
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg. , 20 June 2023
Sec. Visceral Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1206828
This article is part of the Research Topic Hepato-Pancreato-Biliary Surgery: Innovations, Genomics, and Prognostic Management View all 6 articles
Peptic ulcer disease (PUD) is a very common condition, with an annual incidence ranging from 0.1% to 0.3% and a lifetime prevalence ranging from 5% to 10%. If not treated, it can lead to severe complications such as gastro-intestinal bleeding, perforation, or entero-biliary fistula. Entero-biliary fistulas and especially choledocho-duodenal fistula (CDF) are a rare, but relevant and important diagnosis, which can lead to several complications such as gastric outlet obstruction, bleeding, perforation, or recurrent cholangitis. In this article, we present the case of an 85-year-old woman with PUD complicated with gastro-intestinal bleeding and a CDF. We also performed a review of the literature to search for pre-existing cases with this atypical clinical presentation. The aim was to raise awareness among surgeons and clinicians by offering a summary of different types of entero-biliary and especially CDF, existing diagnostic investigations, and management.
Peptic ulcer disease (PUD) is a very common condition, with an annual incidence ranging from 0.1% to 0.3% and a lifetime prevalence ranging from 5% to 10% (1, 2). It is defined as a disruption of the mucosa which extends to the muscularis mucosa, and is usually caused by an Helicobacter pylori infection or the use of non-steroidal anti-inflammatory drugs (NSAID) (1, 2). Other reported risks factors are the use of corticosteroids, the presence of a tumour such as a gastric tumour or a lymphoma, stress, or a Zollinger-Ellison syndrome, caused by an increased acid secretion due to hypergastrinemia (1, 2). PUD may be located in the stomach or, more commonly, in the duodenum (1). Symptoms depend on the location of the ulcer, but patients usually report epigastric pain, fullness, nausea, loss of appetite, weight loss, hematemesis or melena (1, 3). If not treated, PUD can lead to severe complications such as gastro-intestinal bleeding causing haemorrhagic shock, perforation, cancer or entero-biliary fistula, all mostly requiring endoscopic, radiological or surgical treatment (1, 2).
Entero-biliary fistula is an abnormal communication between the biliary tract and the gastro-intestinal tract, and is due to gallstones in 90% of the cases (4, 5). It is a rare condition which can also result from PUD, tumours or Crohn disease involving the duodenum (4, 6). Different types of fistulas can be encountered, the most common being the cholecysto-duodenal fistula (68%–90%) (4, 7–9). There is also the cholecysto-colonic fistula (10%–13.6%), the choledocho-duodenal fistula (8.6%), the cholecysto-gastric fistula and the duodeno-left hepatic fistula, the last two being the rarest (4, 7–9). The diagnosis of entero-biliary fistula is challenging and often made incidentally during endoscopy or radiological imaging, especially because patients usually remain asymptomatic or report non-specific symptoms such as abdominal pain, nausea, fever, bowel obstruction or jaundice (4, 8).
In this article, we present the case of an 85-year-old woman admitted to the emergency department in haemorrhagic shock due to upper gastro-intestinal bleeding. PUD was diagnosed at endoscopy and a choledocho-duodenal fistula (CDF) was incidentally discovered during the examination. We performed a review of the literature to search for pre-existing cases with this atypical clinical presentation and compare the management of CDF.
We searched through MEDLINE and manual search for all existing articles reporting cases of CDF. The following search strategy was used: ((“Hematemesis”[MeSH Terms] OR “gastrointestinal bleeding”[Title/Abstract]) AND (“Biliary Fistula”[MeSH Terms]) OR “choledochoduodenal fistula”[Title/Abstract] OR “enterobiliary fistula”[Title/Abstract]) AND (“Case Reports”[Publication Type]). Only articles written in English or French about patients with a gastro-intestinal bleeding and a proven CDF were included. Duplicates, as well as articles reporting entero-biliary fistulas other than CDF, protocols, abstracts, meta-analysis, systematic reviews, retrospectives and prospective studies were excluded.
Two reviewers (IU, AL) independently selected the articles for inclusion and extracted the data. Discrepancies were solved by a third author (EL). The following data were extracted: country of the first author, date of publication, total number of patients, age and gender of the patient, symptomatology, method of diagnosis of the fistula and treatment.
An 85-year-old woman, known with hypertension and without any history of abdominal surgery, gallstones or allergies, was referred to the emergency department after having an episode of syncope associated with hematemesis and haematochezia. At admission, she showed signs of shock with diaphoresis, low blood pressure (57/42 mmHg), pulse rate 83 of beat/min and saturation of 100% under 2 L of oxygen. The medical history was limited due to acute confusion, but she reported epigastric pain for a week. She also mentioned a regular intake of NSAID for back pain. Physical examination revealed diffuse abdominal tenderness. Blood tests showed a metabolic acidosis (pH value of 7.27 and high lactate level of 5) and anaemia (haemoglobin of 77 g/l). After receiving 2 units of red blood cells, 1 litre of crystalloid solution and after initiation of amine support (Noradrenalin perfusion of 4.8 ml/h), her condition was stabilized, and a CT-scan of the abdomen was carried out. This revealed a CDF, inflammation of the first part of duodenum, signs of cholangitis and the presence of blood in the stomach (Figure 1). No gallstones were identified. A naso-gastric tube was placed, which removed 1.5 litre of blood from the stomach. An urgent esophagogastroduodenoscopy was performed, which showed a Forrest 1A posterior duodenal ulcer with an active bleeding of the gastro-duodenal artery, as well as the presence of air and bile, which was controlled with an Over The Scope Clip (Figures 2, 3). During the endoscopy, the patient developed low blood pressure which was treated with the administration of 1 litre of crystalloid solution, 20 g of albumin, 2 units of red blood cells and 2 g of fibrinogen. The patient was then transferred to the intensive care unit, where she remained stable. Noradrenalin pump was gradually withdrawn, and no further transfusion was needed. A Helicobacter pylori antibody test was carried out, which came out positive. She was therefore put under a treatment of proton pump inhibitors, as well as a course of three antibiotics for 14 days (Bismuth salts, metronidazole, and tetracycline). The patient was discharged 9 days after admission. At one month follow up, the outcome was still uneventful, and the patient was doing well. Since then, she has remained asymptomatic. No further treatment, especially surgical, has been performed.
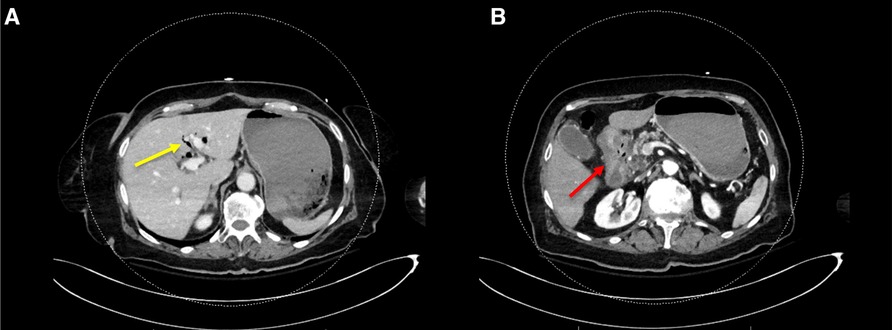
Figure 1. CT-scan of the abdomen showing (A) the presence of blood in the stomach, aerobilia (yellow arrow) and (B) inflammation of the first part of duodenum (red arrow).
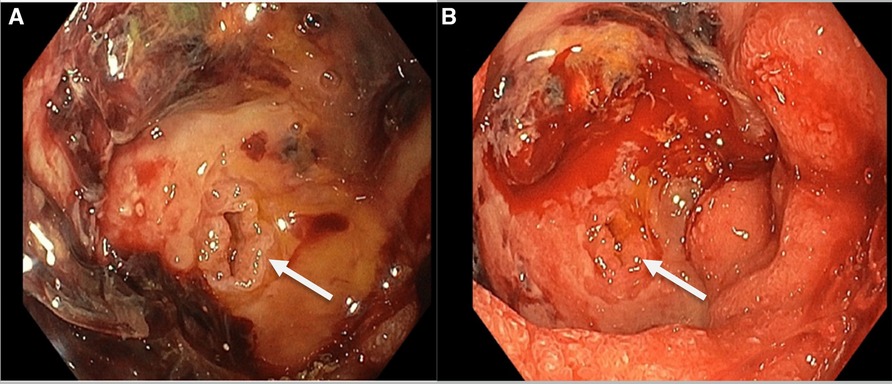
Figure 2. Endoscopic picture showing the 1A Forrest duodenal ulcer with the presence of a choledocho-duodenal fistula (CDF) and an active bleeding of the gastro-duodenal artery around the fistula (B).
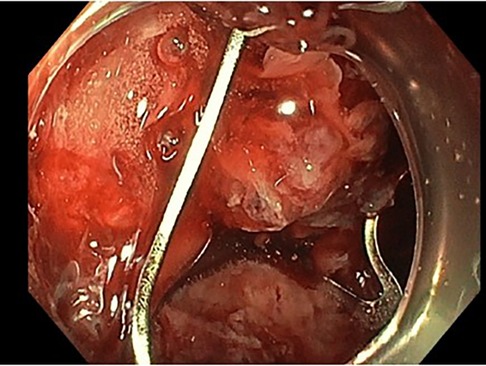
Figure 3. Endoscopic picture showing the 1A forrest duodenal ulcer treated with an over the scope clip.
Regarding the literature search, 155 articles were identified from MEDLINE. No duplicates were removed. Forty-nine articles were excluded after title and abstract screening, and 27 articles were excluded because they were written in a foreign language, leaving 79 articles for full text analysis. Of those, five were excluded because patients presented with an entero-biliary fistula other than the CDF, 52 because patients were admitted in hospital with other symptoms than gastro-intestinal bleeding, and two were editorials. Ten articles were also excluded, because the full text could not be found. Finally, 10 articles were included (Figure 4 and Table 1).
Among the 10 included case reports, four were written in the United States of America (10–13), three in Europe (14–16), one in Nepal (17), one in India (18) and one in Egypt (19) (Table 1). In total, 11 cases were reported. Most of the patients were female (6/11, 54.5%), with ages ranking from 16 to 88 years (mean age 64.2). All patients were admitted in hospital with clinical signs of upper gastro-intestinal bleeding, and five had also abdominal pain (5/11, 45.5%). Except for two patients, all of them had a CT scan, which revealed the presence of pneumobilia. A CDF was identified during gastroscopy in seven cases (7/11, 63.6%). For the other cases, the radiologic tools suggested the presence of the fistula.
Finally, six of the 11 cases of CDF were due to a PUD (6/11, 54.5%), two were related to a metastatic disease of colon and ovarian cancer (2/11, 18.2%), one was due to tuberculosis (1/11, 9.1%), one to gallstones (1/11, 9.1%) and one was iatrogenic (1/11, 9.1%) after metal stent placement in the common bile duct (Table 1).
Among the 11 patients with PUD, five received a conservative treatment with the administration of proton pump inhibitors. Only one patient required surgical treatment.
Peptic ulcer disease (PUD) is a common condition, which can lead to life threatening complications such as gastro-intestinal bleeding. It can also cause entero-biliary fistula, a rare but relevant and important diagnosis. The aim of this article was to highlight this subject, raise awareness among surgeons and clinicians and improve the management of this condition.
To diagnose PUD, the clinician should always get a detailed history and search for symptoms and signs such as epigastric pain, fullness sensation, intake of NSAID or other medication such as corticoids (1, 20). For patients younger than 50 years old without any red flags and if PUD is suspected, a conservative management with administration of a proton pump inhibitor, discontinuation of a possible NSAID treatment and/or eradication of Helicobacter pylori can be proposed (1, 20, 21). For patients older than 50 years old and in case of anaemia, gastro-intestinal bleeding, progressive dysphagia, tumour symptoms or failed medical treatment, esophagogastroduodenoscopy need to be performed (1, 21).
PUD is responsible for most upper gastro-intestinal bleedings (30%–60% of cases) (22–24). Esophagogastroduodenoscopy is an effective first line procedure, which allows to identify the source of haemorrhage and treat it (21, 25). When performed in the early setting (≤24 h of admission), it reduces significantly the rate of blood transfusion and the length of hospital stay (26). It also decreases the need for surgical procedure and mortality rate (27). In 1974, Dr John A.H. Forrest introduced a classification to unify the description of gastro-intestinal bleeding and stratified the risk of recurrent bleeding and the mortality rate (28). The “Forrest classification” includes 3 stages; an active bleeding is present in stage 1 (1a spurting bleeding, 1b oozing bleeding), a recent bleeding in stage 2 (2a visible non-bleeding vessel, 2b adherent clot, 2c hematin covered ulcer) and stage 3 is characterized by the presence of a lesion without signs of haemorrhage (29, 30). Stages 1a to 2a require an endoscopic haemostasis, which can be achieved by several modalities such as epinephrine injection, hemospray or Over The Scope Clip, as in the situation described in this article (25). Endoscopy remains also the treatment of choice in case of recurrent bleeding (25). If the procedure fails, angiography with arterial embolization can be proposed (25). Surgical treatment is also an option, especially in case of hemodynamic instability and/or in the presence of an ulcer larger than 2 cm (25). An open approach, appropriate to location and size of the ulcer, should therefore be preferred (25). Interestingly, the duodenal location is associated with higher reoperation rate and 90 days mortality rate (25, 31).
In the case we reported, the patient did not only have a gastro-intestinal bleeding due to PUD, but also a choledocho-duodenal fistula (CDF), which increases the complexity of the management, especially if a surgical procedure is required.
Most of entero-biliary fistulas and especially cholecysto-duodenal fistulas are caused by the present of gallstones, which induce inflammation, necrosis of the gallbladder or of the common bile duct wall and fistulisation into the adjacent organ (4, 5, 7, 32). PUD of the duodenum is responsible for only 5%–6% of entero-biliary fistulas, but 75%–80% of CDF (32, 33). In our review, 54.5% of CDF cases were due to PUD. Two types of CDF are described in the literature: the proximal and the distal type (7, 34). The proximal type is a single fistula, located more than 2 cm above the papilla and drains into the posterior wall of the duodenum (7, 34). This is usually caused by PUD, but diverticulitis of the duodenum, iatrogenic causes (for instance after laparoscopic cholecystectomy or retrograde cholangio-pancreatogram ERCP), or carcinoma have also been described (7, 35, 36). A carcinoma (for example a cholangio-carcinoma) or a metastasis can lead to a fistula by compressing the common bile duct, therefore increasing the pressure and weakening the duct wall, or by infiltrating the tissues and causing a necrosis (5, 10, 35). In our review, two cases of CDF were related to a metastatic disease of colon and ovarian cancer. Patients with a proximal fistula often complain about dyspepsia, but they can also have gastro-intestinal bleeding, which can have a significant impact if the gastro-duodenal artery is involved, as in this case report (7). The distal type, also called peri-papillar type, can be a single or multiple fistula and is located within 2 cm of the distal common bile duct (7, 34, 37). This is commonly caused by gallstones, eroding the common bile duct and creating a fistula (7).
In 1975, Ikeda et al. also proposed a classification of CDF according to the location of the fistula; the type I and II, as illustrated in Figure 5 (38). The type I is located in the longitudinal fold right next to the papilla (the intra-mural portion of the common bile duct), and results from the impaction of a gallstone not small enough to pass through the papilla (38). The type II forms in the extra-mural portion of the common bile duct and is due to larger stones (38).
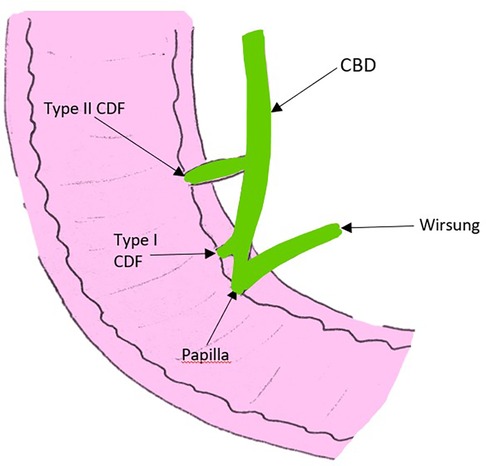
Figure 5. Picture adapted from the article of Ikeda et al., illustrating the two types of CDF according to the location of the fistula; the type I located in the intra-mural portion of the common bile duct right next to the papilla, and the type II located in the extra-mural portion of the common bile duct. CBD, common bile duct; CDF, choledocho-duodenal fistula.
Diagnosis of entero-biliary fistula is made by endoscopy and ERCP, which not only allow to visualize the fistula orifice and tract trough the injection of contrast, but also to diagnose the aetiology and sometimes treat it (4, 7, 39). Computed tomography (CT) is another valuable diagnostic tool, which can reveal the presence of air in the biliary tree (4, 32, 37). According to Shimono et al., it helps to differentiate a gallbladder-enteric fistula from a choledocho-enteric fistula (40). Plain abdominal radiography can also demonstrate air in the biliary tree, but only 5%–10% of the time (40).
Several therapies have been proposed to treat entero-biliary fistula but no guidelines have been established yet (6, 8). It is recommended to treat cholecysto-duodenal fistula surgically with cholecystectomy and closure of the fistula tract (40–42). CDF incidentally diagnosed and caused by PUD could be managed conservatively, with the administration of proton pump inhibitor, as mentioned in our article (5, 32, 39, 43). Our review also showed that most of the cases were managed conservatively. If the fistula is caused by a gallstone and is located distally, ERCP with sphincterotomy can be performed, followed by a cholecystectomy (6, 7, 39). Zhao et al. reported a case where CDF was successfully treated with the implantation of a covered self-expandable metal stent in the common bile duct (44). Neumann et al. also published a case similar to the one we described, where a CDF caused by a PUD was totally closed after using an Over The Scope Clip (45).
According to several authors, a surgical approach should be advised in case of failed medical treatment, gastric outlet obstruction, haemorrhage, perforation, recurrent gallstone ileus or recurrent cholangitis (5, 8, 32, 43). For CDF due to PUD refractory to medical treatment, a vagotomy with an antrectomy and a reconstruction according to Billroth II procedure with a gastrojejunostomy can be performed (7, 32, 33, 39). To note, the fistula should in this case be left intact (7, 32, 33, 39). To determine whether the size of the CDF has an impact on symptoms and treatments, Li et al. reviewed 50 cases during a 14-year period (46). They concluded that large fistulas increase the risk of cholangitis and proposed therefore the following strategies. For orifices larger than 1 cm and common bile duct larger than 2 cm, associated with complications in the biliary system such as strictures of an intrahepatic duct, patients should undergo surgical treatment (for instance Roux-en-Y intrahepatic cholangio-jejunostomy, Roux-en-Y choledocho-jejunostomy or partial liver resection) (46). For orifices between 0.5 and 1 cm with a common bile duct dilated over 2 cm, without complications in the biliary system, a side-to-side choledocho-jejunostomy without a transection of the duct can be achieved (46). For orifices larger than 0.5 cm with a common bile duct dilated over 1.2 cm and caused by a gallstone, a stone removal followed by a cholecystectomy should be performed (46). Finally, for orifices smaller than 0.5 cm, a conservative management can be applied (46).
Through this manuscript, we provide the information available for the reader to recognize, diagnose and manage cases of entero-biliary fistula and especially CDF. We also conducted a review of the literature to search for pre-existing cases of CDF manifesting as gastrointestinal bleeding, offering therefore the first comprehensive review on the subject. The pictures are of good quality, adding a lot of value to this manuscript, compared with other published articles.
Choledocho-duodenal fistulas are a rare condition, often diagnosed incidentally during endoscopy or radiological imaging. Most of the patients remain asymptomatic or report non-specific symptoms such as abdominal pain, fever, or bowel obstruction. To date, no clear guidelines to treat entero-biliary fistula have been established yet, but most of choledocho-duodenal fistulas caused by peptic ulcer disease can be managed conservatively, with the administration of proton pump inhibitor and the eradication of Helicobacter Pylori infection. Endoscopic treatment including ERCP with sphincterotomy, the implantation of a covered self-expandable metal stent in the common bile duct of the use of an Over The Scope Clip can also be proposed. A surgical approach should be advised in case of failed medical treatment or complications, such as gastric outlet obstruction or recurrent cholangitis.
IU and AL were responsible for database search and writing of the article. EL supervised and corrected the article. IU and AL contributed equally to this work. All authors contributed to the article and approved the submitted version.
Open access funding by University of Geneva.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Malik TF, Gnanapandithan K, Singh K. Peptic ulcer disease. StatPearls. Treasure Island, FL: StatPearls Publishing (2022). Available at: http://www.ncbi.nlm.nih.gov/books/NBK534792/ (Cited July 26, 2022).
2. Xie X, Ren K, Zhou Z, Dang C, Zhang H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol. (2022) 22:58. doi: 10.1186/s12876-022-02130-2
3. Peptic ulcer disease. Wikipedia. (2022). Available at: https://en.wikipedia.org/w/index.php?title=Peptic_ulcer_disease&oldid=1098460238 (Cited July 26, 2022).
4. Duzgun AP, Ozmen MM, Ozer MV, Coskun F. Internal biliary fistula due to cholelithiasis: a single-centre experience. World J Gastroenterol. (2007) 13:4606–9. doi: 10.3748/wjg.v13.i34.4606
5. Wu M-B, Zhang W-F, Zhang Y-L, Mu D, Gong J-P. Choledochoduodenal fistula in Mainland China: a review of epidemiology, etiology, diagnosis and management. Ann Surg Treat Res. (2015) 89:240–6. doi: 10.4174/astr.2015.89.5.240
6. Akaydin M, Demiray O, Ferlengez E, Erozgen F, Ersoy YE, Er M. Importance of spontaneous choledochoduodenal fistulas detected during ERCP procedure. Indian J Surg. (2018) 80:216–20. doi: 10.1007/s12262-016-1569-8
7. Fedidat R, Safadi W, Waksman I, Hadary A. Choledochoduodenal fistula: an unusual case of pneumobilia. BMJ Case Rep. (2014) 2014:bcr2014206798. doi: 10.1136/bcr-2014-206798
8. Kachi A, Kanj M, Khaled C, Nassar C, Rached CB, Kansoun A. Choledochoduodenal fistula secondary to peptic ulcer disease: a case report. Am J Case Rep. (2019) 20:398–401. doi: 10.12659/AJCR.915600
9. Spontaneous Choledochoduodenal Fistula Secondary to Long-Standing Ulcer Disease. SMJ. Available at: http://www.smj.org.sg/article/spontaneous-choledochoduodenal-fistula-secondary-long-standing-ulcer-disease (Cited July 26, 2022).
10. Chaudhari D, Saleem A, Murthy R, Baron T, Young M. Choledochoduodenal fistula after biliary placement of a self-expanding metallic stent for palliation of pancreatic cancer. Endoscopy. (2013) 45:E77. doi: 10.1055/s-0032-1326266
11. Misra D, Mirza U, Vakiti A, Padala SA. A rare presentation of choledochoduodenal fistula due to ovarian cancer metastasis. J Investig Med High Impact Case Rep. (2020) 8:2324709620934680. doi: 10.1177/2324709620934680
12. Jacobs JD, Kaplan SJ, Chiorean M. Spontaneous pneumobilia and hematemesis. Gastroenterology. (2019) 157:e8–e9. doi: 10.1053/j.gastro.2019.03.053
13. Antony A, Kramer S, Tzimas D, Saitta P. Pneumobilia resulting from choledochoduodenal fistula secondary to metastatic colon adenocarcinoma. ACG Case Rep J. (2016) 3:112–4. doi: 10.14309/crj.2016.17
14. Sreekumar S, Vithayathil M, Gaur P, Karim S. Choledochoduodenal fistula: a rare complication of acute peptic ulcer bleeding. BMJ Case Rep. (2021) 14:e246532. doi: 10.1136/bcr-2021-246532
15. Jiménez-Rosales R, Caballero-Mateos A, Redondo-Cerezo E. Choledochoduodenal fistula secondary to ulcer disease presenting with gastrointestinal bleeding. Clin Gastroenterol Hepatol. (2018) 16:e104–5. doi: 10.1016/j.cgh.2017.10.013
16. Marsdin EL, Kreckler S, Alzein A, D’Costa H. Choledochal-duodenal fistula presenting as an upper GI bleed. BMJ Case Rep. (2011) 2011:bcr0520114275. doi: 10.1136/bcr.05.2011.4275
17. Yadav TN, Deo KB, Gautam S, Awale L, Pandit N. A complicated peptic ulcer with bleeding, gastric outlet obstruction, and choledochoduodenal fistula. Cureus. (2020) 12:e11189. doi: 10.7759/cureus.11189
18. Mohite A, Somani P, Gambhire P, Rathi P. Tuberculous choledochoduodenal fistula with extrahepatic portal vein obstruction: rare association. J Formosan Med Assoc. (2013) 112:807–9. doi: 10.1016/j.jfma.2013.10.013
19. Naga M, Mogawer MS. Choledochoduodenal fistula: a rare sequel of duodenal ulcer. Endoscopy. (1991) 23:307–8. doi: 10.1055/s-2007-1010700
20. Mazzola Eusébio. Strategies dyspepsies. Geneva: HUG (2017). Available at: https://www.hug.ch/medecine-de-premier-recours/strategies
21. Banerjee S, Cash BD, Dominitz JA, Baron TH, Anderson MA, Ben-Menachem T, et al. The role of endoscopy in the management of patients with peptic ulcer disease. Gastrointest Endosc. (2010) 71:663–8. doi: 10.1016/j.gie.2009.11.026
22. van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. (2008) 22:209–24. doi: 10.1016/j.bpg.2007.10.011
23. Gralnek IM, Dumonceau J-M, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: european society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. (2015) 47:a1–46. doi: 10.1055/s-0034-1393172
24. Agarwal A, Benias P, Brewer Gutierrez OI, Wong V, Hanada Y, Yang J, et al. Endoscopic suturing for management of peptic ulcer-related upper gastrointestinal bleeding: a preliminary experience. Endosc Int Open. (2018) 6:E1439–44. doi: 10.1055/a-0749-0011
25. Tarasconi A, Coccolini F, Biffl WL, Tomasoni M, Ansaloni L, Picetti E, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg. (2020) 15:3. doi: 10.1186/s13017-019-0283-9
26. Lin HJ, Wang K, Perng CL, Chua RT, Lee FY, Lee CH, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol. (1996) 22:267–71. doi: 10.1097/00004836-199606000-00005
27. Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology. (1992) 102:139–48. doi: 10.1016/0016-5085(92)91793-4
28. JohnAH F, Finlayson NDC, Shearman DJC. Endoscopy in gastrointestinal bleeding. Lancet. (1974) 304:394–7. doi: 10.1016/S0140-6736(74)91770-X
29. Endoscopy Campus - Forrest Classification [Internet]. Endoscopy Campus. Available at: https://www.endoscopy-campus.com/en/classifications/forrest-classification/ (Cited July 28, 2022).
30. Forrest classification for upper gastrointesti- nal bleeding [Internet]. ResearchGate.Available from: https://www.researchgate.net/figure/Forrest-classification-for-upper-gastrointesti-nal-bleeding_tbl1_236185556 [Cited July 28, 2022).
31. Lolle I, Møller MH, Rosenstock SJ. Association between ulcer site and outcome in complicated peptic ulcer disease: a Danish nationwide cohort study. Scand J Gastroenterol. (2016) 51:1165–71. doi: 10.1080/00365521.2016.1190398
32. Xeropotamos NS, Nousias VE, Vekris AD, Katsanos KH, Tsianos EV, Kappas AM. Choledochoduodenal fistula: an unusual complication of penetrated duodenal ulcer disease. Ann Gastroenterol. (2004) 1:104–8. Available at: http://annalsgastro.gr/index.php/annalsgastro/article/view/274 (Cited July 29, 2022).
33. Shah PP. Choledochoduodenal fistula complicating duodenal ulcer disease (a report of 3 cases). J Postgrad Med. (1990) 36:167.2102919
34. Duman L, Savas C, Aktas AR, Akcam M. Choledochoduodenal fistula: an unusual cause of recurrent cholangitis in children. J Indian Assoc Pediatr Surg. (2014) 19:172–4. doi: 10.4103/0971-9261.136479
35. Lin C-T, Hsu K-F, Yu J-C, Chu H-C, Hsieh C-B, Fu C-Y, et al. Choledochoduodenal fistula caused by cholangiocarcinoma of the distal common bile duct. Endoscopy. (2009) 41:E319–20. doi: 10.1055/s-0029-1215310
36. Gallagher SP, Imagawa DK. Spontaneous choledochoduodenal fistula in a patient with a bile duct injury following laparoscopic cholecystectomy. J Surg Case Rep. (2019) 2019:rjz141. doi: 10.1093/jscr/rjz141
37. Dadzan E, Akhondi H. Choledochoduodenal fistula presenting with pneumobilia in a patient with gallbladder cancer: a case report. J Med Case Rep. (2012) 6:61. doi: 10.1186/1752-1947-6-61
38. Ikeda S, Okada Y. Classification of choledochoduodenal fistula diagnosed by duodenal fiberscopy and its etiological significance. Gastroenterology. (1975) 69:130–7. doi: 10.1016/S0016-5085(19)32645-9
39. H’ng MWC, Yim HB. Spontaneous Choledochoduodenal Fistula Secondary to Long-Standing Ulcer Disease.:3.
40. Shimono T, Nishimura K, Hayakawa K. CT Imaging of biliary enteric fistula. Abdom Imaging. (1998) 23:172–6. doi: 10.1007/s002619900314
41. Park JM, Kang CD, Kim JH, Lee SH, Nam S-J, Park SC, et al. Cholecystoduodenal fistula presenting with upper gastrointestinal bleeding: a case report. World J Clin Cases. (2021) 9:410–5. doi: 10.12998/wjcc.v9.i2.410
42. Aguilar-Espinosa F, Maza-Sánchez R, Vargas-Solís F, Guerrero-Martínez GA, Medina-Reyes JL, Flores-Quiroz PI. Cholecystoduodenal fistula, an infrequent complication of cholelithiasis: our experience in its surgical management. Rev Gastroenterol Mex. (2017) 82:287–95. doi: 10.1016/j.rgmxen.2017.07.015
43. Mori H, Zakimi M, Kato S, Yamada K, Chinen K, Kubota T, et al. Successful conservative treatment of a cholecystoduodenal fistula caused by a cytomegalovirus-associated duodenal ulcer. Intern Med. (2016) 55:2617–21. doi: 10.2169/internalmedicine.55.6897
44. Zhao S, Wang J, Ge J, Zhang X, Liu J, Zhang A, et al. Implantation of covered self-expandable metal stent in the common bile duct for the treatment of choledochoduodenal Fistula. J Clin Gastroenterol. (2014) 48:383–4. doi: 10.1097/MCG.0000000000000016
45. Neumann H, Nägel A, Bernatik T, Wickles N, Neurath MF, Raithel M. Endoscopic closure of large, spontaneous, choledochoduodenal fistula by using an over-the-scope clip. Gastrointest Endosc. (2011) 74:200–2; discussion 202. doi: 10.1016/j.gie.2011.01.057
Keywords: gastro-intestinal bleeding, hematemesis, biliary fistula, choledochoduodenal fistula, helicobacter pylori
Citation: Uhe I, Litchinko A and Liot E (2023) Peptic ulcer disease complicated with choledocho-duodenal fistula and gastro-intestinal bleeding: a case report and review of the literature. Front. Surg. 10:1206828. doi: 10.3389/fsurg.2023.1206828
Received: 16 April 2023; Accepted: 8 June 2023;
Published: 20 June 2023.
Edited by:
Ahmad Mahamid, Carmel Medical Center, IsraelReviewed by:
Donal Brendan O'Connor, Trinity College Dublin, Ireland© 2023 Uhe, Lichtinko and Liot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabelle Uhe dWhlaTI2MDRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.