
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 20 September 2023
Sec. Otorhinolaryngology - Head and Neck Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1205287
This article is part of the Research Topic No time to die: Or if tomorrow never comes? - What can we draw from the pandemic and general instability? An opportunity for surgeons to grasp View all 6 articles
The brachial cleft carcinoma is an extremely rare head and neck facial malignancy, and there is some disagreement about its differential diagnosis. In this paper, we report a 63-year-old male patient who had a mass on the left side of the neck and diagnosed as the brachial cleft carcinoma by intraoperative biopsy pathology. However, this patient was diagnosed with the carcinoma of the left soft palate more than 20 days after surgery and esophageal cancer 2 years later, and was treated accordingly. Therefore, it is hard to confirm whether the branchial cleft carcinoma is primary or metastatic. In fact, the diagnostic criteria for primary squamous cell carcinoma of branchial cleft cysts are very rigorous. Confirmation of the diagnosis is based on pathological examination of the branchial cleft cyst epithelium lined with squamous cells, meanwhile, a thorough examination should also be performed to exclude the presence of other primary cancers.
Branchial cleft carcinoma originates as a carcinoma of the epithelium lining the branchial cleft cyst, which is extremely low in incidence and difficult to diagnose accurately. The majority of its pathological type is squamous epithelial cell carcinoma. Branchial cleft cysts are congenital malformations caused by malformation of the first to fourth branchial cleft. Depending on the degree of malformation during embryonic development, these abnormalities may appear as fistula, cyst, or sinus tract (1). The most common type of branchial cleft cyst comes from the second cleft, accounting for approximately 40%–90% of the four branchial cyst types (2),while the first, third and fourth cleft have much rarer abnormality. The typical branchial cleft cyst is located on the anterior borderline of the sternocleidomastoid muscle. Although the branchial cleft carcinoma is uncommon, it remains a difficult challenge to carefully differentiate the primary branchial cleft carcinoma and those that metastasize to them. The preferred treatment is radical resection with adjuvant radiotherapy or chemotherapy (3, 4).
A 63-year-old male patient was admitted to our department on December 25, 2017, with a “left neck mass found for 3 years.” The mass was approximately 6 cm in diameter with clear boundaries, protruding from the skin, hard and poorly mobile (Figure 1A), and no enlarged lymph nodes were palpable on the clavicle area behind the ear. Old pulmonary tuberculosis of the left upper lung was found, with no history of surgery, trauma, or drug allergy. According to the CT report, there was a 26 × 18 × 37 mm mass occupying the left upper neck below the parotid gland (Figure 1B), which was considered branchial cleft cyst with infection. In addition, the mucosa of the left piriform fossa was thickened, which was considered to be an inflammatory lesion (Figure 2). Surgical treatment of a left neck mass was performed on December 26, 2017, under intravenous complex general anesthesia with tracheal intubation.
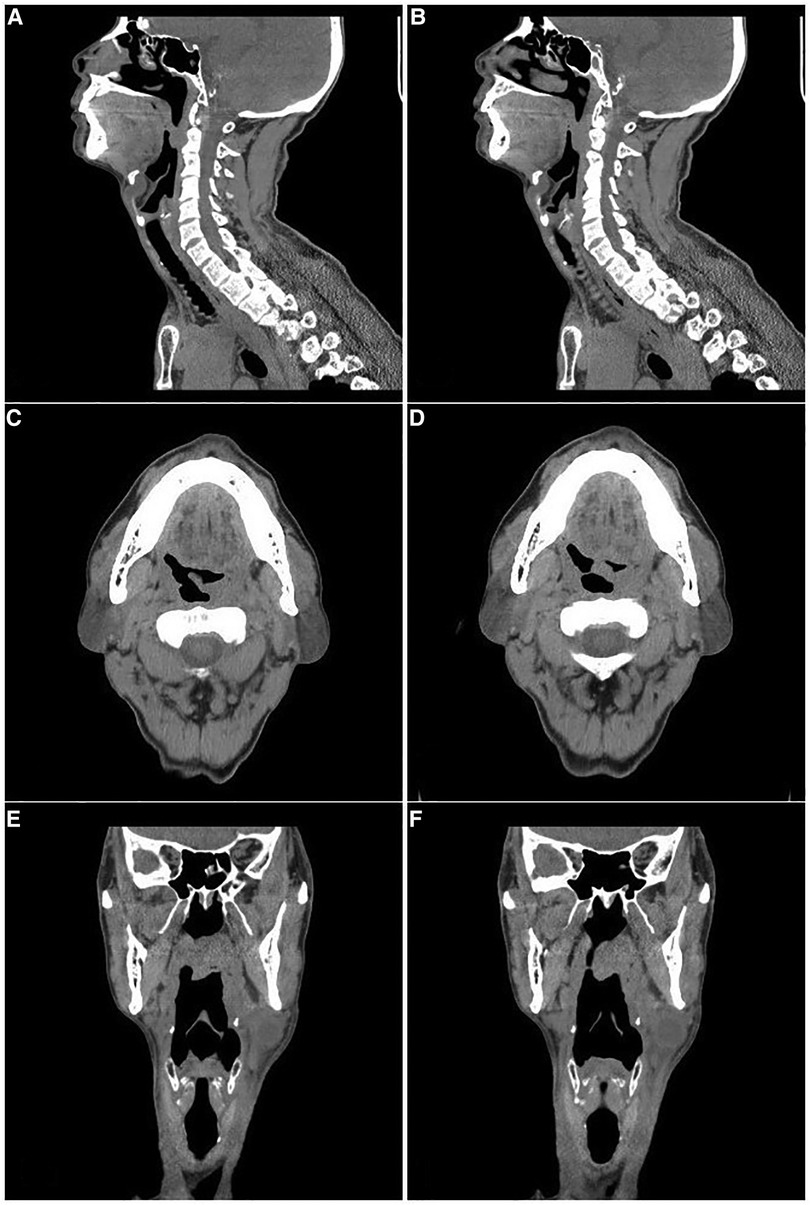
Figure 2. The CT images of the left soft palate. (A)–(B): Sagittal view. (C)–(D): Transverse view. (E)–(F): Coronal view.
Then pathological results were confirmed by the pathology department as a carcinoma of the left cervical branchial cleft cyst, which was a squamous cell carcinoma (branchial cleft carcinoma). Microscopically, cystic structures were seen, lined with squamous epithelium, and lymphocytic bands were seen around the epithelium with lymphoid follicle formation. The lined epithelium was locally heterogeneous, breaking through the basement membrane to infiltrate the surrounding area, and was distributed in nested clusters (Figure 3). The cells were of different sizes, with thickened nuclear chromatin, some cells with obvious nucleoli, and some keratinized beads were visible. The patient was admitted to Cancer Hospital for further treatment.
Twenty days after the first surgery, the patient was diagnosed with the squamous carcinoma of the left soft palate at Cancer Hospital after a tissue biopsy and immunohistochemical examination. Radical excision of left soft palate carcinoma was performed on January 17, 2018, under general anesthesia with pneumonectomy. Pathological examination microscopically revealed highly differentiated squamous cell carcinoma of the left soft palate infiltrating the transverse muscle (Figure 4A). Postoperative radiotherapy was performed after the operation.
On April 7, 2020, the patient was readmitted to our gastroenterology department with “obstruction of swallowing for more than 10 days.” He presented as vomiting after eating, and the vomit was the food eaten. CT in other hospitals showed dilatation of the upper esophagus with irregular wall thickening and luminal narrowing, considering the possibility of esophageal mass occupancy. Then esophageal cancer was diagnosed by pathological examination. A biopsy was taken from the esophagus 20 cm from the incisor, and the pathological diagnosis was squamous cell carcinoma. Microscopically, heterogeneous cells were seen under the squamous epithelium in the form of nesting sheets, with varying cell sizes, increased nucleoplasm ratio, thickened chromatin, eosinophilic nuclei and nuclear fission images, and localized keratinized bead formation (Figure 4B).
The origin of branchial cleft cysts has been debated for a long time, but it is generally considered to be formed by incomplete degeneration of fetal branchial organs during embryonic development. Volkman first reported branchial cleft carcinoma in 1882. The primary branchial cleft carcinoma is extremely rare, and its histopathological diagnosis is based on the transformation of normal epithelium within the branchial cleft cyst to malignant epithelium lined with squamous epithelium (5, 6). Some researchers believe that branchial cleft carcinoma is actually a cystic metastasis of oral cancer rather than a carcinoma originating from the branchial cleft (7, 8). In 1950, Martin (4) and other researchers proposed diagnostic criteria for the cystic carcinoma of the gill slit: (i) the tumor should occur on the line extending from the front of the tragus down to the anterior border of the sternocleidomastoid muscle; (ii) the histology should be consistent with the origin of the residual tissue of the branchial cyst; (iii) the patient must survive at least 5 years through regular examination and must not develop other primary tumors; (iv) the squamous carcinoma is visible within the epithelium of the cyst. However, some scholars (9) still consider the criterion of absence of other primary tumors during the 5-year follow-up too idealistic and of limited diagnostic and therapeutic value.
In this case, the patient was diagnosed with squamous cell carcinoma of the left soft palate more than 20 days after resection for squamous cell carcinoma of the left branchial cleft cyst, and then diagnosed with the esophageal carcinoma 2 years later. It is unclear whether there is an association between the carcinoma of the left soft palate and esophagus and the previous branchial cleft of the left neck. In terms of the diagnostic criteria described above, this case is not diagnostic as primary branchial cleft carcinoma. In addition, according to the pathological results, the patient's esophageal cancer was more likely to be metastatic cancer. It has been shown that in cases of lymph node metastasis of squamous cell carcinoma, approximately 72%–90% of the primary tumors were found to be located in Waldeyer's ring, which is the base of the tongue, palatine tonsils and nasopharyngeal region (5, 10, 11). Resection of the neck mass and histopathological examination is the only accurate basis for the diagnosis of true malignancy, although its origin may remain unknown.
Branchial cleft carcinoma is rare and difficult to diagnose and differentiate, therefore, the current knowledge of this disease is mainly based on case reports. Since the current treatment for this disease is mainly radical surgery with post-operative radiotherapy or chemotherapy to prevent tumor recurrence (12). More relevant clinical studies and histopathological data would be beneficial to deepen the understanding of the branchial cleft carcinoma, which has an important role in improving the guidance of the treatment for this disease.
The confirmation of primary squamous cell carcinoma of branchial cleft cysts is very rigorous, which is based on pathological examination of the branchial cleft cyst epithelium lined with squamous cells. Meanwhile, a thorough examination should also be performed to exclude the presence of other primary cancers. This case report also suggests that it is necessary to pay attention to the physical examination of the oral cavity when diagnosing a neck mass.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YM: writing original draft; HL and LY: collecting and analyzing the data; ST: supervision and editing; LS: revision and writing-reviewing. All authors contributed to the article and approved the submitted version.
I would like to express my great gratitude to the Second Affiliated Hospital Of Shantou University Medical College and Cancer Hospital Of Shantou University Medical College for providing information on this case report, and especially thank to Dr. Changchun Ma of the Department of Radiotherapy, Cancer Hospital, for his help in collecting case data. And I gratefully acknowledged the help of my supervisor LS, who has offered me valuable suggestions in academic studies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SXD declared a shared affiliation with the authors to the handling editor at the time of review
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Papadogeorgakis N, Petsinis V, Parara E, Papaspyrou K, Goutzanis L, Alexandridis C. Branchial cleft cysts in adults. Diagnostic procedures and treatment in a series of 18 cases. Oral Maxillofac Surg. (2009) 13:79–85. doi: 10.1007/s10006-009-0156-6
2. Bahakim A, Francois M, Van Den Abbeele T. Congenital midline cervical cleft and W-plasty: our experience. Int J Otolaryngol. (2018) 2018:5081540. doi: 10.1155/2018/5081540
3. Bradley PT, Bradley PJ. Branchial cleft cyst carcinoma: fact or fiction. Curr Opin Otolaryngol Head Neck Surg. (2013) 21:118–23. doi: 10.1097/MOO.0b013e32835cebde
4. Martin H, Morfit HM, Ehrlich H. The case for branchiogenic cancer (malignant branchioma). Ann Surg. (1950) 132:867–87. doi: 10.1097/00000658-195011000-00002
5. Thompson LD, Heffner DK. The clinical importance of cystic squamous cell carcinomas in the neck: a study of 136 cases. Cancer. (1998) 82:944–56. doi: 10.1002/(sici)1097-0142(19980301)82:5%3C944::aid-cncr21%3E3.0.co;2-#
6. Bilgen C, Ogüt F, Celtiklioglu F. A new case of a branchial cyst of the parapharyngeal space. Ear Nose Throat J. (2001) 80:384, 387–9. doi: 10.1177/014556130108000608
7. Maturo SC, Michaelson PG, Faulkner JA. Primary branchiogenic carcinoma: the confusion continues. Am J Otolaryngol. (2007) 28:25–7. doi: 10.1016/j.amjoto.2006.06.005
8. Mallet Y, Lallemant B, Robin YM, Lefebvre JL. Cystic lymph node metastases of head and neck squamous cell carcinoma: pitfalls and controversies. Oral Oncol. (2005) 41:429–34. doi: 10.1016/j.oraloncology.2004.09.016
9. Zimmermann CE, von Domarus H, Moubayed P. Carcinoma in situ in a lateral cervical cyst. Head Neck. (2002) 24:965–9. doi: 10.1002/hed.10118
10. Gourin CG, Johnson JT. Incidence of unsuspected metastases in lateral cervical cysts. Laryngoscope. (2000) 110:1637–41. doi: 10.1097/00005537-200010000-00012
11. Regauer S, Mannweiler S, Anderhuber W, Gotschuli A, Berghold A, Schachenreiter J, et al. Cystic lymph node metastases of squamous cell carcinoma of Waldeyer’s ring origin. Br J Cancer. (1999) 79:1437–42. doi: 10.1038/sj.bjc.6690229
Keywords: branchial cleft carcinoma, primary branchial cleft carcinoma, metastatic branchial cleft carcinoma, head and neck tumor, head and neck (H&N) cancer
Citation: Ma Y, Liu H, Yang L, Tang S and Shi L (2023) Primary or metastatic branchial cleft carcinoma?: a case report. Front. Surg. 10:1205287. doi: 10.3389/fsurg.2023.1205287
Received: 13 April 2023; Accepted: 7 September 2023;
Published: 20 September 2023.
Edited by:
David T. Liu, Medical University of Vienna/Vienna General Hospital, AustriaReviewed by:
Yong Miao, Southern Medical University, China© 2023 Ma, Liu, Yang, Tang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lungang Shi bHVuZ2FuZ3NoaUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.