
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg. , 26 June 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1186971
This article is part of the Research Topic Body Composition and Malnutrition in Transplant Oncology and in Hepato-Pancreato-Biliary Cancers: Potentially Modifiable Risk-Factors with Major Clinical Impact View all 7 articles
 Svenja Sliwinski1,†
Svenja Sliwinski1,† Elisabeth Werneburg1,†
Elisabeth Werneburg1,† Sara Fatima Faqar-Uz-Zaman1
Sara Fatima Faqar-Uz-Zaman1 Charlotte Detemble1
Charlotte Detemble1 Julia Dreilich2
Julia Dreilich2 Lisa Mohr2
Lisa Mohr2 Dora Zmuc1,2
Dora Zmuc1,2 Katharina Beyer3,4
Katharina Beyer3,4 Wolf O. Bechstein1
Wolf O. Bechstein1 Florian Herrle4,5
Florian Herrle4,5 Patrizia Malkomes1
Patrizia Malkomes1 Christoph Reissfelder4,6
Christoph Reissfelder4,6 Joerg P. Ritz4,7
Joerg P. Ritz4,7 Tim Vilz4,8
Tim Vilz4,8 Johannes Fleckenstein2,9
Johannes Fleckenstein2,9 Andreas A. Schnitzbauer1,4*
Andreas A. Schnitzbauer1,4*
Prehabilitation is a multimodal concept to improve functional capability prior to surgery, so that the patients’ resilience is strengthened to withstand any peri- and postoperative comorbidity. It covers physical activities, nutrition, and psychosocial wellbeing. The literature is heterogeneous in outcomes and definitions. In this scoping review, class 1 and 2 evidence was included to identify seven main aspects of prehabilitation for the treatment pathway: (i) risk assessment, (ii) FITT (frequency, interventions, time, type of exercise) principles of prehabilitation exercise, (iii) outcome measures, (iv) nutrition, (v) patient blood management, (vi) mental wellbeing, and (vii) economic potential. Recommendations include the risk of tumor progression due to delay of surgery. Patients undergoing prehabilitation should perceive risk assessment by structured, quantifiable, and validated tools like Risk Analysis Index, Charlson Comorbidity Index (CCI), American Society of Anesthesiology Score, or Eastern Co-operative Oncology Group scoring. Assessments should be repeated to quantify its effects. The most common types of exercise include breathing exercises and moderate- to high-intensity interval protocols. The program should have a duration of 3–6 weeks with 3–4 exercises per week that take 30–60 min. The 6-Minute Walking Testing is a valid and resource-saving tool to assess changes in aerobic capacity. Long-term assessment should include standardized outcome measurements (overall survival, 90-day survival, Dindo–Clavien/CCI®) to monitor the potential of up to 50% less morbidity. Finally, individual cost-revenue assessment can help assess health economics, confirming the hypothetic saving of $8 for treatment for $1 spent for prehabilitation. These recommendations should serve as a toolbox to generate hypotheses, discussion, and systematic approaches to develop clinical prehabilitation standards.
Major surgeries are one of the top three reasons of deaths in hospitals (1). The World Health Organization analyzed that $1 out of $7 is spent for the treatment of complications in hospitals worldwide (2, 3). Patients undergoing major surgeries have an increased risk of experiencing minor and major complications, which may impact quality of life and lead to short-term failure to rescue and increased mortality (4, 5). Data on the prevalence of such surgical complications range between 15% and 50%, which covers uncertainty unless systematically assessed by the current gold standard, which is the Dindo–Clavien classification of surgical complications or its evolution to the comprehensive complication index (6–10). Taking these scientific aspects into account, major surgery may be considered one of the greatest contributors of clinical deaths despite its lifesaving and curative role being an early therapeutic option in most solid cancers. As the Western societies are getting older, and the average age to get diagnosed with a malignant tumor is currently 65, it is crucial to address a clear strategy to identify and improve modifiable factors before surgery and thus mitigate the risk of experiencing an adverse course after major surgery (11).
Clinical treatment strategies have been constantly improved over the last 20 years to increase the safety and quality surgical treatments have been implementing, e.g., the use of checklists, interdisciplinary board decision-making, treatment at specialized centers, a refinement of neoadjuvant and adjuvant treatment strategies, enhanced recovery after surgery, and minimally invasive techniques (2, 12, 13). One field that is gaining importance and popularity recently is prehabilitation. This means the strategy of adequately preparing the patient for a surgical procedure by identifying and improving modifiable factors in advance of the surgical procedure. In general, prehabilitation is based on three pillars that are physical activity, healthy nutrition, and psychosocial wellbeing. Patient–blood management, a bundle strategy to correct anemia before surgeries, should also be mentioned in this context and contributes to better outcomes after oncologic surgeries (14–17).
To date, there is debate among healthcare professionals as to which systematic and structured assessments are most appropriate for patients to assess their individual risk profile, what kind of prehabilitation modalities should be used, and how long patients need to exercise to see an effect without increasing the risk for progression of the underlying disease and thus worsening prognosis from a potentially beneficial intervention.
Strikingly, prehabilitation is only marginally implemented in clinical settings and still lacks reimbursement or awareness by the stakeholders. The required setup in a hospital is cost intensive as, e.g., dedicated and mostly non-existent personnel are required. The evaluation of cost-effectiveness is the subject of current research, but first theoretical algorithms estimate a small return on investment. In addition, approaches are faced with individual barriers, e.g., patients need to be motivated to come to hospitals/gyms for multiple appointments prior to surgery (18).
In summary, the current overview of studies and recommendations suggests a variety of approaches due to the heterogeneity of provided data. Yet, there is no general recommendation (“toolbox”) mapping the best of prehabilitation concepts. The aim of this review is to systematically screen current literature to identify the best available evidence to obtain structured and useful assessment tools to measure patient risk before surgeries and prehabilitation. In addition, we aim to identify the most promising interventions for the single elements of prehabilitation in addition to the best dose relationships, i.e., the duration of exercise interventions, all this considering the risk–benefit of delaying oncologic surgeries and identifying strategies for a reasonable and broad penetration and traction of measurable prehabilitation in clinical or remote settings. The targeted groups of interest are all adult surgical oncologic indications in abdominal, thoracic, urologic, and gynecologic surgeries.
The systematic scoping review was developed using guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (19).
The electronic databases PubMed, Google Scholar, and AWMF Leitlinienportal (Official German Medical Guidelines) were systematically queried to find applicable articles published between the years 1991 and 2021. The search was structured in three relevant blocks using the following terms, PICO-searches and MeSH terms where applicable: risk assessment (RAI-C scoring, Charlson Comorbidity Index, Patient Blood Management, QLQ-C-30 questionnaire, Timed-Up and Go Testing, 6 Minute walking test, Dindo-Clavien scoring, 90-day surgical mortality (specific search after deciding for the tools that will be used from clinical use), prehabilitation (PICO search: P(surgery) I(prehabilitation) O(survival) to obtain relevant results; (((((((surgery) AND (prehabilitation)) AND (complications)) AND (survival)) NOT (cardiac surgery)) NOT (orthopedic surgery)) NOT (emergency). MESH: (((((“surgery”[MeSH Subheading] OR “surgery”[All Fields] OR “surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “operative surgical procedures”[All Fields] OR “general surgery”[MeSH Terms] OR (“general”[All Fields] AND “surgery”[All Fields]) OR “general surgery”[All Fields] OR “surgery s”[All Fields] OR “surgerys”[All Fields] OR “surgeries”[All Fields]) AND (“prehabilitative”[All Fields] OR “preoperative exercise”[MeSH Terms] OR (“preoperative”[All Fields] AND “exercise”[All Fields]) OR “preoperative exercise”[All Fields] OR “prehabilitation”[All Fields]) AND (“complicances”[All Fields] OR “complicate”[All Fields] OR “complicated”[All Fields] OR “complicates”[All Fields] OR “complicating”[All Fields] OR “complication”[All Fields] OR “complication s”[All Fields] OR “complications”[MeSH Subheading] OR “complications”[All Fields]) AND (“mortality”[MeSH Subheading] OR “mortality”[All Fields] OR “survival”[All Fields] OR “survival”[MeSH Terms] OR “survivability”[All Fields] OR “survivable”[All Fields] OR “survivals”[All Fields] OR “survive”[All Fields] OR “survived”[All Fields] OR “survives”[All Fields] OR “surviving”[All Fields])) NOT (“thoracic surgery”[MeSH Terms] OR (“thoracic”[All Fields] AND “surgery”[All Fields]) OR “thoracic surgery”[All Fields] OR (“cardiac”[All Fields] AND “surgery”[All Fields]) OR “cardiac surgery”[All Fields] OR “cardiac surgical procedures”[MeSH Terms] OR (“cardiac”[All Fields] AND “surgical”[All Fields] AND “procedures”[All Fields]) OR “cardiac surgical procedures”[All Fields] OR (“cardiac”[All Fields] AND “surgery”[All Fields]))) NOT (“orthopaedic surgery”[All Fields] OR “orthopedics”[MeSH Terms] OR “orthopedics”[All Fields] OR (“orthopedic”[All Fields] AND “surgery”[All Fields]) OR “orthopedic surgery”[All Fields])) NOT (“emerge”[All Fields] OR “emerged”[All Fields] OR “emergence”[All Fields] OR “emergences”[All Fields] OR “emergencies”[MeSH Terms] OR “emergencies”[All Fields] OR “emergency”[All Fields] OR “emergent”[All Fields] OR “emergently”[All Fields] OR “emergents”[All Fields] OR “emerges”[All Fields] OR “emerging”[All Fields])) AND (y_5[Filter])), nutrition (Surgery and immunonutrition, AWMF screening, PICO search: P(surgery) I(immunonutrition) O(survival)), delay of surgery (delaying cancer surgery and mortality). Inclusion criteria were clinical trials that published data on prehabilitation in adult oncologic surgery of the abdomen, thoracic, gynecologic oncologic surgery, and urology. Risk assessment data had to include outcome measurement (survival and complication rates). Nutrition data should comprise data from clinical trials, randomized controlled trials, or systematic reviews and meta-analyses. Delaying surgery focused on oncologic treatments as outlined for prehabilitation above. Exclusion criteria were indications other than those mentioned above, pediatric surgeries, cardiovascular surgeries, trauma surgeries, missing data on exercising and assessment modalities, and missing outcome data.
Studies were included when they analyzed risk assessment scores and risk factors. They were included when they used either exercise tests or nutritional therapy, or psychoeducation as a prehabilitation modality and assessed the QoL (Quality of life), mortality, costs, or length of hospital stay postoperatively. Studies for which the full text was not available were excluded, as were studies of patients undergoing orthopedic, pediatric, trauma, and cardiac surgery and opinion, statement, position papers, letters to the editor, guidelines, symposium protocols, study protocols, advisory reports, manuals, commentaries, or recommendation papers. Case reports, opinion papers, animal studies, and studies other than the English language were also excluded. After the removal of double hits from the search results, three reviewers (EW, SS, and AAS) independently screened and selected potentially eligible studies. After consensus was reached in this initial selection procedure, the reviewers independently reviewed the full text of the selected studies to determine the final suitability for inclusion based on the established inclusion criteria. To include additional relevant studies, after full-text assessment, the references sections of papers were screened, and relevant papers were chosen based on the above-described criteria.
Risk of bias is regarded high due to the high number of lower level of evidence, especially large cohorts (20).
Based on the evidence found in the literature, statements were derived from the findings. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) for the clinical guidelines pathway was followed, which is shown in Table 1 (21).
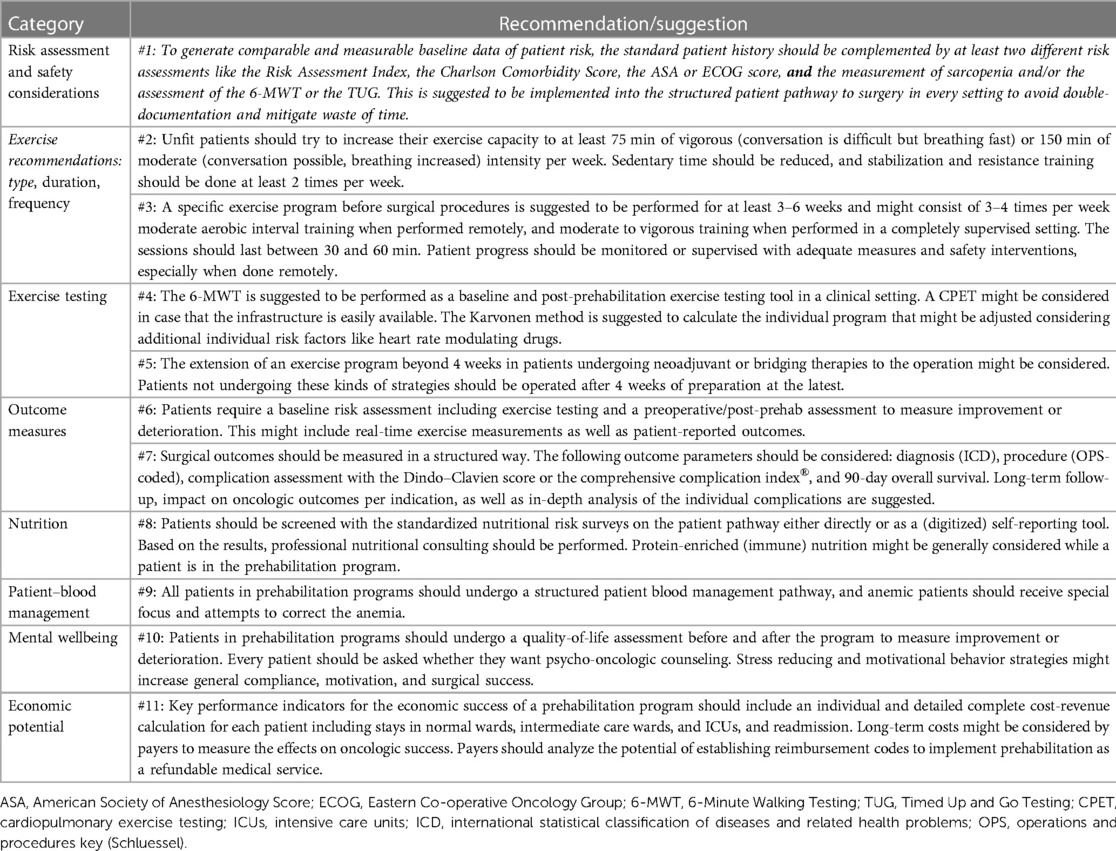
Table 1. Recommendations and suggestions for use, further exploration, and generation of hypotheses to strengthen prehabilitation as a standard of care and generate the political will to reimburse the medical intervention.
The literature search report identified 1,559 manuscripts of which 134 were doubles; 1,132 were not suitable after screening the titles and abstracts and another 195 were not suitable because of the defined criteria outlined above. The selection process is outlined in the PRISMA flowchart in Figure 1 (19). A total of 93 studies were identified to be suitable for the review.
The Oxford levels for evidence-based medicine were applied to classify the quality of studies identified. From 93 identified studies, 13 were defined as class 1 evidence delivering solid meta-analysis of high-quality trials; 41 trials were defined as class 2 delivering data from randomized controlled trials or large cohorts with a dramatic effect; 23 trials were class 3 consisting of a cohort and observational studies; 15 were class 4 cohort, observational, and case (control) series with low patient numbers; and finally, 1 publication was classified as class 5 evidence as it depicted a standpoint about the topic. Bias was high in the screened trials, as most trials were simply not randomized, blinded, or dropouts, and withdrawals were not described adequately.
Finally, only class 1 and 2 articles with one selected class 3 evidence article (in total n = 55) (class 3 selected because of high-quality economic work-up) were considered for a deeper analysis and as a valid source to build recommendations and suggestions about the investigated topics. Notably, one article could cover several of the following topics so the following numbers don’t add up to the total.
The topics covered by this review were “risk assessment tools” (k = 13; n = 3,686,465), “prehabilitation, exercise testing, and physical activity” (k = 23; n = 352,898), “delay of surgery and risk of oncologic progression” (k = 10; n = 1,846,995), “nutrition” (k = 20; n = 565,843), “patient blood management” (k = 2; n = 3,008), mental wellbeing (k = 10; n = 8,100), and “economics and prehabilitation” (k = 4; n = 290,522). A closer description can be found in Table 2.
We identified activity of daily living (ADL), Age, Risk Analysis Index (RAI score), Charlson Comorbidity Index (CCI), Clinical frailty scale (CSHA), Sarcopenia, (modified) frailty index (mFI), American Society of Anesthesiology Score (ASA), Timed Up and Go Testing (TUG), 6-Minute Walking Testing (6-MWT), Eastern Co-operative Oncology Group (ECOG), and Psoas muscle Index being the most frequently used risk assessment tools in the published literature. There is no general international agreement or highly evident recommendation, on which scores should be used to identify the individual risk profile of (major) surgical patients. Thirteen of the 26 analyses have an Oxford level of evidence of 2 or better and accumulate almost 3.7 Mio. patients analyzed (5, 24, 25, 42, 44, 45, 50, 54, 59, 63, 64, 70, 71). Six include the ADL, 10 evaluate age as a risk factor, 5 studies highlight the RAI score, 4 picked up the CCI, 5 used ASA, 4 assessed the 6-MWT, and 2 measured sarcopenia by muscle density in imaging (multiple answers).
The RAI score consists of age, clinical risk factors, and a modified ADL checklist that generates a scoring that was validated in multimillion patients in the United States. It has shown a highly significant association between the risk groups in various studies and is an excellent marker for frailty. Moreover, it was validated as a highly correlative marker for failure to rescue after major surgical procedures with rising scores (5, 24, 45, 63, 64). Similar results were obtained by the ASA and the CCI score, which, however, do consider fewer variables than the RAI score that can be assessed within 2–5 min and delivers a highly valuable clinical assessment.
The literature research revealed 24 out of 55 studies with Oxford evidence levels of 1 and 2 focusing on prehabilitation prior to intra-abdominal or thoracic surgery predominantly in the oncologic area and including 353,014 patients for analysis (27, 29–32, 34, 36–39, 43, 48, 49, 51, 52, 55, 56, 58, 60, 65, 68, 69, 72). One guideline was related to the general population with recommendations for physical activity (22).
A total of 18 (78%) studies investigated different types of exercise interventions and exercise testing. Thirteen studies applied inspiratory muscle training (IMT) to prepare patients for surgery (30, 32, 36, 43, 48, 49, 52, 55, 58, 60, 68, 69, 72). Five studies reported high intense interval training (HIIT) as modality (27, 29, 34, 60, 68), and in five studies, the intensity was controlled with various target measurements of intensity (27, 29, 55, 56, 72). Two interventions were performed as purely home based (55, 68); all other trials were carried out as hybrid trials, and only five trials offered individualized exercising programs tailored to each patient (27, 29, 49, 56, 68). Most trials were supervised for at least the first session or for the HIIT exercises by a physician, physiotherapist, or other qualified medical staff.
Frequency and duration differed between the evaluated clinical trials. There was a range between 3 times per day to 5 times per week using the time period of 1–6 weeks before the surgical procedure. The individual session lasted between 20 and 75 min. The most popular load was moderate- to high-intensity interval training [common protocols: (i) 2 min high intensity vs. 3 min low intensity, (ii) 15 s of very high intensity vs. 15 s of passive rest for 4 min and additional 4 min rest, or (iii) continuous moderate]. Patients were instructed personally or with leaflets. In most studies, the patients received a standard of care control group intervention explaining the benefits of breathing or recommending some low-intensity exercise before surgery.
A variety of methods to assess aerobic capacity and endurance following prehabilitation programs has been described among the 23 studies: 6-MWT (k = 17) (22, 25, 27, 29, 32, 34, 36, 43, 44, 48, 49, 51, 55, 56, 58, 68, 72). Cardiopulmonary exercise testing (CPET) analyzed the VO2max or Metabolic Equivalents of Task (k = 9) (22, 27, 29, 32, 34, 44, 50, 68, 72), patient-reported outcomes [PROMS, i.e., perceived exertion with the Borg scales or other comparable scales like the Visual Analog Scale (VAS) or Numeric Rate Scale (NRS); k = 7] (22, 25, 29, 34, 55, 56, 72), a combination of vital signs (e.g., HR, the BP, or both; k = 7) (22, 27, 29, 36, 43, 50), or the Forced 1 Sec. Expiratory Volume (FEV1, k = 6) (22, 27, 29, 36, 43, 50). Other tests such as muscle strength, oxygen saturation, or Diffusion Capacity of the Lungs for Carbon Monoxide (DLCO) played a minor role (22, 25, 27, 29, 34, 47, 60).
To better understand the duration of prehabilitation modalities, especially in surgical oncology, the data on tumor progression before surgical procedures have been analyzed. Ten studies were identified as grade 1 or 2 Oxford level of evidence classified. Those studies evaluated most solid cancers in the thorax and abdomen and found that a delay of surgery for 30 days is not associated with adverse outcomes in surgical patients (26, 33, 40, 41, 57, 61, 66, 67, 74). However, a meta-analysis by Hanna et al. revealed an increased risk for additional tumor-associated deaths by 6%–8% for every 4 weeks of every oncologic treatment delay, which must be weighed against the potential benefits of prehabilitation and its morbidity-reducing effects (46).
Outcome measurements were heterogeneous and clinically not necessarily meaningful. For a clinically relevant outcome measure, it is important to be easily implemented in the clinical workflow and pathway. Overall, there is a general agreement that good cardiopulmonary fitness is associated with a decreased risk for cardiovascular risk in the general population (37). Patients with heart failure have lower functional capacity and should be assessed routinely with ergometer-based methods (maximal exercise test) prior to exercise interventions. From a pragmatic point of view, ergometer-based assessment is often replaced by 6-MWT and has been used in 10 out of 19 studies reporting outcome measures as well as 23 out of 37 studies investigating exercise testing. This decision is based on strong to moderate correlations between methods (44) and can thus be transferred into the surgical setting, where a lower anaerobic threshold is associated with a higher risk for 90-day mortality after e.g., esophagectomies (65). We identified four RCTs included in this analysis that revealed significant improvements for perioperative complications: The studies showed complications to be reduced by 51% [relative risk (RR): 0.51; 95% CI: 0.3–0.8; p = 0.001], including shorter ICU and hospital stays, lower rates of hospital readmission rates (drop from 18% to 3%, p = 0,009), and delivering high compliance (up to 87%). Other studies revealed pulmonary complications to be reduced by 50% (HR 0.48; 95% CI: 0.30–0.75; p = 0.001), or functional capacity to be improved by 130% [interquartile range (IQR): 112–137; p < 0.001], as assessed by 6-MWTs (27, 29, 30, 43).
Importantly, there were only single reports about intervention-related adverse events in predominantly patients who were older than 60 years, which displays strong safety for the patients in a high-intensity interval training advocating for a patient-empowering home-based setting with an unsupervised moderate to vigorous interval training in case of exclusion of major cardiopulmonary risk factors. Endpoint measurements are heterogeneous and should include measurable and meaningful clinical endpoints for short-term outcome quality and long-term oncologic outcome stratified to the underlying disease.
Nutritional assessment tools are important to identify patients with an impaired nutritional status and support those that require medically indicated nutritional supplementation. This is important as it is known that an impaired nutritional status may be associated with increased complication rates like surgical site infections.
A total of 20 articles with an Oxford level of evidence of 2 or better analyzed nutritional recommendations before surgical procedures (23, 27, 32, 34, 35, 37–39, 43, 49, 51–53, 55, 56, 62, 69, 72, 73, 75), of which 12 used a nutritional assessment tool or self-reporting to measure the nutritional status of an individual patient (22, 27, 35, 37–39, 43, 53, 56, 58, 69, 72, 73). Fourteen studies recommended a specific nutrition support mode that consisted predominantly of specific protein supplementation varying between the publication and/or the regular supplementation with immunonutrition, ranging between 3 days and 6 weeks before surgery. Compliance ranged between 72% and 100%. Outcome measures like the length of stay or the occurrence of surgical site infections could be reduced in some trials. Generally, most authors are advocating for a protein-enriched (immune-) nutritional protocol before major surgery, focusing on mitigating the risk of sarcopenia, post-aggressive metabolism, and malnutrition.
Anemia is associated with adverse outcomes after surgical procedures. In more than 95% of all cases, iron deficiency is the leading reason for anemia and those can be corrected with intravenous application of iron. Indeed, several studies have shown that the oncologic outcomes of patients who are not anemic are better than the outcomes of those who are. However, there are contradicting data from purely iron supplementing clinical trials as well (76). In contrast, the structured implementation of patient blood management (PBM) was shown to reduce the requirements for transfusions, i.e., the ratio of anemic patients undergoing operations, and was associated with better oncologic outcomes in multiple real-life cohorts and scenarios (14–17). Only two high-quality studies in abdominal surgery identified anemic patients and tried to correct the anemia with i.v. iron injections in accordance with the recommendations of the patient blood management associations (14, 15, 27, 32).
Mental (or psychosocial) wellbeing is key to success in any medical treatment. However, personalities, resilience, and coping mechanisms are as heterogeneous as patient risk factors. Psychosocial factors can be measured; stress and other adverse factors can be mitigated systematically, and thus may have a positive influence on the patients’ experience before and after a surgical intervention. Only 10 high-quality studies out of 55 hits considered mental wellbeing as an outcome measure in their program (22, 27, 34, 43, 49, 51, 55, 56, 69, 72). There was a heterogeneous mix of behavioral strategies to improve the quality of life, motivational interviews, psychological support, anxiety, stress-reducing approaches, and relaxation strategies as the main tools to improve or keep psychosocial wellbeing in patients. The most frequently used tools include the SF-36 (short-form 36), HADS (hospital anxiety and depression scale), and CGA (comprehensive geriatric assessment). Only 4 of the 10 studies evaluated the effect of the intervention and concluded that behavioral strategies can increase compliance to exercise (70%–90%) by increased motivation and significantly reduced anxiety in patients during their surgical experience (reduction in anxiety score, p = 0.03).
Prehabilitation to date is not reimbursed by any payers in any healthcare system, to our knowledge. This means that surgeons, dedicated care nurses, and other healthcare professionals use extra time, extra effort, and extra money to improve patient outcomes. For the evaluation of the economic potential, all four studies with an Oxford level of evidence level III or higher were included in the evaluation (28, 31, 42, 47). There was a significant benefit of prehabilitation on postoperative complications reflected by the cost-efficiency of a preoperative intervention. Simply expressed, every 1$ that was invested in prehabilitation led to a saving of 8$ in the postoperative course, which is a tremendous return on investment (31). These findings were just confirmed by the group of Howard, having established a trimodal prehabilitation program at their hospital reducing minor and major complications, leading to an economic advantage of prehabilitated patients of $65,000 vs. emergency patients and of $25,000 vs. routine elective patients (47).
Based on the findings from the extractions, recommendations were made for application, further systematic research, and evaluation and are displayed in Table 1.
As surgeons and anesthesiologists, we do care about our patients and try to avoid harm. Surgery is among the top risks of hospital deaths after nonemergent operations (1). This requires careful selection and a risk–benefit assessment that weighs in existing and modifiable risk factors and the potential of failure to rescue after surgery. Prehabilitation has, therefore, been recognized as a potential game-changer not only for selecting but also for increasing the ratio of patients who do not experience preventable harm, which still accounts for 1 out of 3 adverse events that occur in a hospital (13). Data that promote the establishment of prehabilitation are promising and show that there is a high potential to reduce the number of complications by 50% and that there is a realistic chance to simultaneously save $8 for care after surgery for every $1 invested into prehabilitation and proper evaluation and preparation of the patient before a major surgical procedure (27, 31).
To our best knowledge, prehabilitation is not an established and reimbursed treatment in healthcare systems worldwide, and it is dependent on enthusiastic clinical champions that shape a better understanding of the field by generating class 1 and 2 evidence. The field, however, is thriving at this moment making it challenging to keep up with the new evidence that emerges every month. Prehabilitation should be embedded in the whole clinical pathway and needs to be established for the patient immediately and fast. The establishment of an individualized prehabilitation program requires the knowledge of measurable and comparable patient risk factors. In this review, multiple highly evident risk assessment tools were identified, which in combination deliver a meaningful, reproducible, and structured assessment and have been proven to be associated with outcomes.
Exercise testing, the interventions of prehabilitation, and their duration remain the biggest challenges of definition in the literature. Multiple authors recommend dedicated pretraining exercise diagnostics to assess exercise capacity, likely comparable to professional sports. The type, frequency, duration, and intensity of each exercise are also the subject of discussion and ongoing research. However, real-life infrastructure to perform this kind of performance diagnostics in all patients is barely available considering the number of patients requiring surgical procedures. Most high-intensity exercises may appear too challenging, especially in view of the elderly. To date, prehabilitation has been shown to be effective when performed in clinical trials but is highly cost intensive, and lacks infrastructure as well as personnel in most hospitals. Patients, nonetheless, are motivated and compliance seems high with a proposed adherence ratio ranging from 70% to 100%. Therefore, based on our findings, we suggest a pragmatic approach (“toolbox”) for the clinical implementation of prehabilitation concepts (see also Figure 2).
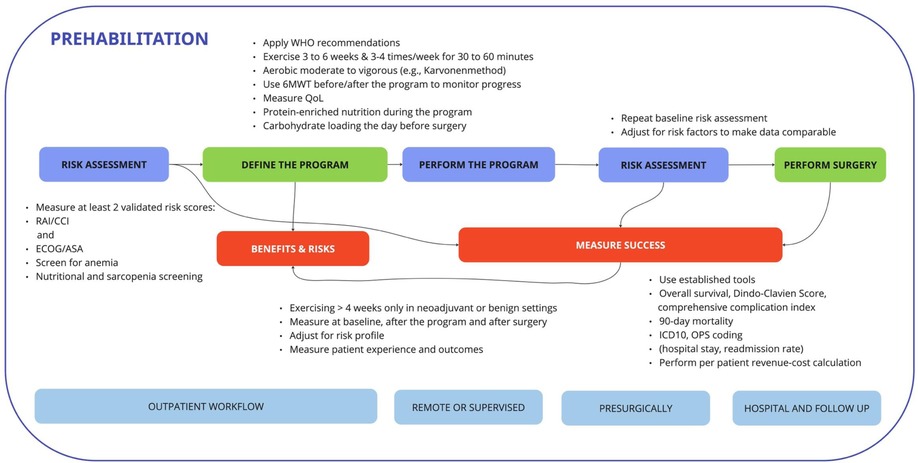
Figure 2. A workflow for the prehabilitation pathway that when implemented into routine procedures of the hospital workflow is likely to decrease the workload of surgeons, anesthesiologists, physiotherapists, and specialized nurses. Measurement of success based on baseline and reassessments as well as meaningful outcome measurements is key to success when implemented.
The assessment of the risk factors with validated scores like the RAI score, the Charlson comorbidity index, the ASA score, or the ECOG score leads to highly reliable discrimination between patients (5, 54, 59, 63). Precise patient history is still critically important and needs to be regarded as the gold standard in patient and doctor interaction as Faqar-Uz-Zaman et al. have shown in a large double-blinded trial in patients with abdominal pain in an emergency room setting (77, 78). Although exercise testing such as CPET is the gold standard for assessing functional capacity, the 6-MWT can provide reliable information about the patient's daily activity and short-term prognosis, especially in patients with heart failure (HF) (chronic stable or acute decompensation). The 6-MWT is an easy-to-perform, widely available, and well-tolerated test for assessing the functional performance of patients with HF in daily clinical practice (44).
However, contraindications against the performance of a 6-MWT and/or a moderate to vigorous aerobic exercising program should be excluded using the recommendations of the ATS (American Thoracic Society) (25), which majorly includes acute and decompensated cardiac, vascular, and pulmonary diseases. Patients with an increased cardiovascular risk are recommended for CPET on an ergometer or any other maximal exercise test. Taking the considerably low serious adverse event rates into account, i.e., 1 out of 10,000 cardiac events and 2–5 deaths out of 100,000 in large cohorts (50), allows for a risk-adapted ergometry testing in high-risk, (borderline) symptomatic patients before assigning them to a prehabilitation program. The exercises can be based on the recommendations of the World Health Organization for physical activity (79). The duration, frequency, and elements of the program can be built around these recommendations and adapted after risk–benefit analysis in an ongoing fashion. Special attention and risk–benefit estimation should be put on the progress of malignancies as every 4 weeks of treatment delay (not only surgical) will increase the risk for tumor progression, which calls for programs that last up to 3 weeks in patients without neoadjuvant strategies and up to 6 weeks in benign or neoadjuvant settings before surgery (46).
Nutrition, patient–blood management, and psychosocial wellbeing have been described as fundamental pillars of prehabilitation. Still, considering this analysis, these items feel like side dishes when compared to the effects mediated by exercise interventions. Screening helps identify patients at increased risk for surgical site infections and other perioperative complications. Perioperative nutrition is critical to fill protein resources, and immunonutrition has been shown to be associated with beneficial and adverse event-reducing effects after surgery (23, 75). PBM as a bundle program can help reduce the number of operations in anemic patients, as anemia is known to be associated with adverse outcomes. It can help reduce the number of transfusions, which are associated with increased mortality (14, 15), and psychosocial wellbeing is important to keep the patients motivated and stay focused before, during, and after the surgical treatment (34, 43, 49, 69). Even if there are contradicting and heterogeneous data on the effect of the above-mentioned items, they might have a granular influence on the complete patient experience, but their effects are hard to measure. In the perception of the authors, they are positive cofounding and surrogate factors for the success and penetration of prehabilitation and should be definitively communicated and implemented in each clinical program. Currently, factors like a stable quality of life and the increase in motivation reflected by high compliance seem to be the clinically best accessible factors.
One aspect that has not been specifically highlighted in the systematic review is smoking cessation. Indeed, this is a critical aspect of improved clinical outcome after surgery and is strongly recommended in the European Code Against Cancer (https://cancer-code-europe.iarc.fr/index.php/en/), and there is a specific World Health Organization Knowledge Summary on key facts about smoking cessation that should be recommended before every surgery that are beneficial for our patients (80).
The economic potential of prehabilitation has not yet been systematically evaluated, but there seems to be a significant return on investment for hospitals that try to get their patients involved in prehabilitation. The data that were analyzed in this systematic and pragmatic review justify an investment into prehabilitation by hospitals as they reduce complications and generate better outcomes for their surgical cohorts. It affects patients that require abdominal operations just as it affects patients with thoracic indications. A lower number of complications usually go hand in hand with less use of intensive care resources, shorter hospital stays, and reduced overall treatment costs. The published data indicate a potential of up to 800% in return on investment (28, 31, 42, 47). An example of an return on investment (ROI) is the availability of hospital days that can be used for additional patients. Staff experience less trauma or frustration due to better outcomes, better quality, and increased safety for the patients (27, 81–83). These are only two among a broad spectrum of factors becoming more and more important in the political demand and obligation toward transparent and risk-adjusted hospital quality reports per indication in most healthcare systems. This is to empower patients to choose the best available treatment location and to shift the payment system to a pay-for-performance approach. Insurances and other stakeholders should establish a reimbursement system for qualified prehabilitation as soon as possible to enable surgeons and anesthesiologists to modify early determinants (physical, nutritional, and psychological state) of late outcomes (morbidity and mortality). This could already start at the referring General Practitioner (GP) level and would foster the collaboration between the ambulatory and hospital sector in a proactive way. The potential of remote digitized solutions should be explored to empower and involve patients and decompress infrastructures on the GP level and at hospitals.
The limitation of this review is its reduced specification, as suggestions are based on the inclusion of all oncologic and major pathologies in the abdomen and thorax. However, patients in these indications are comparable with each other, and the increase in exercise capacity can be regarded as the main goal in improving resistance to postoperative complications. Reasons for excluding other indications were obvious. Cardiosurgical patients often have contraindications against a potentially unsupervised moderate to vigorous aerobic exercise program although the major aim is to increase their functional capacity. Trauma and orthopedic patients, on the other hand, have injuries or physical limitations that indicate a more muscle-strength-focused program. A definite strength of the review is that class 1 and 2 evidence studies were included in the data extraction and evaluation process, indicating high reliability of the published data, except for one exceptional class 3 trial in the economy. Finally, the proposal of a clinical pathway with synergistic and complementary parts shows how prehabilitation might optimally be implemented into the daily hospital and outpatient workflow, including a focused risk management and outcome data measurement to enable penetration and reduce barriers. There are numerous options for further deployment of the tools identified. The authors are currently working on the development of a medical device and have tested it in a pilot study (https://drks.de/search/de/trial/DRKS00026985). A randomized controlled trial is currently set up by the group to explore the potential of remote exercising. In the future, artificial intelligence (AI) applications could analyze the baseline assessments as well as the exercising data and create an AI-based program or directly intervene, tailor, and adapt the individual programs (84).
In conclusion, prehabilitation is a new field in surgery and perioperative medicine requiring definition, assessment, and active quality- and evidence-based approaches, as well as rapid action by stakeholders to establish prehabilitation as a reimbursable instrument for better patient care and increased safety and quality of surgical care. It is not a lifestyle but a critical mosaic stone in a professional and successful surgical treatment strategy with a tremendous economic potential serving for better patient care.
Study concepts: AS, SS, EW, LM, JD, JF, and SF-U-Z. Study design: AS, JF, EW, and SS. Data acquisition: AS, SS, EW, and JF. Quality control of data and algorithms: AS and JF. Data analysis and interpretation: AS, SS, DZ, CD, KB, WB, FH, PM, CR, JR, TV, and JF. Manuscript preparation: AS, JF, EW, SF-U-Z, SS, and DZ. Manuscript editing and review: All authors. All authors contributed to the article and approved the submitted version.
AAS, CD and DZ have founded a company for the development of an app-based prehabilitation program. They did not receive any specific funding for this review.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RROS declared a shared affiliation with the author KB to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nepogodiev D, Martin J, Biccard B, Makupe A, Bhangu A, Nepogodiev D, et al. Global burden of postoperative death. Lancet. (2019) 393:401. doi: 10.1016/S0140-6736(18)33139-8
2. WHO. WHO guidelines for safe surgery: safe surgery saves lives. WHO (n.d.). Available at: https://www.who.int/patientsafety/safesurgery/tools_resources/9789241598552/en/ (Accessed May 23, 2020).
3. WHO. 10 facts on patient safety. WHO (n.d.). Available at: http://www.who.int/features/factfiles/patient_safety/en/ (Accessed May 23, 2020).
4. Shinall MC, Arya S, Youk A, Varley P, Shah R, Massarweh NN, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. (2020) 155:e194620. doi: 10.1001/jamasurg.2019.4620
5. Shinall MC, Youk A, Massarweh NN, Shireman PK, Arya S, George EL, et al. Association of preoperative frailty and operative stress with mortality after elective vs emergency surgery. JAMA Netw Open. (2020) 3:e2010358. doi: 10.1001/jamanetworkopen.2020.10358
6. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
7. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
8. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien P-A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. (2013) 258:1–7. doi: 10.1097/SLA.0b013e318296c732
9. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BPL, Breitenstein S, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. (2014) 260:757–62; discussion 762–3. doi: 10.1097/SLA.0000000000000948
10. Clavien P-A, Vetter D, Staiger RD, Slankamenac K, Mehra T, Graf R, et al. The comprehensive complication index (CCI®): added value and clinical perspectives 3 years “down the line.” Ann Surg. (2017) 265:1045–50. doi: 10.1097/SLA.0000000000002132
11. (n.d.). Available at: https://gco.iarc.fr/.
12. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. (2009) 360:491–9. doi: 10.1056/NEJMsa0810119
13. Bates DW, Levine DM, Salmasian H, Syrowatka A, Shahian DM, Lipsitz S, et al. The safety of inpatient health care. N Engl J Med. (2023) 388:142–53. doi: 10.1056/NEJMsa2206117
14. Meybohm P, Fischer DP, Geisen C, Müller MM, Weber CF, Herrmann E, et al. Safety and effectiveness of a patient blood management (PBM) program in surgical patients—the study design for a multi-centre prospective epidemiologic non-inferiority trial. BMC Health Serv Res. (2014) 14:576. doi: 10.1186/s12913-014-0576-3
15. Meybohm P, Herrmann E, Steinbicker AU, Wittmann M, Gruenewald M, Fischer D, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg. (2016) 264:203–11. doi: 10.1097/SLA.0000000000001747
16. Keding V, Zacharowski K, Bechstein WO, Meybohm P, Schnitzbauer AA. Patient blood management improves outcome in oncologic surgery. World J Surg Oncol. (2018) 16:159. doi: 10.1186/s12957-018-1456-9
17. Schnitzbauer AA, Eberhard J, Bartsch F, Brunner SM, Ceyhan GO, Walter D, et al. The MEGNA score and preoperative anemia are major prognostic factors after resection in the German intrahepatic cholangiocarcinoma cohort. Ann Surg Oncol. (2019) 27(4):1147–55. doi: 10.1245/s10434-019-07968-7
18. Martin D, Besson C, Pache B, Michel A, Geinoz S, Gremeaux-Bader V, et al. Feasibility of a prehabilitation program before major abdominal surgery: a pilot prospective study. J Int Med Res. (2021) 49:03000605211060196. doi: 10.1177/03000605211060196
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
20. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. “The 2011 Oxford CEBM Evidence Levels of Evidence (Introductory Document)”. Oxford Centre for Evidence-Based Medicine. Available at: http://www.cebm.net/index.aspx?o=5653. (Accessed February 23, 2023)
21. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
22. Current Guidelines | health.gov (n.d.). Available at: https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/current-guidelines (Accessed January 1, 2023).
23. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr Edinb Scotl. (2017) 36:11–48. doi: 10.1016/j.clnu.2016.07.015
24. Arya S, Varley P, Youk A, Borrebach JD, Perez S, Massarweh NN, et al. Recalibration and external validation of the risk analysis index: a surgical frailty assessment tool. Ann Surg. (2020) 272:996–1005. doi: 10.1097/SLA.0000000000003276
25. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (2002) 166:111–7. doi: 10.1164/ajrccm.166.1.at1102
26. Bagaria SP, Heckman MG, Diehl NN, Parker A, Wasif N. Delay to colectomy and survival for patients diagnosed with colon cancer. J Investig Surg. (2019) 32:350–7. doi: 10.1080/08941939.2017.1421732
27. Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. (2018) 267:50–6. doi: 10.1097/SLA.0000000000002293
28. Barberan-Garcia A, Ubre M, Pascual-Argente N, Risco R, Faner J, Balust J, et al. Post-discharge impact and cost-consequence analysis of prehabilitation in high-risk patients undergoing major abdominal surgery: secondary results from a randomised controlled trial. Br J Anaesth. (2019) 123:450–6. doi: 10.1016/j.bja.2019.05.032
29. Bhatia C, Kayser B. Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: a randomized clinical trial. J Rehabil Med. (2019) 51:712–8. doi: 10.2340/16501977-2592
30. Boden I, Skinner EH, Browning L, Reeve J, Anderson L, Hill C, et al. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: pragmatic, double blinded, multicentre randomised controlled trial. Br Med J. (2018) 360:j5916. doi: 10.1136/bmj.j5916
31. Boden I, Robertson IK, Neil A, Reeve J, Palmer AJ, Skinner EH, et al. Preoperative physiotherapy is cost-effective for preventing pulmonary complications after major abdominal surgery: a health economic analysis of a multicentre randomised trial. J Physiother. (2020) 66:180–7. doi: 10.1016/j.jphys.2020.06.005
32. Bolshinsky V, Li MH-G, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum. (2018) 61:124–38. doi: 10.1097/DCR.0000000000000987
33. Bourgade V, Drouin SJ, Yates DR, Parra J, Bitker M-O, Cussenot O, et al. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol. (2014) 32:475–9. doi: 10.1007/s00345-013-1045-z
34. Briggs LG, Reitblat C, Bain PA, Parke S, Lam N-Y, Wright J, et al. Prehabilitation exercise before urologic cancer surgery: a systematic and interdisciplinary review. Eur Urol. (2022) 81:157–67. doi: 10.1016/j.eururo.2021.05.015
35. Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat A-HS, Dellinger EP, et al. Oral nutrition as a form of pre-operative enhancement in patients undergoing surgery for colorectal cancer: a systematic review. Surg Infect. (2018) 19:1–10. doi: 10.1089/sur.2017.143
36. Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. (2017) 6:CD012020. doi: 10.1002/14651858.CD012020.pub2
37. Ekblom-Bak E, Ekblom B, Söderling J, Börjesson M, Blom V, Kallings LV, et al. Sex- and age-specific associations between cardiorespiratory fitness, CVD morbidity and all-cause mortality in 266.109 adults. Prev Med. (2019) 127:105799. doi: 10.1016/j.ypmed.2019.105799
38. Ekblom-Bak E, Stenling A, Salier Eriksson J, Hemmingsson E, Kallings LV, Andersson G, et al. Latent profile analysis patterns of exercise, sitting and fitness in adults—associations with metabolic risk factors, perceived health, and perceived symptoms. PLoS One. (2020) 15:e0232210. doi: 10.1371/journal.pone.0232210
39. Ekblom-Bak E, Halldin M, Vikström M, Stenling A, Gigante B, de Faire U, et al. Physical activity attenuates cardiovascular risk and mortality in men and women with and without the metabolic syndrome—a 20-year follow-up of a population-based cohort of 60-year-olds. Eur J Prev Cardiol. (2021) 28:1376–85. doi: 10.1177/2047487320916596
40. Elit LM, O’Leary EM, Pond GR, Seow H-Y. Impact of wait times on survival for women with uterine cancer. J Clin Oncol. (2013) 32(1):27–33. doi: 10.1200/JCO.2013.51.3671
41. Figueiredo N, Panteleimonitis S, Popeskou S, Cunha JF, Qureshi T, Beets GL, et al. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J Surg Oncol. (2018) 44:484–9. doi: 10.1016/j.ejso.2018.01.088
42. Fuertes-Guiró F, Viteri Velasco E. The impact of frailty on the economic evaluation of geriatric surgery: hospital costs and opportunity costs based on meta-analysis. J Med Econ. (2020) 23(8):819–30. doi: 10.1080/13696998.2020.1764965
43. Fulop A, Lakatos L, Susztak N, Szijarto A, Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia. (2021) 76:82–90. doi: 10.1111/anae.15215
44. Giannitsi S, Bougiakli M, Bechlioulis A, Kotsia A, Michalis LK, Naka KK. 6-minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis. (2019) 13:1753944719870084. doi: 10.1177/1753944719870084
45. Hall DE, Arya S, Schmid KK, Blaser C, Carlson MA, Bailey TL, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. (2017) 152:175–82. doi: 10.1001/jamasurg.2016.4202
46. Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. Br Med J. (2020) 371:m4087. doi: 10.1136/bmj.m4087
47. Howard R, Yin YS, McCandless L, Wang S, Englesbe M, Machado-Aranda D. Taking control of your surgery: impact of a prehabilitation program on major abdominal surgery. J Am Coll Surg. (2019) 228:72–80. doi: 10.1016/j.jamcollsurg.2018.09.018
48. Lambert JE, Hayes LD, Keegan TJ, Subar DA, Gaffney CJ. The impact of prehabilitation on patient outcomes in hepatobiliary, colorectal, and upper gastrointestinal cancer surgery: a PRISMA-accordant meta-analysis. Ann Surg. (2021) 274:70–7. doi: 10.1097/SLA.0000000000004527
49. Lau CSM, Chamberlain RS. Prehabilitation programs improve exercise capacity before and after surgery in gastrointestinal cancer surgery patients: a meta-analysis. J Gastrointest Surg. (2020) 24:2829–37. doi: 10.1007/s11605-019-04436-1
50. Levett DZH, Jack S, Swart M, Carlisle J, Wilson J, Snowden C, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. (2018) 120:484–500. doi: 10.1016/j.bja.2017.10.020
51. Liu C, Lu Z, Zhu M, Lu X. Trimodal prehabilitation for older surgical patients: a systematic review and meta-analysis. Aging Clin Exp Res. (2022) 34:485–94. doi: 10.1007/s40520-021-01929-5
52. Looijaard SMLM, Slee-Valentijn MS, Otten RHJ, Maier AB. Physical and nutritional prehabilitation in older patients with colorectal carcinoma: a systematic review. J Geriatr Phys Ther. (2018) 41:236–44. doi: 10.1519/JPT.0000000000000125
53. McKenna NP, Bews KA, Al-Refaie WB, Colibaseanu DT, Pemberton JH, Cima RR, et al. Assessing malnutrition before major oncologic surgery: one size does not fit all. J Am Coll Surg. (2020) 230:451–60. doi: 10.1016/j.jamcollsurg.2019.12.034
54. Meng X, Press B, Renson A, Wysock JS, Taneja SS, Huang WC, et al. Discriminative ability of commonly used indexes to predict adverse outcomes after radical cystectomy: comparison of demographic data, American Society of Anesthesiologists, Modified Charlson Comorbidity Index, and Modified Frailty Index. Clin Genitourin Cancer. (2018) 16:e843–50. doi: 10.1016/j.clgc.2018.02.009
55. Minnella EM, Awasthi R, Gillis C, Fiore JF, Liberman AS, Charlebois P, et al. Patients with poor baseline walking capacity are most likely to improve their functional status with multimodal prehabilitation. Surgery. (2016) 160:1070–9. doi: 10.1016/j.surg.2016.05.036
56. Minnella EM, Awasthi R, Bousquet-Dion G, Ferreira V, Austin B, Audi C, et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus. (2021) 7:132–8. doi: 10.1016/j.euf.2019.05.016
57. Mirkin KA, Hollenbeak CS, Wong J. Time to surgery: a misguided quality metric in early stage pancreatic cancer. J Gastrointest Surg. (2018) 22:1365–75. doi: 10.1007/s11605-018-3730-0
58. Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. (2016) 160:1189–201. doi: 10.1016/j.surg.2016.05.014
59. Palumbo C, Knipper S, Pecoraro A, Rosiello G, Luzzago S, Deuker M, et al. Patient frailty predicts worse perioperative outcomes and higher cost after radical cystectomy. Surg Oncol. (2020) 32:8–13. doi: 10.1016/j.suronc.2019.10.014
60. Piraux E, Reychler G, de Noordhout LM, Forget P, Deswysen Y, Caty G. What are the impact and the optimal design of a physical prehabilitation program in patients with esophagogastric cancer awaiting surgery? A systematic review. BMC Sports Sci Med Rehabil. (2021) 13:33. doi: 10.1186/s13102-021-00260-w
61. Polverini AC, Nelson RA, Marcinkowski E, Jones VC, Lai L, Mortimer JE, et al. Time to treatment: measuring quality breast cancer care. Ann Surg Oncol. (2016) 23:3392–402. doi: 10.1245/s10434-016-5486-7
62. Probst P, Ohmann S, Klaiber U, Hüttner FJ, Billeter AT, Ulrich A, et al. Meta-analysis of immunonutrition in major abdominal surgery. Br J Surg. (2017) 104:1594–608. doi: 10.1002/bjs.10659
63. Shah R, Attwood K, Arya S, Hall DE, Johanning JM, Gabriel E, et al. Association of frailty with failure to rescue after low-risk and high-risk inpatient surgery. JAMA Surg. (2018) 153:e180214. doi: 10.1001/jamasurg.2018.0214
64. Shah R, Borrebach JD, Hodges JC, Varley PR, Wisniewski MK, Shinall MC, et al. Validation of the risk analysis index for evaluating frailty in ambulatory patients. J Am Geriatr Soc. (2020) 68:1818–24. doi: 10.1111/jgs.16453
65. Sheill G, Reynolds S, O’Neill L, Mockler D, Reynolds JV, Hussey J, et al. Cardiopulmonary exercise testing in oesophagogastric surgery: a systematic review. J Gastrointest Surg. (2020) 24:2667–78. doi: 10.1007/s11605-020-04696-2
66. Simunovic M, Rempel E, Thériault M-E, Baxter NN, Virnig BA, Meropol NJ, et al. Influence of delays to nonemergent colon cancer surgery on operative mortality, disease-specific survival and overall survival. Can J Surg J Can Chir. (2009) 52:E79–86. PMID: 19680502; PMCID: 2724831
67. Strohl AE, Feinglass JM, Shahabi S, Simon MA. Surgical wait time: a new health indicator in women with endometrial cancer. Gynecol Oncol. (2016) 141:511–5. doi: 10.1016/j.ygyno.2016.04.014
68. Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery. Anaesthesia. (2018) 73:750–68. doi: 10.1111/anae.14177
69. Thillainadesan J, Yumol MF, Hilmer S, Aitken SJ, Naganathan V. Interventions to improve clinical outcomes in older adults admitted to a surgical service: a systematic review and meta-analysis. J Am Med Dir Assoc. (2020) 21:1833–43.e20. doi: 10.1016/j.jamda.2020.03.023
70. van Kooten RT, Bahadoer RR, Peeters KCMJ, Hoeksema JHL, Steyerberg EW, Hartgrink HH, et al. Preoperative risk factors for major postoperative complications after complex gastrointestinal cancer surgery: a systematic review. Eur J Surg Oncol. (2021) 47:3049–58. doi: 10.1016/j.ejso.2021.07.021
71. Varley PR, Borrebach JD, Arya S, Massarweh NN, Bilderback AL, Wisniewski MK, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. (2021) 274:e1230–7. doi: 10.1097/SLA.0000000000003808
72. Waterland JL, McCourt O, Edbrooke L, Grangessr CL, Ismail H, Riedel B, et al. Efficacy of prehabilitation including exercise on postoperative outcomes following abdominal cancer surgery: a systematic review and meta-analysis. Front Surg. (2021) 8:628848. doi: 10.3389/fsurg.2021.628848
73. Weimann A, Breitenstein S, Breuer JP, Gabor SE, Holland-Cunz S, Kemen M, et al. Clinical nutrition in surgery. Guidelines of the German Society for Nutritional Medicine. Chir Z Alle Geb Oper Medizen. (2014) 85:320–6. doi: 10.1007/s00104-014-2737-7
74. Xu K, Watanabe-Galloway S, Rochling FA, Farazi PA, Monirul Islam KM, Wang H, et al. Surgical delay is associated with improved survival in hepatocellular carcinoma: results of the national cancer database. J Gastrointest Surg. (2019) 23:933–43. doi: 10.1007/s11605-018-3925-4
75. Yu K, Zheng X, Wang G, Liu M, Li Y, Yu P, et al. Immunonutrition vs standard nutrition for cancer patients: a systematic review and meta-analysis (part 1). JPEN J Parenter Enteral Nutr. (2020) 44:742–67. doi: 10.1002/jpen.1736
76. Richards T, Baikady RR, Clevenger B, Butcher A, Abeysiri S, Chau M, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet. (2020) 396:1353–61. doi: 10.1016/S0140-6736(20)31539-7
77. Faqar-Uz-Zaman SF, Filmann N, Mahkovic D, von Wagner M, Detemble C, Kippke U, et al. Study protocol for a prospective, double-blinded, observational study investigating the diagnostic accuracy of an app-based diagnostic health care application in an emergency room setting: the eRadaR trial. BMJ Open. (2021) 11:e041396. doi: 10.1136/bmjopen-2020-041396
78. Faqar-Uz-Zaman SF, Anantharajah L, Baumartz P, Sobotta P, Filmann N, Zmuc D, et al. The diagnostic efficacy of an app-based diagnostic health care application in the emergency room: eRadaR-trial. A prospective, double-blinded, observational study. Ann Surg. (2022) 276:935–42. doi: 10.1097/SLA.0000000000005614
79. Physical activity (n.d.). Available at: https://www.who.int/news-room/fact-sheets/detail/physical-activity (Accessed January 22, 2023).
80. Yoong SL, Tursan d’Espaignet E, Wiggers J, St Claire S, Mellin-Olsen J, Grady A, et al. Tobacco and postsurgical outcomes: WHO tobacco knowledge summaries. Geneva: World Health Organization (2020). Available at: https://www.google.com/search?client=firefox-b-d&q=Yoong+SL%2C+Tursan+d%E2%80%99Espaignet+E%2C+Wiggers+J%2C+St+Claire+S%2C+Mellin-Olsen+J%2C+Grady+A+et+al.+Tobacco+and+postsurgical+outcomes%3A+WHO+tobacco+knowledge+summaries.+Geneva%3A+World+Health+Organization%3B+2020.+Licence%3A+CC+BY-NC-SA+3.0+IGO (Accessed April 5, 2023).
81. Joliat G-R, Demartines N, Uldry E. Systematic review of the impact of patient death on surgeons. Br J Surg. (2019) 106:1429–32. doi: 10.1002/bjs.11264
82. Bohnen JD, Lillemoe KD, Mort EA, Kaafarani HMA. When things go wrong: the surgeon as second victim. Ann Surg. (2019) 269:808–9. doi: 10.1097/SLA.0000000000003138
83. Han K, Bohnen JD, Peponis T, Martinez M, Nandan A, Yeh DD, et al. The surgeon as the second victim? Results of the Boston intraoperative adverse events surgeons’ attitude (BISA) study. J Am Coll Surg. (2017) 224:1048–56. doi: 10.1016/j.jamcollsurg.2016.12.039
Keywords: surgical oncology, prehabilitation, morbidity, risk assessment—methods, exercising, nutrition, mental wellbeing, PBM
Citation: Sliwinski S, Werneburg E, Faqar-Uz-Zaman SF, Detemble C, Dreilich J, Mohr L, Zmuc D, Beyer K, Bechstein WO, Herrle F, Malkomes P, Reissfelder C, Ritz JP, Vilz T, Fleckenstein J and Schnitzbauer AA (2023) A toolbox for a structured risk-based prehabilitation program in major surgical oncology. Front. Surg. 10:1186971. doi: 10.3389/fsurg.2023.1186971
Received: 15 March 2023; Accepted: 17 April 2023;
Published: 26 June 2023.
Edited by:
Quirino Lai, Sapienza University of Rome, ItalyReviewed by:
Heba Taher, Cairo University, Egypt© 2023 Sliwinski, Werneburg, Faqar-Uz-Zaman, Detemble, Dreilich, Mohr, Zmuc, Beyer, Bechstein, Herrle, Malkomes, Reissfelder, Ritz, Vilz, Fleckenstein and Schnitzbauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas A. Schnitzbauer YW5kcmVhcy5zY2huaXR6YmF1ZXJAa2d1LmRl
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.