
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 23 May 2023
Sec. Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1198837
This article is part of the Research TopicArtificial Intelligence and Advanced Technologies in Neurological SurgeryView all 5 articles
 Michel Roethlisberger1,2,3,4,†
Michel Roethlisberger1,2,3,4,† Noëmi Elisabeth Eberhard1,†
Noëmi Elisabeth Eberhard1,† Jonathan Rychen1*
Jonathan Rychen1* Saif Al-Zahid4,5
Saif Al-Zahid4,5 Ronie Romelean Jayapalan3
Ronie Romelean Jayapalan3 Christian Zweifel1,2,6
Christian Zweifel1,2,6 Ravindran Karuppiah3
Ravindran Karuppiah3 Vicknes Waran3*
Vicknes Waran3*
Background: Cerebellar contusion, swelling and herniation is frequently encoutered upon durotomy in patients undergoing retrosigmoid craniotomy for cerebellopontine angle (CPA) tumors, despite using standard methods to obtain adequate cerebellar relaxation.
Objective: The aim of this study is to report an alternative cerebrospinal fluid (CSF)-diversion method using image-guided ipsilateral trigonal ventriculostomy.
Methods: Single-center retro- and prospective cohort study of n = 62 patients undergoing above-mentioned technique. Prior durotomy, CSF-diversion was performed to the point where the posterior fossa dura was visibly pulsatile. Outcome assessment consisted of the surgeon's intra- and postoperative clinical observations, and postoperative radiological imaging.
Results: Fifty-two out of n = 62 (84%) cases were eligible for analysis. The surgeons consistently reported successful ventricular puncture and a pulsatile dura prior durotomy without cerebellar contusion, swelling or herniation through the dural incision in n = 51/52 (98%) cases. Forty-nine out of n = 52 (94%) catheters were placed correctly within the first attempt, with the majority of catheter tips (n = 50, 96%) located intraventricularly (grade 1 or 2). In n = 4/52 (8%) patients, postoperative imaging revealed evidence of a ventriculostomy-related hemorrhage (VRH) associated with an intracerebral hemorrhage [n = 2/52 (4%)] or an isolated intraventricular hemorrhage [n = 2/52 (4%)]. However, these hemorrhagic complications were not associated with neurological symptoms, surgical interventions or postoperative hydrocephalus. None of the evaluated patients demonstrated radiological signs of upward transtentorial herniation.
Conclusion: The method described above efficiently allows CSF-diversion prior durotomy to reduce cerebellar pressure during retrosigmoid approach for CPA tumors. However, there is an inherent risk of subclinical supratentorial hemorrhagic complications.
Surgery for tumors of the cerebellopontine angle (CPA) requires meticulous care of the cerebellar cortex, which is known to be softer than the cerebral cortex (1). A higher resistance of the venous outflow caused by the mass lesion and the patient's positioning potentially leads to a raised pressure within the posterior fossa. Preoperative measures to reduce said complications are temporary cerebrospinal fluid (CSF)-diversion with a lumbar drain, and the semi-sitting or sitting position of the patient, however, each with their own spectrum of advantages and disadvantages. Standard methods of obtaining adequate cerebellar relaxation during surgery are anesthesiologic management and the microsurgical puncture of arachnoid cisterns or cerebellar fissures (2–4). In certain cases, however, the surgeon encouters cerebellar contusion, swelling and herniation upon durotomy in which the release of CSF remains difficult (2, 5). Excessive retraction maneuvers cause tissue trauma, and the resulting cortical contusions promote further cerebellar swelling (2, 6). All of the above-mentioned measures have their own risk-benefit profile, potentially making it unnecessary to perform a supratentorial ventriculostomy. To further widen the surgical armamentarium and evaluate the safety of such a reserve strategy, the authors used a supratentorial CSF-diversion method using image-guided ipsilateral trigonal ventriculostomy to reduce cerebellar pressure prior to durotomy when resecting CPA-tumors via the retrosigmoid approach (7, 8). To date, no cohort study determining this methods accuracy and outcome has been reported in the English literature. The aim of this study is to report technical nuances of the above-mentioned technique, and to report surgical outcomes and complications in a retrospectively and prospectively collected consecutive single-center cohort.
The data that support the findings of this study are reported according to the STROBE guidelines and are available on reasonable request (9).
Local ethical committee approval was obtained from the last authors' institution (MREC ID NO: 2017127-4864) and the requirement for written informed consent was waived (justification: disproportionality). No clinical trial registration was required.
Single-center case series based on data collected between March 2012 and May 2019 (retrospective 2012–2017 and prospective 2018–2019).
Patients undergoing supratentorial CSF-diversion using image-guided trigonal ventriculostomy prior to retrosigmoid craniotomy for the resection of a CPA-tumor, irrespective of the ventricular size on preoperative imaging (Figure 1). To minimize selection bias within the reported cohort, the technique was used within a consecutive series patients and pathologies (Table 1). Regular follow-up consisted of clinical postoperative intermediate care visits and outpatient follow-up performed by the last author 4–6 weeks after surgery and then once per year.
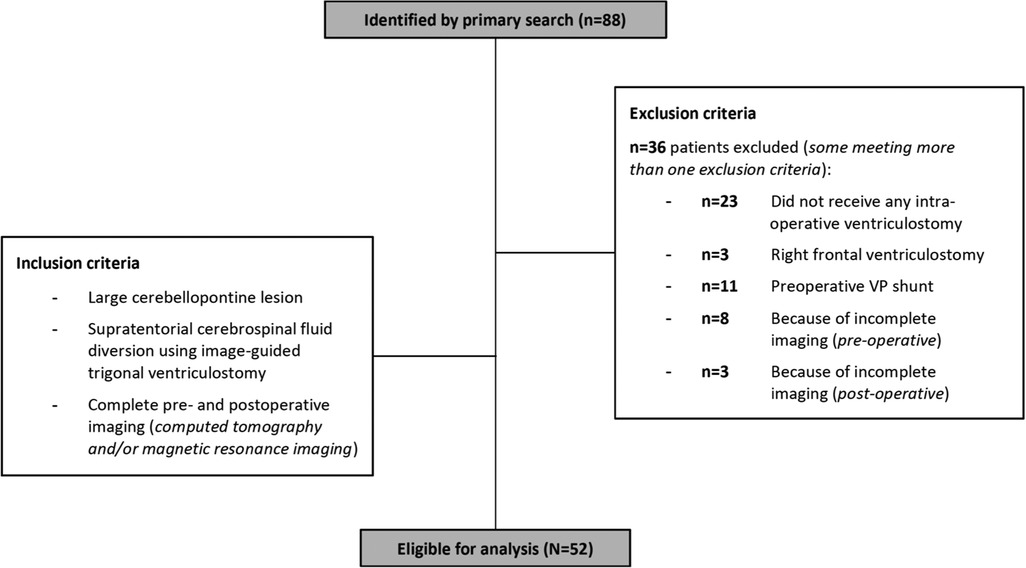
Figure 1. Patient inclusion profile: Patients, where standard methods of CSF-release without a ventriculostomy (n = 23) or a ventriculostomy via a right frontal trajectory (n = 3) was used were excluded from the study, resulting in n = 62 eligible patients. Additionally, patients with a pre-existing permanent CSF-diversion (n = 11), and patients with missing information on pre- (n = 8) or postoperative imaging (n = 3), were excluded. Certain patients met more than one exclusion criteria. Eventually, n = 52 patients were included into the final analysis.
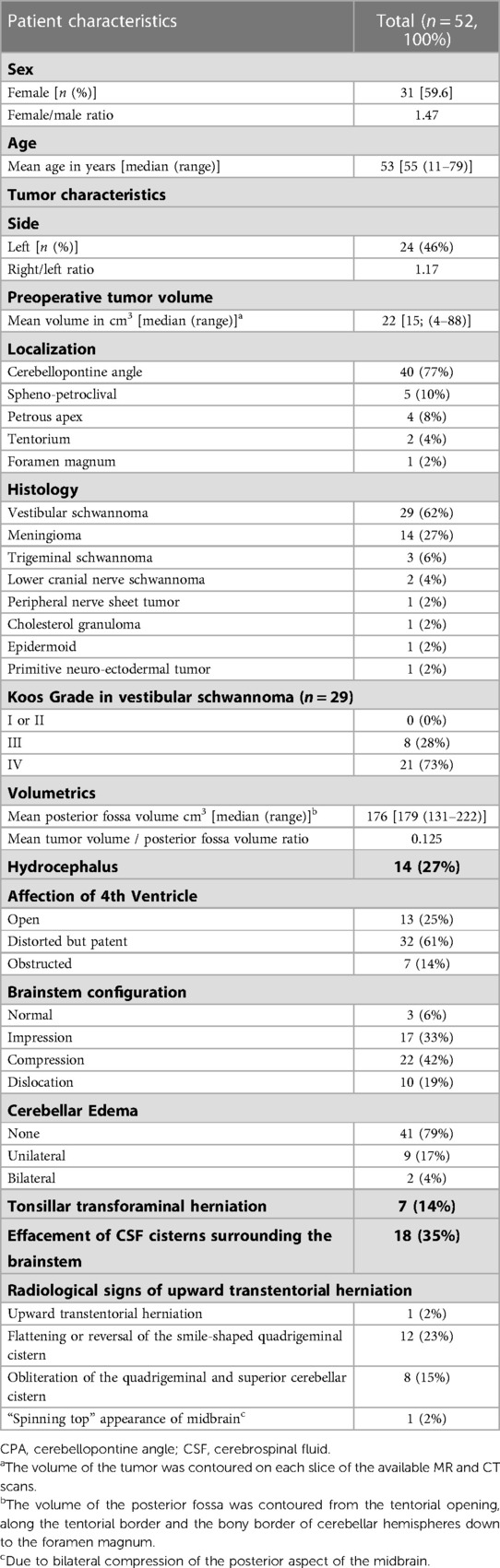
Table 1. Baseline characteristics of n = 52 patients recieving supratentorial cerebrospinal fluid diversion using image-guided trigonal ventriculostomy during retrosigmoid craniotomy for cerebellopontine angle tumors.
The patients are positioned supine with their head rotated and slightly latero-flexed to the contralateral side. Image-guided surgery (IGS) is used based on preoperative computed tomography (CT) or magnetic resonance imaging (MRI) scans. Following a superiorly extended post-auricular “C” shaped incision and delineation of the dural sinuses, a standard retrosigmoid craniotomy is performed. A burr hole is then placed based on IGS to be included within primary incision approximately 2.5 cm superior and posterior to the pinna of the ear. Prior to durotomy, an image-guided ventriculostomy of the ipsilateral trigone of the lateral ventricle is performed using a sterile image guidance probe aimed in a slight cephalic direction, and the external ventricular drain (EVD) is advanced 4–5 cm perpendicular to the cortex as it penetrates the proximal wall of the ventricle (7, 8). A loss of resistance is usually felt after 4–5 cm, and from that point, the catheter is advanced in soft pass technique after removing the image guidance probe. In most of the cases, there is some CSF flow out of the ventriculostomy track and CSF flow is observed while the ventricular catheter is softly advanced (usually 6–7 cm). The amount of CSF released is performed to the point where the posterior fossa dura is visibly pulsatile. The EVD is then tunneled out about 2–5 cm away from the surgical field. Following a standard durotomy, cerebellar relaxation was confirmed in cases where the brain has spontaeously fallen off the dura (Figures 2–4 and Supplementary Video S1).
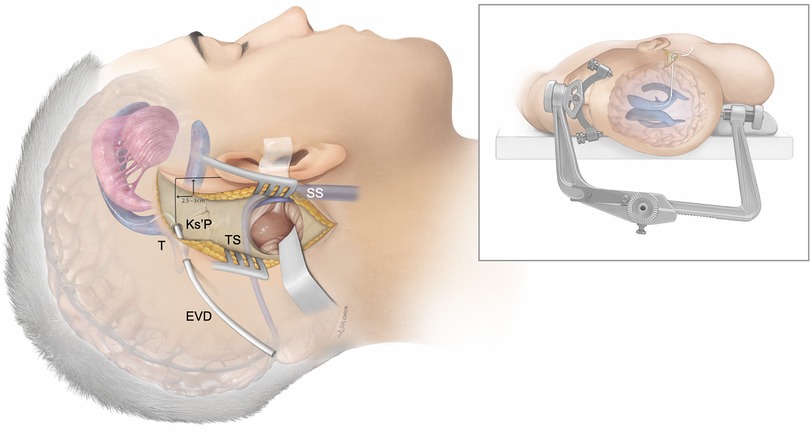
Figure 2. Patient positioning and general concept: Artistic rendition of the supratentorial cerebrospinal fluid diversion method using image-guided trigonal (T) ventriculostomy via the Keen’s point (Ks’P) to achieve cerebellar relaxation during retrosigmoid craniotomy for cerebellopontine angle tumors. The patient is in a supine position and the head fixated in rotation and slightly latero-flexed to the contralateral side using a standard Mayfield clamp. Transverse sinus (TS); sigmoid sinus (SS).
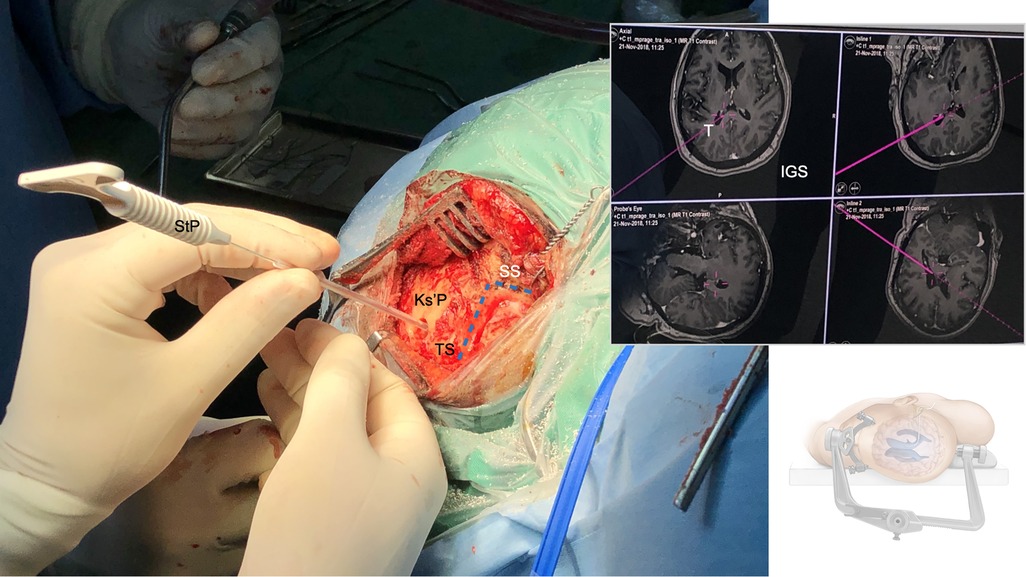
Figure 3. Supratentorial image-guided trigonal ventriculostomy: After myocutaneous skin incision, the course (blue lines) of the transverse (TS) and sigmoid sinus (SS) and the transverso-sigmoid junction (TSJ) are defined superficially along the bony surface. A burr hole is placed with the trajectory aimed towards ipsilateral occipital horns of the lateral ventricle, the dura is opened and a small corticotomy is performed. A sterile image-guidance probe (StP) replacing the supply trocar of the external ventricular drain (EVD) is inserted into the ipsilateral occipital horn of the lateral ventricle (usually with a loss of resistance after 4–5 cm), aimed in a slight cephalic direction, and under constant image-guidance (IGS). The ventricular catheter is further advanced in soft pass technique after positive CSF-outflow out of the catheter (usually up to 6–7 cm).
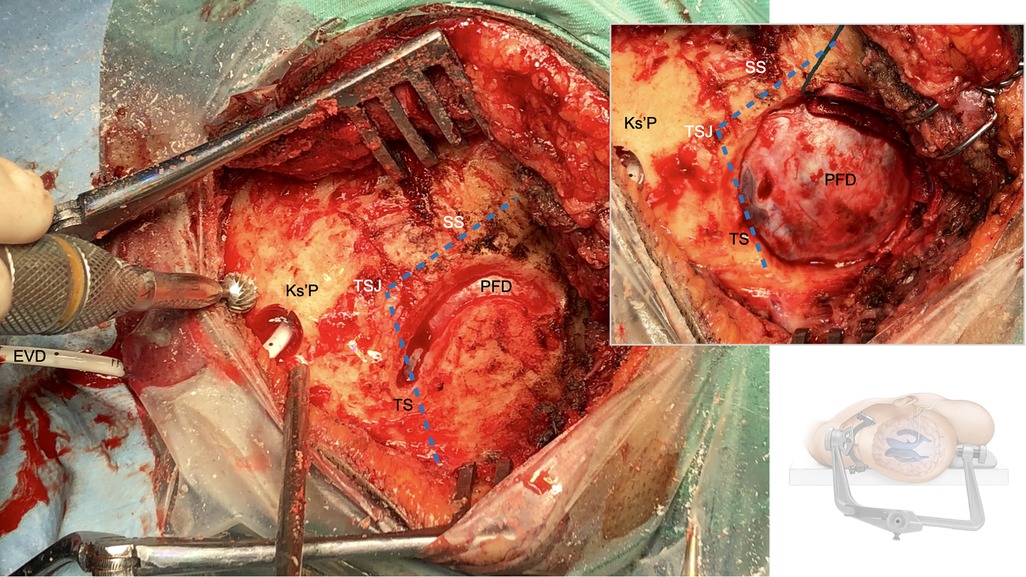
Figure 4. Retrosigmoid craniotomy: The transverse (TS) and the sigmoid (SS) sinus as well as the transverso-sigmoid junction (TSJ) are dlineated and skeletonized. The bone flap is then raised using a craniotome and the posterior fossa dura (PFD) is visualized. The external ventricular drain (EVD) is then tunneled from the Keen's point (Ks'P) out about 5 cm away from the surgical field.
Baseline variables: patient characteristics; initial tumor characteristics [volume (cm3), morphology, pathology]; posterior fossa characteristics (volume [cm3] and tumor/posterior fossa ratio (10); volumetric analysis of the preoperative tumor volume, the posterior fossa and their ratio was performed on preoperative MRI sequences (T1-Gd, T2) using a volume rendering software (BrainLab AG. Release date 2013. iPlan® Cranial, Version 3.0. Feldkirchen, Germany) (11); affection of the fourth ventricle (open, distorted but patent or obstructed) and brainstem (normal, impressed, compressed or dislocated) (12); preoperative tonsillar transforaminal herniation (effacement of the CSF-cisterns surrounding the brainstem, inferior descent of the cerebellar tonsils below the level of the foramen magnum); Surgical outcome variables: relaxation of the surgical field upon durotomy: intraoperative assessment on successful ventricle puncture, CSF-diversion and a pulsatile dura prior durotomy (Supplementary Video S1) without signs of cerebellar contusion, swelling or herniation through the dural incision. To reduce selection bias, a consecutive series of patients irrespective of the underlying pathology was included where the last author (VW) was involved in the surgical procedure; pre- or postoperative hydrocephalus (malresorptive or obstructive) based on clinical and radiological examination, pre- or postoperative permanent CSF-diversion; Radiographic outcome variables: Catheter malposition, defined as grade 1 (ipsilateral intraventricular), grade 2 (contralateral intraventricular), grade 3 (parenchymal or deep/eloquent areas) (13, 14); ventriculostomy-related hemorrhage (VRH); intracerebral hemorrhage; intraventricular hemorrhage; cortical cortical scarring along the ventriculostomy canal; cortical atrophy and scaring caused by hemorrhage; signs of infarction; pre- and postoperative signs of upward transtentorial herniation (1. flattening or reversal of the smile-shaped quadrigeminal cistern; 2. obliteration of the quadrigeminal and superior cerebellar cistern; 3. “spinning top” appearance of midbrain due to bilateral compression of the posterior aspect of the midbrain) (15, 16); posterior cerebral or superior cerebellar artery infarction. Clinical outcome variables: Ventriculostomy-related infections (VRI), ventriculostomy-related seizures and clinically relevant ventriculostomy-related visual field defects in the follow-up examinations (14).
For descriptive analyses, we report medians and ranges for continuous variables, and percentages for categorical variables. The primary endpoint of the study was the detection of any supratentorial hemorrhagic complication associated with the ventriculostomy in postoperative imaging. The secondary endpoints were signs of upward transtentorial herniation and the frequency of postoperative hydrocephalus. To obtain associations between the primary and secondary endpoints, the binary logistic regression model was used. Risk factors that had more than 10% increase or decrease in OR (OR ≤ 0.9 or ≥1.10) and which were considered clinically relevant for the endpoints of interest (Supplementary Table S1) were included in the multivariable model. Confidence intervals were calculated with the profile likelihood method based on the Wald test statistic. Statistical significance was set at p ≤ .05. Statistical analysis was performed using SPSS [IBM SPSS Statistics 28.0.1.0 (142), 2013, New York, USA].
Fifty-two out of n = 62 cases (84%) were eligible for analysis (Figure 1). Mean patient age was 53 (range 11–79 years). The cohort consisted of n = 34/52 (65%) cranial nerve schwannoma's, n = 14/52 (27%) meningioma's, and n = 4/52 (8%) rare pathologies of the CPA. Fourteen out of n = 52 patients (27%) suffered from preoperative obstructive hydrocephalus. 4th ventricle compression or obstruction was present in n = 39/52 (75%), and brainstem compression or dislocation in n = 32/52 (62%) cases. Radiological signs of tonsillar transforaminal herniation were found in n = 7/52 cases (14%), and cerebellar upward transtentorial herniation only in one single case (2%) that met the predefined radiological criteria (Table 1) (15, 16).
Successful ventricle puncture and a pulsatile dura prior durotomy without cerebellar contusion, swelling or herniation through the dural incision was consistently reported except for one case (2%) in a very short necked patient with a medial third petroclival meningioma, where protracted and uncontrollable cerebellar swelling occurred throughout the whole procedure. No failed punctures were recorded, with n = 49/52 (94%) correctly placed EVD's on the first, and n = 3/52 (6%) on the second attempt. The postoperatively closed EVD was removed in n = 40/52 patients (77%) within 48 h after surgery. Twelve out of n = 52 patients (23%) needed prolonged EVD weaning because of postoperative clinical and/or radiological signs of hydrocephalus. Eventually, n = 5/52 patients (10%) needed a permanent CSF-diversion in the longer term (mean 91 days, range 3–246) (Table 2).
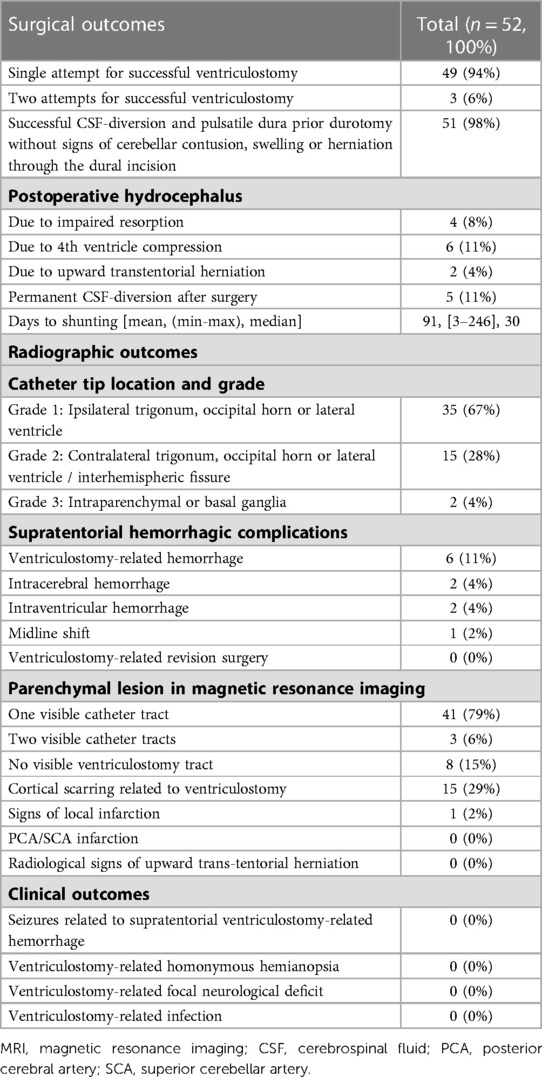
Table 2. Surgical, radiographic and clinical outcome variables of n = 52 patients patients recieving supratentorial cerebrospinal fluid diversion using image-guided trigonal ventriculostomy during retrosigmoid craniotomy for cerebellopontine angle tumors.
No significant signs of cerebellar contusion or swelling were noted in the post-operative scans. In the majority of patients (n = 50, 96%), the catheter tip was intraventricular (grade 1 or 2) except for two cases (4%), where the catheter tip was found intraparenchymal (e.g., basal ganglia) (13, 14). In n = 4/52 patients (8%), evidence of a VRH in association with an intracerebral hemorrhage [n = 2/52 (4%)] or an isolated intraventricular hemorrhage [n = 2/52 (4%)] was found in the postoperative imaging. In most of the patients, a ventriculostomy track was visible in the postoperative follow-up MRI. A supratentorial cortical scar along the ventriculostomy canal was seen in n = 15/52 cases (29%). None of the included patients showed radiological signs of upward transtentorial herniation postoperatively (Table 2 and Figure 5). Uni- and multivariable regression analysis did not detect any association of VRH, neither with one of the relevant baseline variables, postoperative hydrocephalus nor permanent CSF-diversion (Table 3 and Supplementary Table S1).
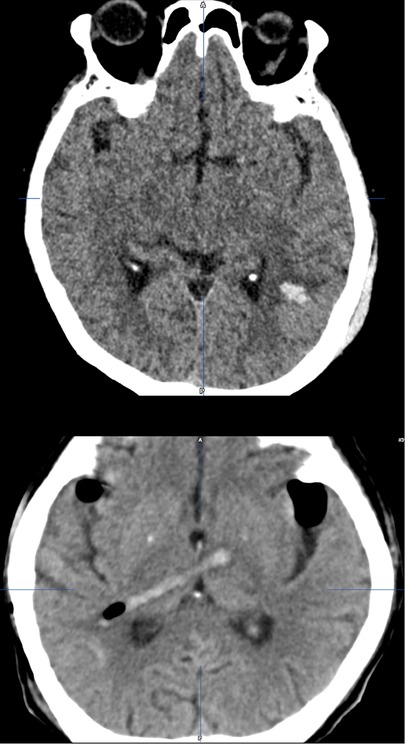
Figure 5. Ventriculostomy-related supratentorial complications: ventriculostomy-related intracerebral hemorrhage in the left parieto-occipital parenchyma within the area of the trigonal ventriculostomy [upper]; ventriculostomy-related hemorrhage along the catheter track; grade 3 malposition (catheter through parenchyma and tip position in the basal ganglia) [lower].
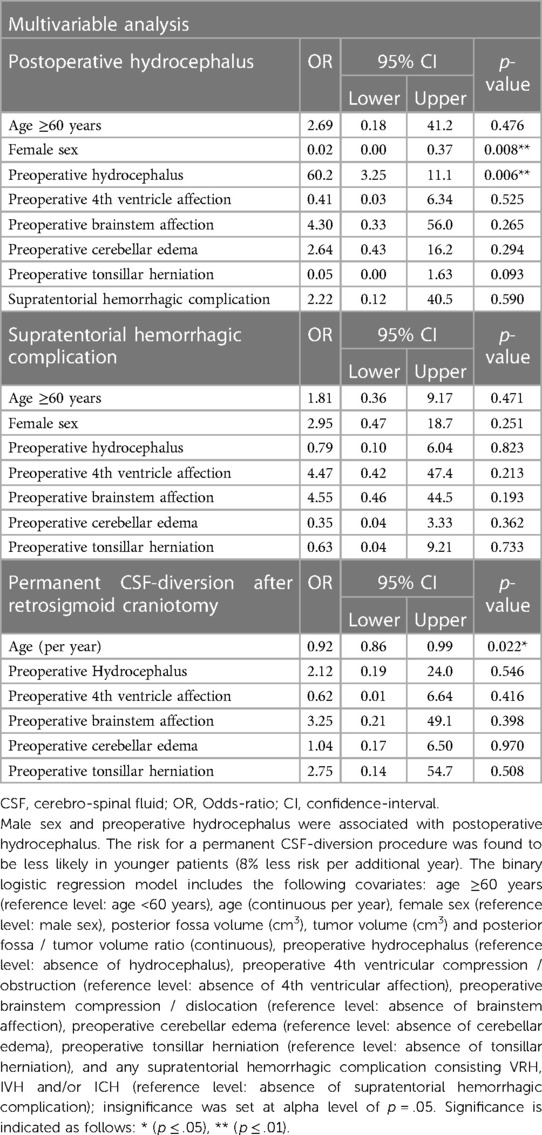
Table 3. Multivariable covariate binary logistic regression analysis on risk-factors associated with postoperative hydrocephalus; any supratentorial hemorrhagic complication (including ventriculostomy-related, intraventricular and intracerebral hemorrhage); and permanent CSF-diversion in n = 52 patients undergoing supratentorial CSF-diversion using image-guided trigonal ventriculostomy during retrosigmoid craniotomy for CPA-tumors.
Neither of the above-described hemorrhagic complications resulted in clinical symptoms including seizures or clinically relevant homonymous hemianopsia related to the cortical bleeding, or focal neurological deficits related to malpositioning of the catheters in the follow-up period (Table 2).
This cohort study reports the elective use of image-guided trigonal ventriculostomy during retrosigmoid craniotomy for CPA-tumors. We found a pulsatile dura prior durotomy without signs of cerebellar contusion, swelling or herniation through the dural opening in n = 51/52 (98%) cases, however, with an inherent risk of supratentorial hemorrhagic complications.
Brain relaxation has been defined by Li et al. as the relationship between the volume of intracranial contents and the capacity of the intracranial space upon durotomy (3). Hence, optimal brain relaxation is achieved when the volume of intracranial contents equals or is less than the capacity of the intracranial space, while inadequate brain relaxation is the result of an intracranial content volume surpassing this capacity (3). Optimal brain relaxation is a routinely assessed aspect of anesthetic care during neurosurgical procedures, leading to improved operating conditions and reduced necessity for brain retraction (3). This is of special importance when operating on large lesions of the CPA, where a higher resistance of the venous outflow and the patient's positioning leads to a raised pressure within the posterior fossa (3, 17). The importance of having cerebellar relaxation upon durotomy and non-traumatic retraction of the cerebellum to expose CPA-tumors cannot be over emphasized. The surgeon routinely evaluates how tight or relaxed the cerebellum is, or if swelling or even cerebellar herniation occurs. Standard maneuvers to soften the cerebellum and obtaining an optimal volume of the intracranial contents in relationship to the capacity of the intracranial space include anesthesiologic interventions and the microsurgical puncture of the horizontal fissure or the lateral cerebellopontine or cerebellomedullary cistern (1–3, 5). Release of CSF from the cisternal spaces is the commonest and most effective surgical maneuver, however, it can remain difficult when there is evident cerebellar compression and edema due to the posterior mass lesion or when hydrocephalus is present at the time of the dural opening. Heavy-handed retraction when attempting to open the basal cisterns can often lead to injury to the cerebellar hemisphere. Retraction and manipulation of the cerebellar cortex, known to be softer than the cerebral cortex, is unavoidable in these cases, and frequently results in retraction injuries or ischemia from compression (1, 3). Lumbar CSF-drains are often placed just prior to posterior fossa craniotomies to improve mean approach time to reach the cisterns, duration of hemostasis and to prevent CSF-leaks (18). However, the rate for moderate to severe complications associated with lumbar CSF-drains is not negligible, intraoperative obstruction occurs, and placement can be difficult in certain cases (19, 20). An expansion of the standard retrosigmoid craniotomy is a well-described option to avoid cerebellar retraction. Removal of the bone covering the sigmoid sinus allows its reflection and increases the angle of exposure to the cerebellopontine cistern by 50% while shortening the distance to the internal acoustic meatus (21). Additionally, a decreased cerebellar retraction pressure of 50%–60% was demonstrated (22). However, exposing and mobilizing the sigmoid sinus can lead to hemorrhagic and thrombotic complications (23). The retrosigmoid craniotomy can also be extended to the foramen magnum with exposure of the cerebellomedullary cistern. This approach overlaps the so called far lateral- or extended suboccipital approach and might be particularly helpful in tumors extending down to the foramen magnum (24). Cerebellar swelling can be prevented not only by extended exposure, but also with the optimal positioning of the patient. Our patients were operated in the supine position. It is likely that the venous pressure is raised in a supine position with the head rotated away, especially when compared to the semi-sitting or sitting position. The latter may be advantageous for larger masses in the posterior fossa, since the venous brain compartment is relieved and the brain is less prone to swelling, however, with the disadvantage of elaborate positioning and the inherent risk of air-embolism (4). Finally, the prophylactic placement of a frontal EVD or preoperative ventriculo-peritoneal shunt has been advocated if there is any concern in posterior fossa tenseness without obvious hydrocephalus, however, with the disadvantage of additional surgery and the potential risk of overtreatment (25).
The Keen's point was first described in 1890 for emergent CSF-diversion during posterior fossa surgery (7). The regular use of a supratentorial ventriculostomy to release CSF from the trigonum and achieve a relaxed field for large posterior fossa lesions was later described by Dandy in 1925 (26). The Keen's point, anatomically referring to the posterior parietal point, thus found common use for the elective proximal placing of ventriculoperitoneal shunt catheters, endoscopic evacuation of spontaneous intracerebral hemorrhage, and ventriculostomy for traumatic brain injury (7, 27–30). However, there is a lack of evidence on its accuracy, safety and radiological outcomes within a contemporary cohort of elective CPA-surgeries (7). The intraoperative integration of a trigonal ventriculostomy during retrosigmoid craniotomies requires a slightly larger incision, which does not seem to have any noticeable cosmetic effect on the patient (Figures 2–4). Both the burr hole for the EVD and the retrosigmoid craniotomy can be neatly contained within a single incision.
Freehand puncture of the ventricular system has inherent limitations in its accuracy and the potential for multiple attempts, thus carrying a higher risk of intracranial injury and hemorrhagic complications (14, 31, 32). Accurate placement after a primary freehand puncture of the frontal lateral ventricle is reported to be between 57%–91% (31–34), with a 10% rate of functional placement in the contralateral lateral ventricle or non-eloquent cortex, and an up to 13% rate of suboptimal placement in the eloquent cortex or nontarget cerebrospinal fluid space, with or without functional drainage (13). The given limitations of freehand trigonal puncture are even more relevant during retrosigmoid approaches, where the patients are positioned supine with the head being rotated away from the side of the lesion, a certain degree of neck flexion and the vertex oriented downwards. This position can be disorientating especially once the patient has been draped (Figures 2–4). Intraoperative image-guidance has significantly improved accuracy of catheter placement of ventricular catheters for temporary and permanent CSF-diversion (35–38). This is confirmed in our cohort study where only 3 out of 49 (6%) patients needed a 2nd attempt (Table 2). However, catheter localization errors can cause significant variations at the target and along the insertion trajectory, caused by anatomical differences between the image and the patient space or transformation errors of the surgical tool (39, 40). Our study confirmed a 28% (n = 15/52) rate of grade 2 (contralateral ventricular) and a 4% (n = 2/52) rate of grade 3 (parenchymal, eloquent) catheter malposition, being in line with a recently published contemporary multicenter register study on EVDs complications (Table 2 and Figure 5) (13, 14). In terms of trigonal ventriculostomy, not inserting the image-guidance probe more than 4–5 cm without a loss of resistance, and not advancing the catheter in soft pass technique more than 6–7 cm if there is no observable CSF-outflow, might reduce the risk of catheter malposition (Figures 2–4 and Supplementary Video S1).
Hemorrhagic and parenchymal injuries from cortical vessel damage are reported from 1% up to 20%–46% after supratentorial ventriculostomies. Known risk-factors include female sex, mean systolic blood pressure, amount of infused mannitol during anesthesia, smaller catheter diameter, antiplatelet intake and bed-side placement (34, 41–46). Hemorrhage is likely caused by cortical vein injury, explaining why neurological symptoms and severe complications by VRH remain extremely rare and are reported in only 0.4% of the affected patients (47, 48). Using trigonal ventriculostomy, VRH was reported in 7.7% of patients with aneurysmal subarachnoid hemorrhage prior to pterional craniotomy (49), and confirmed by our study on patients undergoing retrosigmoid craniotomy for the resection of a CPA-tumor, where 6/52 (11%) of patients were detected to have VRH without neurological symptoms or related complications (Table 2 and Figure 5).
Cerebellar upward transtentorial herniation was only detected in one patient (2%) preoperatively and is a well-known phenomenon in CPA-tumors (15, 50). The reported risk of clinical worsening of patients after ventriculostomy due to accelerated upward transtentorial herniation remains very low (51). In line with these results, none of our included patients showed radiological signs or sequelae of accelerated upward transtentorial herniation postoperatively (Table 2). Hydrocephalus requiring permanent CSF-diversion following CPA-surgery has been reported in up to 5%–7% for vestibular schwannoma (25, 52). Multivariable analysis revealed no association of hemorrhagic events with the 10% permanent CSF diversion rate in our case series (Tables 2, 3).
The current literature and our findings support, that supratentorial CSF-diversion should not be routinely used for smaller lesions, but only considered in large posterior fossa masses to facilitate resection after standard methods of obtaining adequate cerebellar relaxation remain insufficient.
A strength of the present cohort study lies in the homogeneity of the surgical procedure in a well-defined cohort with strict exclusion criteria. Some data were missing and could not be obtained despite all efforts, leading to exclusion of some patients. The safety of trigonal puncture for CSF-diversion is well established, thus, we did not perform systematic postoperative visual field examinations, but focused on clinically relevant homonymous hemianopsia in the postoperative follow-up period. The results are limited by the retrospective nature of the data and the single-center design, and larger studies are needed to confirm our results.
Supratentorial CSF-diversion using image-guided trigonal ventriculostomy during retrosigmoid craniotomy for CPA-tumors efficiently allows to avoid cerebellar contusion, swelling or herniation through the dural incision. However, there is an inherent risk of subclinical supratentorial hemorrhagic complications.
The abstract of this study was presented as a poster at the 4th SFCNS Congress (Swiss Federation of Clinical Neuro-Societies) at the Swiss Tech Convention Center, EPFL (École Polytechnique Fédérale de Lausanne), Lausanne, Switzerland (10/25/2019); as a poster at the Virtual Annual SSNS Meeting (Swiss Society of Neurosurgery), Switzerland (09/17/2022); and as a scientific short-talk at the 14th ESBS Congress (European Skull Base Society Meeting) in Riva del Garda, Italy (04/22/2022).
The raw data supporting the conclusions of this article will be made available by the authors on reasonable request, without undue reservation.
This study involving human participants was reviewed and approved by the ethical committee of the University of Malaya, Kuala Lumpur, Malaysia (MREC ID NO: 2017123-4864). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Study conception and design were performed by MR and VW. Material preparation, data collection and analysis were performed by MR and NE. The first draft of the manuscript was written by MR and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Martin Allgöwer Foundation, the Departments of Surgery and Neurosurgery of the University Hospital Basel (Basel, Switzerland), and the Gottfried and Julia Bangerter-Rhyner Foundation (Bern, Switzerland). The University of Basel (Basel, Switzerland) and the University of Malaya Medical (UMMC) and Specialist (UMSC) Center (Kuala Lumpur, Malaysia) supported this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1198837/full#supplementary-material.
1. Jannetta PJ, McLaughlin MR, Casey KF. Technique of microvascular decompression. Technical note. Neurosurg Focus. (2005) 18(5):E5. PMID: 1591328115913281
2. Park CK, Lee SH, Rhee BA, Choi SK. Puncture of cerebellar horizontal fissure for retrosigmoid approach: a prospective and quantitative analysis. Oper Neurosurg (Hagerstown). (2017) 13(6):689–92. doi: 10.1093/ons/opx048
3. Li J, Gelb AW, Flexman AM, Ji F, Meng L. Definition, evaluation, and management of brain relaxation during craniotomy. Br J Anaesth. (2016) 116(6):759–69. doi: 10.1093/bja/aew096
4. Tatagiba M, Roser F, Schuhmann MU, Ebner FH. Vestibular schwannoma surgery via the retrosigmoid transmeatal approach. Acta Neurochir (Wien). (2014) 156(2):421–5; discussion 5. doi: 10.1007/s00701-013-1915-6
5. Mostafa BE, El Sharnoubi M, Youssef AM. The keyhole retrosigmoid approach to the cerebello-pontine angle: indications, technical modifications, and results. Skull Base. (2008) 18(6):371–6. doi: 10.1055/s-0028-1087220
6. Li Z, Lan Q. Retrosigmoid keyhole approach to the posterior cranial fossa: an anatomical and clinical study. Eur Surg Res. (2010) 44(1):56–63. doi: 10.1159/000264636
7. Morone PJ, Dewan MC, Zuckerman SL, Tubbs RS, Singer RJ. Craniometrics and ventricular access: a review of kocher’s, kaufman’s, paine’s, menovksy’s, tubbs’, keen's, frazier’s, dandy’s, and sanchez’s points. Oper Neurosurg (Hagerstown). (2020) 18(5):461–9. doi: 10.1093/ons/opz194
8. Roethlisberger M, Prepageran N, Waran V. Chapter 67. Retrosigmoid craniotomy with stereotactic supratentorial cerebrospinal fluid diversion. In: Narayanan J, Prepageran N, editors. Atlas of 360 degree skull base surgery. 1st ed. Uttar Pradesh, India: Thieme and Scientific Publishers Private Limited (2021). p. 884–8.
9. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (Strobe) Statement: Guidelines for Reporting Observational Studies. Lancet. (2007) 370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X
10. Macielak RJ, Harris MS, Kirsch CF, Prevedello LM, Adunka OF. Influence of posterior fossa volume on clinical outcomes after vestibular schwannoma resection. Otol Neurotol. (2016) 37(8):1155–61. doi: 10.1097/MAO.0000000000001128
11. Vurdem UE, Acer N, Ertekin T, Savranlar A, Inci MF. Analysis of the volumes of the posterior cranial fossa, cerebellum, and herniated tonsils using the stereological methods in patients with Chiari type I malformation. ScientificWorldJournal. (2012) 2012:616934. doi: 10.1100/2012/616934
12. Koos WT, Day JD, Matula C, Levy DI. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg. (1998) 88(3):506–12. doi: 10.3171/jns.1998.88.3.0506
13. Kakarla UK, Kim LJ, Chang SW, Theodore N, Spetzler RF. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery. (2008) 63(1 Suppl 1):ONS162-6; discussion ONS6-7. doi: 10.1227/01.neu.0000335031.23521.d0
14. Dakson A, Kameda-Smith M, Staudt MD, Lavergne P, Makarenko S, Eagles ME, et al. A nationwide prospective multicenter study of external ventricular drainage: accuracy, safety, and related complications. J Neurosurg. (2021):1–9. doi: 10.3171/2021.7.JNS21421. [Epub ahead of print]34826821
15. Laine FJ, Shedden AI, Dunn MM, Ghatak NR. Acquired intracranial herniations: MR imaging findings. AJR Am J Roentgenol. (1995) 165(4):967–73. doi: 10.2214/ajr.165.4.7677003
16. Osborn AG, Heaston DK, Wing SD. Diagnosis of ascending transtentorial herniation by cranial computed tomography. AJR Am J Roentgenol. (1978) 130(4):755–60. doi: 10.2214/ajr.130.4.755
17. Whittle IR, Viswanathan R. Acute intraoperative brain herniation during elective neurosurgery: pathophysiology and management considerations. J Neurol Neurosurg Psychiatry. (1996) 61(6):584–90. doi: 10.1136/jnnp.61.6.584
18. Hamasaki T, Takezaki T, Yano S, Ueda R, Mukasa A. Efficacy of lumbar spinal drainage for straightforward approach in reoperation via lateral suboccipital retrosigmoid craniotomy. Interdisciplinary Neurosurgery. (2021) 23:100915. doi: 10.1016/j.inat.2020.100915
19. Guo X, Zhu Y, Hong Y. Efficacy and safety of intraoperative lumbar drain in endoscopic skull base tumor resection: a meta-analysis. Front Oncol. (2020) 10:606. doi: 10.3389/fonc.2020.00606
20. Rong LQ, Kamel MK, Rahouma M, White RS, Lichtman AD, Pryor KO, et al. Cerebrospinal-fluid drain-related complications in patients undergoing open and endovascular repairs of thoracic and thoraco-abdominal aortic pathologies: a systematic review and meta-analysis. Br J Anaesth. (2018) 120(5):904–13. doi: 10.1016/j.bja.2017.12.045
21. Basma J, Anagnostopoulos C, Tudose A, Harty M, Michael LM 2nd, Teo M, et al. History, variations, and extensions of the retrosigmoid approach: anatomical and literature review. J Neurol Surg B Skull Base. (2022) 83(Suppl 2):e324–35. doi: 10.1055/s-0041-1729177
22. Liebelt BD, Huang M, Britz GW. A comparison of cerebellar retraction pressures in posterior Fossa surgery: extended retrosigmoid versus traditional retrosigmoid approach. World Neurosurg. (2018) 113:e88–e92. doi: 10.1016/j.wneu.2018.01.173
23. Mattei TA, Ramina R. Critical remarks on the proposed “extended retrosigmoid approach”. Neurosurg Rev. (2011) 34(4):527–30. doi: 10.1007/s10143-011-0339-0
24. Cui H, Zhou CF, Bao YH, Wang MS, Wang Y. Extended suboccipital retrosigmoid surgical approach is effective for resection of petrous apex meningioma. J Craniofac Surg. (2016) 27(5):e429–33. doi: 10.1097/SCS.0000000000002705
25. di Russo P, Fava A, Vandenbulcke A, Miyakoshi A, Kohno M, Evins AI, et al. Characteristics and management of hydrocephalus associated with vestibular schwannomas: a systematic review. Neurosurg Rev. (2021) 44(2):687–698. doi: 10.1007/s10143-020-01287-2. 32266553
26. Dandy WE. Contributions to brain surgery: A. Removal of certain deep-seated brain tumors B. Intracranial approach with concealed incisions. Ann Surg. (1925) 82(4):513–25. doi: 10.1097/00000658-192510010-00001
27. Junaid M, Ahmed M, Rashid MU. An experience with ventriculoperitoneal shunting at keen’s point for hydrocephalus. Pak J Med Sci. (2018) 34(3):691–5. doi: 10.12669/pjms.343.14081
28. Huang KT, Chavakula V, Gormley WB. Keen’s point for external ventricular drainage in traumatic brain injury patients: an uncommon indication for an old technique. World Neurosurg. (2017) 102:694 e1–e7. doi: 10.1016/j.wneu.2017.03.145
29. Wang WH, Hung YC, Hsu SP, Lin CF, Chen HH, Shih YH, et al. Endoscopic hematoma evacuation in patients with spontaneous supratentorial intracerebral hemorrhage. J Chin Med Assoc. (2015) 78(2):101–7. doi: 10.1016/j.jcma.2014.08.013
30. Chen CC, Lin HL, Cho DY. Endoscopic surgery for thalamic hemorrhage: a technical note. Surg Neurol. (2007) 68(4):438–42; discussion 42. doi: 10.1016/j.surneu.2006.11.054
31. Thomale UW, Schaumann A, Stockhammer F, Giese H, Schuster D, Kastner S, et al. GAVCA study: randomized, multicenter trial to evaluate the quality of ventricular catheter placement with a mobile health assisted guidance technique. Neurosurgery. (2018) 83(2):252–62. doi: 10.1093/neuros/nyx420
32. AlAzri A, Mok K, Chankowsky J, Mullah M, Marcoux J. Placement accuracy of external ventricular drain when comparing freehand insertion to neuronavigation guidance in severe traumatic brain injury. Acta Neurochir (Wien). (2017) 159(8):1399–411. doi: 10.1007/s00701-017-3201-5
33. Enriquez-Marulanda A, Ascanio LC, Salem MM, Maragkos GA, Jhun R, Alturki AY, et al. Accuracy and safety of external ventricular drain placement by physician assistants and nurse practitioners in aneurysmal acute subarachnoid hemorrhage. Neurocrit Care. (2018) 29(3):435–42. doi: 10.1007/s12028-018-0556-2
34. Ellens NR, Fischer DL, Meldau JE, Schroeder BA, Patra SE. External ventricular drain placement accuracy and safety when done by midlevel practitioners. Neurosurgery. (2019) 84(1):235–41. doi: 10.1093/neuros/nyy090
35. McLean AL, Jamjoom AAB, Poon MTC, Wang D, Phang I, Okasha M, et al. Utility of image-guided external ventriculostomy: analysis of contemporary practice in the United Kingdom and Ireland. J Neurosurg. (2021):1–9. doi: 10.3171/2020.8.JNS20321. [Epub ahead of print]
36. Xu LW, Sussman ES, Li G. Frameless, electromagnetic image-guided ventriculostomy for ventriculoperitoneal shunt and Ommaya reservoir placement. Clin Neurol Neurosurg. (2016) 147:46–52. doi: 10.1016/j.clineuro.2016.05.024
37. Nesvick CL, Khan NR, Mehta GU, Klimo P Jr. Image guidance in ventricular cerebrospinal fluid shunt catheter placement: a systematic review and meta-analysis. Neurosurgery. (2015) 77(3):321–31; discussion 31. doi: 10.1227/NEU.0000000000000849
38. Azeem SS, Origitano TC. Ventricular catheter placement with a frameless neuronavigational system: a 1-year experience. Neurosurgery. (2007) 60(4 Suppl 2):243–7; discussion 7–8. doi: 10.1227/01.NEU.0000255387.03088.53
39. Shamir RR, Joskowicz L, Spektor S, Shoshan Y. Target and trajectory clinical application accuracy in neuronavigation. Neurosurgery. (2011) 68(1 Suppl Operative):95–101; discussion -2. doi: 10.1227/NEU.0b013e31820828d9
40. Wang MN, Song ZJ. Classification and analysis of the errors in neuronavigation. Neurosurgery. (2011) 68(4):1131–43; discussion 43. doi: 10.1227/NEU.0b013e318209cc45
41. Moon HH, Kim JH, Kang HI, Moon BG, Lee SJ, Kim JS. Brain injuries during intraoperative ventriculostomy in the aneurysmal subarachnoid hemorrhage patients. J Korean Neurosurg Soc. (2009) 46(3):215–20. doi: 10.3340/jkns.2009.46.3.215
42. Rowe AS, Rinehart DR, Lezatte S, Langdon JR. Intracerebral hemorrhage after external ventricular drain placement: an evaluation of risk factors for post-procedural hemorrhagic complications. BMC Neurol. (2018) 18(1):22. doi: 10.1186/s12883-018-1030-7
43. Miller C, Tummala RP. Risk factors for hemorrhage associated with external ventricular drain placement and removal. J Neurosurg. (2017) 126(1):289–97. doi: 10.3171/2015.12.JNS152341
44. Sussman ES, Kellner CP, Nelson E, McDowell MM, Bruce SS, Bruce RA, et al. Hemorrhagic complications of ventriculostomy: incidence and predictors in patients with intracerebral hemorrhage. J Neurosurg. (2014) 120(4):931–6. doi: 10.3171/2013.12.JNS131685
45. Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. (2009) 110(5):1021–5. doi: 10.3171/2008.9.JNS17661
46. Ko JK, Cha SH, Choi BK, Lee JI, Yun EY, Choi CH. Hemorrhage rates associated with two methods of ventriculostomy: external ventricular drainage vs. ventriculoperitoneal shunt procedure. Neurol Med Chir (Tokyo). (2014) 54(7):545–51. doi: 10.2176/nmc.oa.2013-0178
47. Robertson FC, Abd-El-Barr MM, Mukundan S Jr, Gormley WB. Ventriculostomy-associated hemorrhage: a risk assessment by radiographic simulation. J Neurosurg. (2017) 127(3):532–6. doi: 10.3171/2016.8.JNS16538
48. Roa JA, Fakih R, Zanaty M, Pazour A, Howard MA, Hasan DM, et al. Quantitative assessment of ventriculostomy-related hemorrhage: a volume-based classification system to predict new neurological symptoms. Oper Neurosurg (Hagerstown). (2021) 20(2):198–205. doi: 10.1093/ons/opaa319
49. Kim JH, Kang HI. Intraoperative ventriculostomy using K point in surgical management of aneurysmal subarachnoid hemorrhage. World Neurosurg. (2019) 122:e248–52. doi: 10.1016/j.wneu.2018.09.228
50. Ecker A. Upward transtentorial herniation of the brain stem and cerebellum due to tumor of the posterior fossa with special note on tumors of the acoustic nerve. J Neurosurg. (1948) 5(1):51–61. doi: 10.3171/jns.1948.5.1.0051
51. Braksick SA, Himes BT, Snyder K, Van Gompel JJ, Fugate JE, Rabinstein AA. Ventriculostomy and risk of upward herniation in patients with obstructive hydrocephalus from posterior Fossa mass lesions. Neurocrit Care. (2018) 28(3):338–43. doi: 10.1007/s12028-017-0487-3
Keywords: ventriculostomy, cerebrospinal fluid diversion, trigonal, retrosigmoid craniotomy, cerebellopontine angle, image guided surgery, hemorrhage, upward transtentorial herniation
Citation: Roethlisberger M, Eberhard NE, Rychen J, Al-Zahid S, Jayapalan RR, Zweifel C, Karuppiah R and Waran V (2023) Supratentorial cerebrospinal fluid diversion using image-guided trigonal ventriculostomy during retrosigmoid craniotomy for cerebellopontine angle tumors. Front. Surg. 10:1198837. doi: 10.3389/fsurg.2023.1198837
Received: 2 April 2023; Accepted: 24 April 2023;
Published: 23 May 2023.
Edited by:
Wai S. Poon, The Chinese University of Hong Kong, ChinaReviewed by:
Zuohong Zhang, The University of Hong Kong, China© 2023 Roethlisberger, Eberhard, Rychen, Al-Zahid, Jayapalan, Zweifel, Karuppiah and Waran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Rychen Sm9uYXRoYW4uUnljaGVuQHVzYi5jaA== Vicknes Waran Y212d2FyYW5AZ21haWwuY29t
†These authors have contributed equally to this work
Abbreviations CPA, Cerebellopontine Angle; CSF, Cerebrospinal Fluid; EVD, External Ventricular Drain; CT, Computed Tomography; MRIv Magnetic Resonance Imaging; IGS, Image Guided Surgery; STROBE, STrengthening the Reporting of OBservational studies in Epidemiology; VRH, Ventriculostomy-related Hemorrhage; OR, Odds Ratio; CI, Confidence Interval.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.