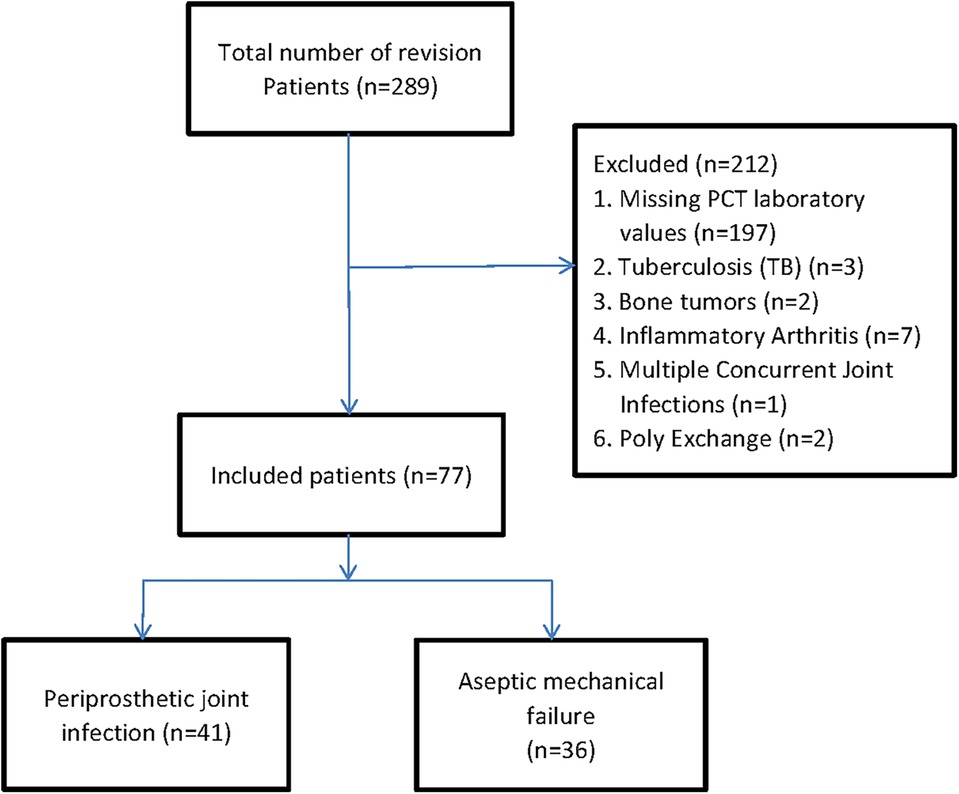
We identified patients with revision hip or knee arthroplasties using the International Classification of Diseases, Tenth Revision, and Clinical Modification procedure codes (17). A total of 289 patients with revision arthroplasties were originally included into our retrospective cohort study. The primary causes of joint revision and clinical symptoms before surgery are pain, systemic or local joint fever, joint swelling or sinus formation, etc. Firstly, patients without serum PCT at revision arthroplasties were excluded. In order to diminish the possibility of bias associated with comorbidities, we excluded 12 patients with the history of tuberculosis (TB) (n = 3), bone tumors (n = 2), and inflammatory arthritis (n = 7). And those patients who had undergone multiple concurrent joint Infections (n = 1), Poly exchange surgery (n = 2) also were excluded due to its intricate source of pathogens and undetermined duration of infection (18). Finally, 77 patients were included in the analysis, which was divided into two groups: 41 patients in the periprosthetic joint infection group (PJI group) and 36 patients in the aseptic loosening group (AL group) (Figure 1). Patient's age, gender, and other baseline data were compared between the two groups (Table 1).
Diagnostic criteria of infection and data extraction
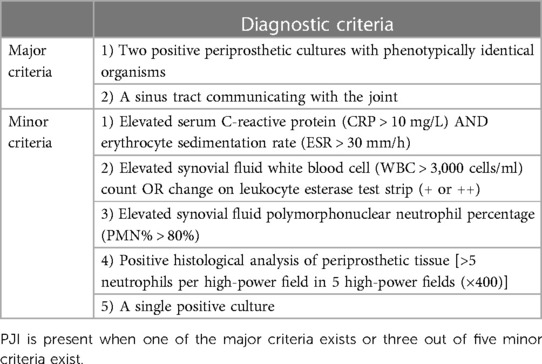
The final diagnosis of PJI was based on MSIS criteria (Table 2) (6, 7). Using patients' electronic medical records, we carefully extracted the following baseline data: demographic information, diagnoses, treatments, the involved joint, symptoms and signs, time from primary arthroplasty to the first reoperation (years), time from symptom onset to the first reoperation (months), laboratory results, culture results, comorbidities, and medication use.
Laboratory evaluations
Patients' cubital fasting venous blood samples were obtained by nurses the day before revision surgery, routinely. The samples were immediately tested by our hospital's laboratory within 1–2 h for PLT and FIB levels. Nurses also took blood samples for serum PCT, ESR, and CRP evaluation at the same time.
In our hospital, at least 3 tissue culture specimens were collected and cultured for 3–7 days. More than one periprosthetic tissues were selected and sent for biopsy and immediate histological analysis by the chief surgeon during the revision surgeries. After that, Vancomycin or a sensitive antibiotic was used to prevent or treat the infection for 2 weeks after the operation. In addition, rivaroxaban was used to prevent deep vein thrombosis in lower limbs. The follow-up time was at least 1 year.
Statistical analyses
We analyzed clinical and laboratory values by using basic descriptive statistics. As far as quantitative data is concerned, there were two situations. We used the Independent-samples test to compare continuous variables between the PJI and AL groups for normally distributed continuous data which were shown as mean ± standard deviation (SD). On the other hand, we conducted the Mann–Whitney U-test to compare continuous variables between groups for non-normally distributed continuous data which were shown as quartiles. In terms of qualitative data, frequencies, and constituent ratios were evaluated to perform the Pearson chi-square test or Continuity correction chi-square test between the two groups. P < 0.05 was considered as statistical significance.
The receiver operating characteristic curves (ROC) were plotted to evaluate the diagnostic performance of each serological marker. The areas under the curve (AUC) and 95% confidence intervals (CI) were calculated via ROC analysis. The AUC values were shown as excellent (0.900–1.000), good (0.800–0.899), fair (0.700–0.799), poor (0.600–0.699), or noneffective (0.500–0.599) (19). Youden's index was used to make sure of the optimal predictive cut-offs for each marker. All data were conducted by SPSS software version 23 and MedCalc Software version 15.0.
Results
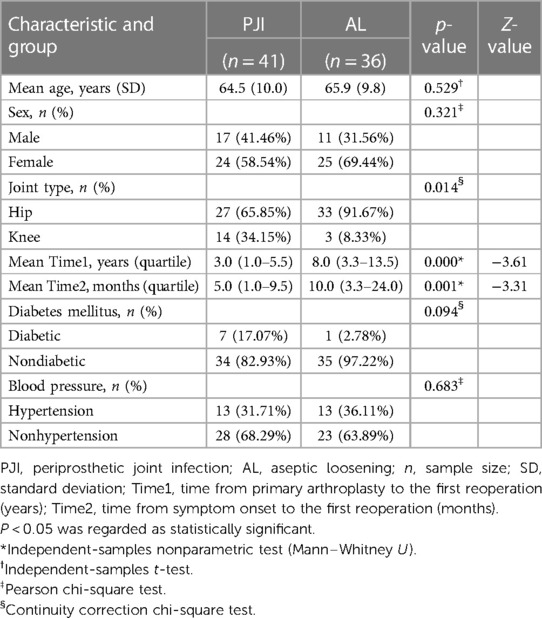
A total of 77 patients were included for the final analysis. According to the MSIS criteria, 41 patients were identified as the periprosthetic joint infection (PJI) group (27 hips and 14 knees). In comparison, 36 patients were identified as the aseptic loosening (AL) group (33 hips and 3 knees). The mean age in the PJI group was 64.5 ± 10.0 years; of them, 17 were men and 24 were women. The mean age in the AL group was 65.9 ± 9.8 years; of them, 11 were men, and 25 were women. The two cohorts did not differ statistically in age (p = 0.529) and gender (p = 0.321). At the same time, there were no statistically significant differences between groups with diabetes mellitus (p = 0.094) or hypertension (p = 0.683). However, a statistical significance was shown in joint type (p = 0.014), time from primary arthroplasty to the first reoperation (p = 0.001), and time from symptom onset to the first reoperation (p = 0.001) between the two groups. The characteristics of the recruited patients were depicted in Table 1.
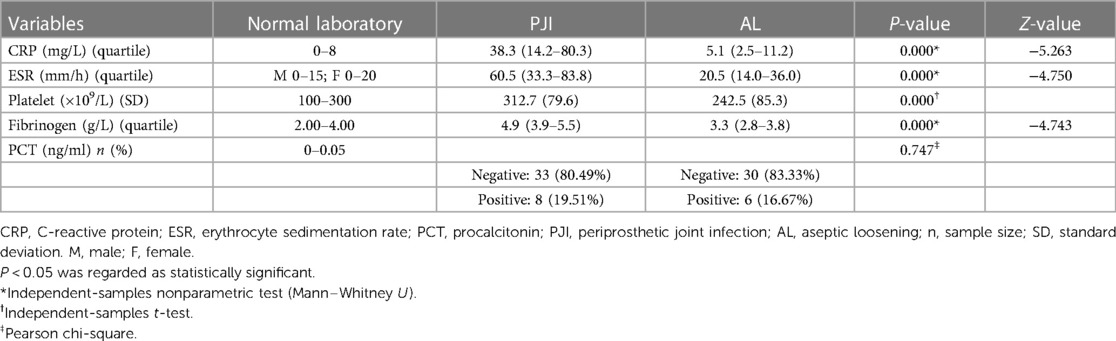
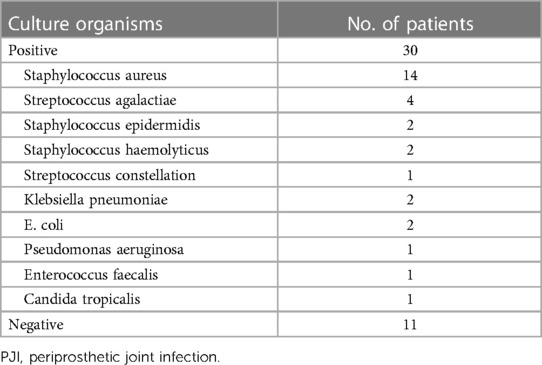
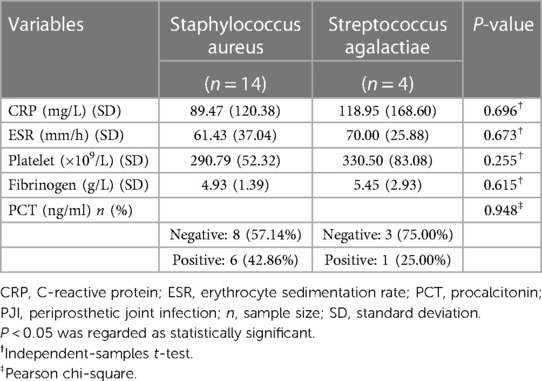
We evaluated the tested markers (CRP, ESR, PLT, FIB, and PCT) for all included patients. All patients in the PJI group had significantly higher values for the four markers (CRP, ESR, PLT, and FIB) compared with the AL group (all P < 0.05). Unfortunately, there was no significant difference for PCT between the two groups (P = 0.747). The details were shown in Table 3. We also listed the normal ranges for these tested markers in Table 3. As shown in Table 4 for culture organisms in the PJI group, Staphylococcus aureus and Streptococcus agalactiae were the two most common pathogens. We classified these pathogens into two groups (Table 5). There were no significant differences in all tested markers (CRP, ESR, PLT, FIB, and PCT) between the two groups (P > 0.05). That is to say, the results of the tested markers had nothing to do with the species of pathogens.
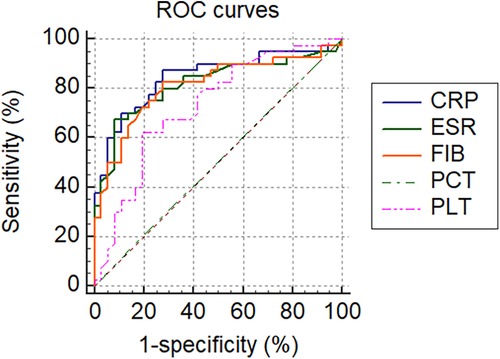
All tested markers (CRP, ESR, PLT, FIB, and PCT) were evaluated and plotted in the ROC curves (Figure 2). The AUCs for CRP, ESR, PLT, FIB, and PCT were 0.845 (95% CI 0.755–0.936, p < 0.001), 0.817 (95% CI 0.718–0.916, p < 0.001), 0.728 (95% CI 0.613–0.843, p < 0.001), 0.810 (95% CI 0.710–0.910, p < 0.001) and 0.504 (95% CI 0.373–0.635, p = 0.950), respectively (Table 6). The ROC curves showed that CRP had the highest AUC, followed by ESR, FIB, PLT, and PCT. The AUCs of CRP, ESR, and FIB ranged from 0.800–0.899, which demonstrates a good diagnostic value for PJI. The AUC of FIB was between 0.7 and 0.8, indicating a fair diagnostic value for PJI. In contrast, PCT had an AUC of 0.504 (lower than 0.6), the lowest value, indicating an inferior diagnostic value for PJI. Further analyses of the diagnostic value of PCT combined with other markers for PJI were conducted in order to improve their diagnostic accuracies. Higher AUC values indicating better diagnostic accuracy were obtained for the combinations of PCT and CRP (AUC = 0.870) (95% CI, 0.774–0.936), PCT and ESR (AUC = 0.817) (95% CI, 0.712–0.896), PCT and PLT (AUC = 0.731) (95% CI, 0.617–0.825), PCT and FIB (AUC = 0.815) (95% CI, 0.710–0.894). Among them, the combined analysis of PCT with CRP had the highest AUC. In conclusion, combining serum PCT with one of the other markers can improve their diagnostic accuracies (Table 6).
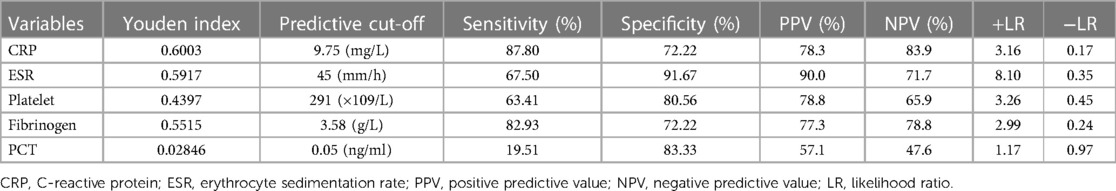
The serum PCT indicated a sensitivity of 19.51% and a specificity of 83.33% for diagnosing PJI. When the optimal cut-off value for PCT was set as 0.05 ng/ml, its positive likelihood ratio (PPV) and negative likelihood ratio (NPV) were 57.1% and 47.6%, respectively. Based on the ROC analysis, when CRP was above 9.75 mg/L, the sensitivity, specificity, PPV, and NPV were 87.80%, 72.22%, 78.3%, and 83.9%, respectively. Using a cut-off value for ESR of 45 mm/h, the sensitivity, specificity, PPV, and NPV were 67.50%, 91.67%, 90.0%, and 71.7%. Using a cut-off value for PLT at 291 × 109/L, the sensitivity, specificity, PPV, and NPV were 63.41%, 80.56%, 78.8%, and 65.9%, respectively. Using Youden's index, the optimal cut-off value was 3.58 g/L, resulting in sensitivity, specificity, PPV, and NPV of 82.93%, 72.22%, 77.3%, and 78.1%, respectively (Table 7). From the above results, the value of PCT for diagnosing PJI was inferior to other markers. As shown in Table 7, PCT with the lowest Youden's index (0.02846) has the lowest PLR (1.17) but the highest NLR (0.97). Therefore, the same conclusion can be drawn from Youden's index, positive likelihood ratio (PLR), and negative likelihood ratio (NLR).
Discussion
PJI is a catastrophic complication after total joint arthroplasty (20). Currently, there is an international consensus for the diagnosis of PJI, but no “gold standard” (21). The differentiation between PJI and aseptic loosening (AL) is challenging in orthopedic surgery because the treatment of PJI is entirely different to the treatment of aseptic loosening (22). ESR and CRP are initial markers recommended by the current guidelines due to the low false-negative and high sensitivity rates (23–25). CRP is a protein made by the liver. When there is acute inflammation, it responds to increased macrophages (26). Erythrocyte sedimentation rate (ESR) refers to the rate at which red blood cells sink under certain conditions. Although CRP and ESR have shown abilities for diagnosing PJI after primary replacement, the efficacy is limited (27, 28). Research by Paziuk T et al. showed that initial platelet (PLT) counts could be used for distinguishing between PJI and AL (29). Related studies reported that fibrinogen (FIB) with high sensitivity and specificity might become a novel biomarker for diagnosing PJI (19, 30).
PCT, which is produced by Thyroid C cells, consists of 116 amino acids. During infection, serum PCT levels rise with the bacterial endotoxin (31). Therefore, it is helpful to diagnose systemic infection (32). PCT is an undefined biomarker for diagnosing local infections such as PJI (33). The reports about PCT as a marker for the diagnosis of PJI are controversial. Sa-Ngasoongsong et al. showed that PCT was a very specific marker with low sensitivity for diagnosing PJI (34). In contrast, Glehr et al. demonstrated PCT is a sensitive, but not specific marker for diagnosis of PJI (35). Yoon et al. come to a conclusion that PCT is not a promising maker for diagnosing PJI (36). After reviewing reports for the diagnosis of PJI, we found Several studies have illustrated the role of PCT in diagnosing patients with PJI. These studies showed PCT is a sensitive and specific marker of bacterial infection (37–39). We want to investigate whether PCT is a superior maker. Therefore, we performed a sensitivity analysis to further validate the diagnostic performance of PCT. Finally, we found different results which showed the limited efficacy of PCT for diagnosing PJI before revision arthroplasties.
There was no significant difference for PCT between PJI group and the AL group (P = 0.747). The AUC for PCT was 0.504 (95% CI 0.373–0.635, p = 0.950). The serum PCT indicated a sensitivity of 19.51% and a specificity of 83.33% for diagnosing PJI. When the optimal cut-off value for PCT was set as 0.05 ng/ml, its PPV and NPV were 57.1% and 47.6%, respectively. The results in our study showed that PCT is a specific, but less sensitive biomarker for diagnosing PJI. The AUCs of other biomarkers increased significantly as they were combined with PCT. In conclusion, the combination of serum PCT with one of the other markers can improve their diagnostic accuracies.
Different reasons may result in the limited efficacy of PCT for diagnosing PJI. Firstly, PCT is not necessarily released into the blood if patients suffering from PJI do not show bacteremia (40). It is conceivable that the grade and virulence of the majority of PJI is too low to trigger PCT release. It was pointed out that the high rate of false negatives is associated with local low-virulence organisms (41). Secondly, in healthy adults, even after tooth brushing, transient bacteremia may lead to low-grade PCT release (42–44). Thirdly, since the penetration of PCT into the blood is different in each patient, the cut-off values set for the PCT may affect the study results. The cut-off value (0.05 ng/ml) may not be optimal in our cohort. In addition, we performed only the measurement of serum PCT, while we did not conduct the measurement of synovial fluid PCT.
Some limitations should be considered in our study. Firstly, the modified MSIS criteria used in this study may produce bias for assessing the diagnostic accuracy. Secondly, this was a retrospective cohort study, so its own inherent selection bias may affect its results. Thirdly, only 77 patients were recruited for the study. Thus, a multi-center study with a larger sample size is needed to be carried out for further analyses. This also diminished the sample size. Finally, our study has shown only serum PCT biomarkers, not including synovial fluid PCT biomarkers.
Conclusions
We detected multiple biomarkers for their diagnostic performance. In conclusion, this study demonstrates that the value of serum PCT has limited efficacy in differentiating PJI from aseptic loosening before revision arthroplasties. However, PCT combined with other biomarkers further increases diagnostic accuracy. Further multiple-center studies with large-size samples are needed to improve its diagnostic rate and validate our results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The First Clinical Medical School, Guangzhou University of Chinese. Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
Conceptualization: XS and YZ; Literature review and search: XS, HZ, and YL; Data collection: HZ and XS; Data analysis and interpretation: ZL and YL; Manuscript preparation & editing: XS, YL, and HZ; Revision and Validation: XS, HZ, and YZ. All authors contributed to the article and approved the submitted version.
Acknowledgments
One of the first authors (XS) wants to show heartedly thanks to his wife YL, who provided much help for grammar expression.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PJI, periprosthetic joint infection; CRP, C-reactive protein; PCT, procalcitonin; SD, standard deviation; CI, confidence interval; AAOS, The American Academy of Orthopedic Surgeon; MSIS, Musculoskeletal Infection Society; LR, likelihood ratio; ROC, receiver operating characteristic; AUC, area under the curve.
References
1. Leitner L, Posch F, Amerstorfer F, Sadoghi P, Leithner A, Glehr M. The dark side of arthroplasty: competing risk analysis of failed hip and knee arthroplasty with periprosthetic joint infection. J Arthroplasty. (2020) 35(9):2601–6.e1. doi: 10.1016/j.arth.2020.04.078
2. Morgenstern C, Cabric S, Perka C, Trampuz A, Renz N. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. (2018) 90(2):115–9. doi: 10.1016/j.diagmicrobio.2017.10.016
3. Lum ZC, Natsuhara KM, Shelton TJ, Giordani M, Pereira GC, Meehan JP. Mortality during total knee periprosthetic joint infection. J Arthroplasty. (2018) 33(12):3783–8. doi: 10.1016/j.arth.2018.08.021
4. Lima AL, Oliveira PR, Carvalho VC, Saconi ES, Cabrita HB, Rodrigues MB. Periprosthetic joint infections. Interdiscip Perspect Infect Dis. (2013) 2013:542796. doi: 10.1155/2013/542796
5. Hansen E, Tetreault M, Zmistowski B, Della Valle CJ, Parvizi J, Haddad FS, et al. Outcome of one-stage cementless exchange for acute postoperative periprosthetic hip infection. Clin Orthop Relat Res. (2013) 471(10):3214–22. doi: 10.1007/s11999-013-3079-3
6. Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. (2010) 18(12):760–70. doi: 10.5435/00124635-201012000-00006
7. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. (2011) 12(1):1–10. doi: 10.1186/1471-2474-12-222
8. Zhang W, Guo Y, Kuss M, Shi W, Aldrich AL, Untrauer J, et al. Platelet-rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Eng Part B Rev. (2019) 25(3):225–36. doi: 10.1089/ten.TEB.2018.0309
9. Klim SM, Amerstorfer F, Gruber G, Bernhardt GA, Radl R, Leitner L, et al. Fibrinogen—a practical and cost efficient biomarker for detecting periprosthetic joint infection. Sci Rep. (2018) 8(1):8802. doi: 10.1038/s41598-018-27198-3
10. Song Z, Huang J, Wang Q, Wang D, Feng J, Cao Q, et al. An exciting performance of established and novel biomarkers in diagnosing periprosthetic joint infections: a single-center retrospective cohort study. Orthop Surg. (2023) 15(9):2328–33. doi: 10.1111/os.13810
11. Lin KH, Wang FL, Wu MS, Jiang BY, Kao WL, Chao HY, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. (2014) 80(1):72–8. doi: 10.1016/j.diagmicrobio.2014.03.029
12. Spapen HD, Hachimi-Idrissi S, Corne L, Huyghens LP. Diagnostic markers of sepsis in the emergency department. Acta Clin Belg. (2006) 61(3):138–42. doi: 10.1179/acb.2006.022
13. Lee M, Snyder A. The role of procalcitonin in community-acquired pneumonia: a literature review. Adv Emerg Nurs J. (2012) 34(3):259–71. doi: 10.1097/TME.0b013e318261338d
14. Yuan K, Li WD, Qiang Y, Cui ZM. Comparison of procalcitonin and C-reactive protein for the diagnosis of periprosthetic joint infection before revision total hip arthroplasty. Surg Infect (Larchmt). (2015) 16(2):146–50. doi: 10.1089/sur.2014.034
15. Tarabichi S, Goh GS, Baker CM, Chisari E, Shahi A, Parvizi J. Plasma D-dimer is noninferior to Serum C-reactive protein in the diagnosis of periprosthetic joint infection. J Bone Joint Surg Am. (2023) 105(7):501–8. doi: 10.2106/JBJS.22.00784
16. Wu Y, Sun K, Liu R, Wu L, Zeng Y, Li M, et al. C-reactive protein/albumin and C-reactive protein/fibrinogen ratios for the diagnosis of periprosthetic joint infection in revision total joint arthroplasty. Int Immunopharmacol. (2023) 115:109682. doi: 10.1016/j.intimp.2023.109682
17. Harris ST, Zeng X, Ford L. International classification of diseases, 10th revision: it’s coming, ready or not. Health Care Manag (Frederick). (2011) 30(3):227–35. doi: 10.1097/HCM.0b013e318225e0a2
18. Chen MF, Chang CH, Chiang-Ni C, Hsieh PH, Shih HN, Ueng SWN, et al. Rapid analysis of bacterial composition in prosthetic joint infection by 16S rRNA metagenomic sequencing. Bone Joint Res. (2019) 8(8):367–77. doi: 10.1302/2046-3758.88.BJR-2019-0003.R2
19. Li R, Shao HY, Hao LB, Yu BZ, Qu PF, Zhou YX, et al. Plasma fibrinogen exhibits better performance than plasma D-dimer in the diagnosis of periprosthetic joint infection: a multicenter retrospective study. J Bone Joint Surg Am. (2019) 101(7):613–9. doi: 10.2106/JBJS.18.00624
20. Zhang H, Xie S, Li Y, Li J, Deng P, Zeng H, et al. The potential performance of serum albumin to globulin ratio, albumin and globulin in the diagnosis of periprosthetic joint infection and prediction of reinfection following reimplantation. BMC Musculoskelet Disord. (2022) 23(1):730. doi: 10.1186/s12891-022-05533-0
21. Patel R, Alijanipour P, Parvizi J. Advancements in diagnosing periprosthetic joint infections after total hip and knee arthroplasty. Open Orthop J. (2016) 10:654–61. doi: 10.2174/1874325001610010654
22. Ellenrieder M, Lenz R, Haenle M, Bader R, Mittelmeier W. Two-stage revision of implant-associated infections after total hip and knee arthroplasty. GMS Krankenhhyg Interdiszip. (2011) 6(1):Doc17. doi: 10.1016/j.arth.2023.05.025
23. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. (2018) 33(5):1309–14.e2. doi: 10.1016/j.arth.2018.02.078
24. Yu JS, Bornes TD, Youssef MP, Tam KW, Nocon AA, Sculco PK, et al. Which combination is the best? A comparison of the predictive potential of Serum biomarker combinations to diagnose periprosthetic joint infection. J Arthroplasty. (2023) 38(7 Suppl 2):S381–8. doi: 10.1016/j.arth.2023.05.025
25. Shi W, Jiang Y, Tian H, Wang Y, Zhang Y, Yu T, et al. C-reactive protein-to-albumin ratio (CAR) and C-reactive protein-to-lymphocyte ratio (CLR) are valuable inflammatory biomarker combination for the accurate prediction of periprosthetic joint infection. Infect Drug Resist. (2023) 16:477–86. doi: 10.2147/IDR.S398958
26. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. (2012) 470(1):54–60. doi: 10.1007/s11999-011-1991-y
27. Stambough JB, Curtin BM, Odum SM, Cross MB, Martin JR, Fehring TK. Does change in ESR and CRP guide the timing of two-stage arthroplasty reimplantation? Clin Orthop Relat Res. (2019) 477(2):364–71. doi: 10.1097/01.blo.0000533618.31937.45
28. Dupont C, Rodenbach J, Flachaire E. The value of C-reactive protein for postoperative monitoring of lower limb arthroplasty. Ann Readapt Med Phys. (2008) 51(5):348–57. doi: 10.1016/j.annrmp.2008.01.014
29. Paziuk T, Rondon AJ, Goswami K, Tan TL, Parvizi J. A novel adjunct indicator of periprosthetic joint infection: platelet count and mean platelet volume. J Arthroplasty. (2020) 35(3):836–9. doi: 10.1016/j.arth.2019.10.012
30. Wu H, Meng Z, Pan L, Liu H, Yang X, Yongping C. Plasma fibrinogen performs better than plasma d-dimer and fibrin degradation product in the diagnosis of periprosthetic joint infection and determination of reimplantation timing. J Arthroplasty. (2020) 35(8):2230–6. doi: 10.1016/j.arth.2020.03.055
31. Chen A, Fei J, Deirmegian C. Diagnosis of periprosthetic infection: novel developments. Orthop Clin North Am. (2016) 47(1):1–9. doi: 10.1016/j.ocl.2015.08.003
32. Wang H, Yin F, Shen D-X, Zhang Y-J, Luo Y-P, Liu C-J, et al. Predictive value of procalcitonin for excluding bloodstream infection: results of a retrospective study and utility of a rapid, quantitative test for procalcitonin. J Int Med Res. (2013) 41(5):1671–81. doi: 10.1177/0300060513497558
33. Bouaicha S, Blatter S, Moor BK, Spanaus K, Dora C, Werner CML. Early serum procalcitonin level after primary total hip replacement. Mediators Inflamm. (2013) 2013:927636. doi: 10.1155/2013/927636
34. Sa-Ngasoongsong P, Wongsak S, Jarungvittayakon C, Limsamutpetch K, Channoom T, Kawinwonggowit V. Comparison of synovial fluid and Serum procalcitonin for diagnosis of periprosthetic joint infection: a pilot study in 32 patients. BioMed Res Int. (2018) 2018:8351308. doi: 10.1155/2018/8351308
35. Glehr M, Friesenbichler J, Hofmann G, Bernhardt GA, Zacherl M, Avian A, et al. Novel biomarkers to detect infection in revision hip and knee arthroplasties. Clin Orthop Relat Res. (2013) 471(8):2621–8. doi: 10.1007/s11999-013-2998-3
36. Vicenti G, Bizzoca D, Nappi V, Pesce V, Solarino G, Carrozzo M, et al. Serum biomarkers in the diagnosis of periprosthetic joint infection: consolidated evidence and recent developments. Eur Rev Med Pharmacol Sci. (2019) 23(2 Suppl):43–50. doi: 10.26355/eurrev_201904_17473
37. Fuchs A, Gotta V, Decker ML, Szinnai G, Baumann P, Bonhoeffer J, et al. Cytokine kinetic profiles in children with acute lower respiratory tract infection: a post hoc descriptive analysis from a randomized control trial. Clin Microbiol Infect. (2018) 24(12):1341.e1–.e7. doi: 10.1016/j.cmi.2018.03.016
38. van der Does Y, Limper M, Jie KE, Schuit SCE, Jansen H, Pernot N, et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: a multicentre non-inferiority randomized clinical trial (HiTEMP study). Clin Microbiol Infect. (2018) 24(12):1282–9. [1469-0691 (Electronic)]. doi: 10.1016/j.cmi.2018.05.011
39. van der Does Y, Rood PPM, Ramakers C, Schuit SCE, Patka P, van Gorp ECM, et al. Identifying patients with bacterial infections using a combination of C-reactive protein, procalcitonin, TRAIL, and IP-10 in the emergency department: a prospective observational cohort study. Clin Microbiol Infect. (2018) 24(12):1297–304. doi: 10.1016/j.cmi.2018.09.007
40. Klement MR, Siddiqi A, Rock JM, Chen AF, Bolognesi MP, Seyler TM. Positive blood cultures in periprosthetic joint infection decrease rate of treatment success. J Arthroplasty. (2018) 33(1):200–4.e1. doi: 10.1016/j.arth.2017.08.034
41. Kheir MM, Tan TL, Shohat N, Foltz C, Parvizi J. Routine diagnostic tests for periprosthetic joint infection demonstrate a high false-negative rate and are influenced by the infecting organism. J Bone Joint Surg Am. (2018) 100(23):2057–65. doi: 10.2106/JBJS.17.01429
42. Lucas VS, Gafan G, Dewhurst S, Roberts GJ. Prevalence, intensity and nature of bacteraemia after toothbrushing. J Dent. (2008) 36(7):481–7. doi: 10.1016/j.jdent.2008.03.005
43. Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. (2008) 117(24):3118–25. doi: 10.1161/CIRCULATIONAHA.107.758524
Keywords: periprosthetic joint infection, total joint arthroplasty, serum procalcitonin, complication, diagnostic performance
Citation: Sun X, Zhang H, Liu Y, Lai Z and Zeng Y (2023) Serum procalcitonin has no significance in the diagnosis of periprosthesis joint infection before total hip and knee replacement. Front. Surg. 10:1216103. doi: 10.3389/fsurg.2023.1216103
Received: 3 May 2023; Accepted: 16 October 2023;
Published: 6 November 2023.
Edited by:
Tristan Ferry, Hospices Civils de Lyon, FranceReviewed by:
Albert Sotto, University of Nîmes, FranceVincent Dubee, Centre Hospitalier Universitaire d'Angers, France
© 2023 Sun, Zhang, Liu, Lai and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobo Sun Z3pzdW54aWFvYm9AMTYzLmNvbQ== Yirong Zeng emVuZzE5NjZ6ZW5nQDEyNi5jb20=
†These authors have contributed equally to this work
 Xiaobo Sun
Xiaobo Sun Haitao Zhang
Haitao Zhang Yuting Liu3,†
Yuting Liu3,† Yirong Zeng
Yirong Zeng