
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Surg. , 24 May 2023
Sec. Visceral Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1175633
 Mehmet Kostek1*†
Mehmet Kostek1*† Mehmet Taner Unlu1
Mehmet Taner Unlu1 Ozan Caliskan1
Ozan Caliskan1 Nurcihan Aygun1
Nurcihan Aygun1 Yalin Iscan2
Yalin Iscan2 Ahmet Cem Dural3
Ahmet Cem Dural3 Ismail Cem Sormaz2
Ismail Cem Sormaz2 Fatih Tunca2
Fatih Tunca2 Yasemin Giles Senyurek2
Yasemin Giles Senyurek2 Mehmet Uludag1
Mehmet Uludag1
Adrenal schwannomas are rare benign tumors with no specific imaging and laboratory findings to diagnose preoperatively. Due to the limited number of cases in the literature, clinical, imaging, and pathological findings are presented in this study. Case 1 is a 61-year-old woman patient who has a 31-mm mass in the right adrenal gland. This mass was nonfunctional; in imaging studies, this mass had a cystic necrotic component, and high 18-fluorodeoxyglucose (FDG) uptake was seen. There was no metaiodobenzylguanidine (MIBG) uptake. Laparoscopic transabdominal right adrenalectomy was performed, and the pathology result was consistent with adrenal schwannomas. Case 2 is a 63-year-old man patient who presented with a 38-mm mass in the left adrenal gland. This mass was nonfunctional and similar to that in Case 1; this mass had a cystic component. Laparoscopic transabdominal left adrenalectomy was performed. The diagnosis of adrenal schwannoma with degeneration was revealed. Case 3 was a 72-year-old woman patient admitted to the hospital for a 125-mm left adrenal mass. Similar to Case 1, this mass also had a cystic necrotic component in imaging studies. High FDG uptake was seen, and the patient underwent conventional adrenalectomy due to the suspicion of malignancy. After pathological evaluation, a diagnosis of adrenal schwannoma was made. A main diagnostic challenge in adrenal schwannomas is the preoperative diagnosis. These masses have no pathognomonic finding or specific hormonal function. Imaging findings of these masses may increase the suspicion of malignancy, which may affect decisions for surgery and the surgical technique.
Schwannomas are mostly benign tumors that comprise Schwann cells neighboring to myelinated parts of central or peripheral neurons. These rare tumors generally appear at flexor surfaces of extremities, mediastinum, head, and neck regions. Infrequently, these tumors are seen in the retroperitoneal area (1, 2). Retroperitoneal schwannomas constitute 1% of all retroperitoneal masses (3).
Common benign tumors originating from adrenal tissue are benign adenomas and pheochromocytomas; however, schwannomas rarely originate from adrenal tissue and are considered as only 0.7% of benign adrenal tumors (4). Due to the lack of pathognomonic findings, preoperative diagnosis is challenging, and postoperative pathology results are needed for the final diagnosis.
Adrenal schwannomas may be confused with adrenocortical cancer, adrenal metastasis, or pheochromocytoma due to their imaging findings (5). In this case series, we present characteristics, preoperative clinical, radiologic, and postoperative findings of three different adrenal schwannomas with suspicious findings in imaging studies for malignancy or pheochromocytoma; however, after adrenal surgery, the diagnosis of benign adrenal schwannoma was revealed in pathological evaluation.
A 61-year-old woman patient was admitted to the outpatient clinic because of a 31-mm mass in the right adrenal gland after undergoing a computed tomography (CT) scan for COVID-19. The patient has no medical history other than COVID-19. The CT scan showed a cystic necrotic component that caused suspicion of adrenocortical carcinoma or pheochromocytoma. The patient was evaluated for excess hormone production with the 1-mg dexamethasone suppression test, serum and urine metanephrines, plasma renin activity, and serum aldosterone. There was no excess hormone production by the adrenal mass. Several imaging studies, in addition to computed tomography scans, were performed. Magnetic resonance imaging (MRI) showed a semisolid mass with cystic and necrotic components and peripheral diffusion restriction, which increased suspicion of adrenocortical carcinoma (Figures 1A,B). Therefore, the patient underwent 18-fluorodeoxyglucose positron emission tomography combined with computed tomography (FDG-PET/CT). In this imaging study, an enhancement of FDG at the level of malignancy was seen, and the maximum standardized uptake value (SUVmax) was 9.94 (Figure 2). Due to heterogeneous imaging findings, to exclude silent pheochromocytoma or any metastasis of pheochromocytoma, the patient also underwent metaiodobenzylguanidine (MIBG) scintigraphy; however, there was no enhancement throughout body.
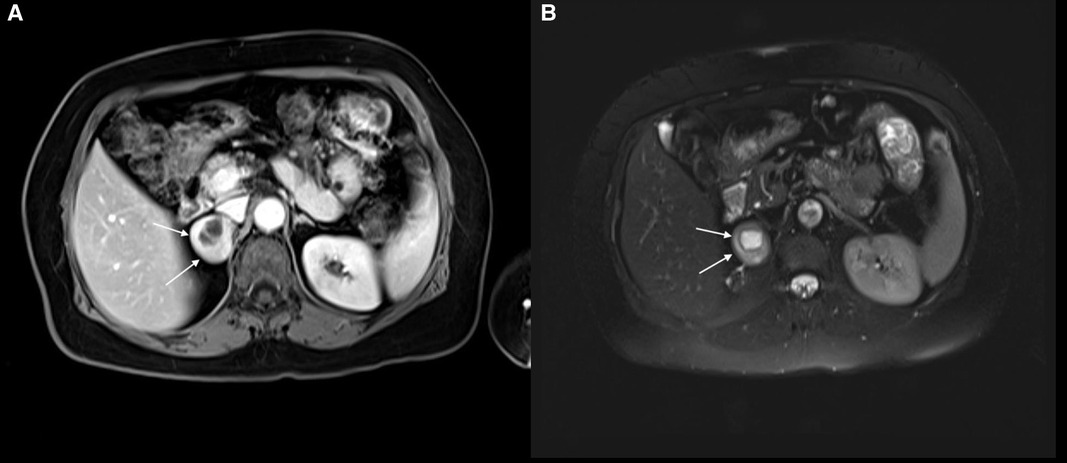
Figure 1. (A) right adrenal schwannoma in the T1 sequence of MRI. The cystic necrotic component can be seen in this imaging study. The schwannoma was shown by a white arrow. (B) Right adrenal schwannoma in the T2 sequence of MRI. The schwannoma was shown by a white arrow.
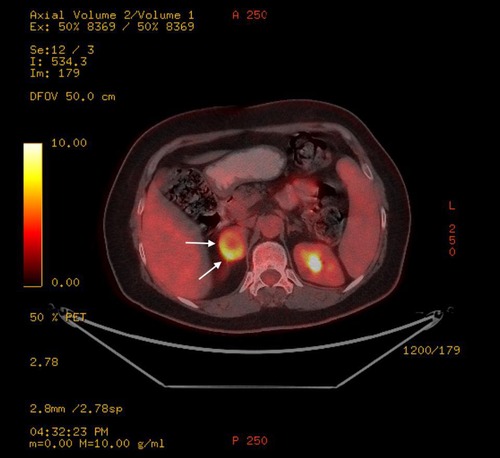
Figure 2. Right adrenal schwannoma in the FDG-PET/CT scan. This mass had an increased FDG uptake. The schwannoma was shown by a white arrow.
Owing to the suspicious findings in the imaging studies, surgical excision was planned. Because of the small mass size, laparoscopic transabdominal adrenalectomy was applied. During gross examination of the excised material, two components were easily seen (Figure 3). One cystic component with suspected mass and unaffected adrenal gland. After the operation, the patient had nothing remarkable and was discharged postoperative on the third day. Postoperative follow-up was continued until 8 months, and there was no recurrence or any complaints after surgery.
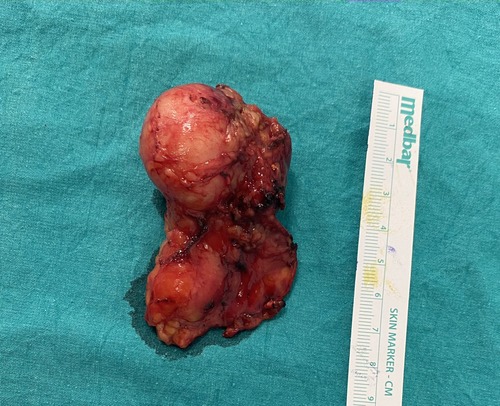
Figure 3. Right adrenal schwannoma after laparoscopic transabdominal right adrenalectomy. Two components of adrenal mass can be observed.
At the end of the pathological examination, the final diagnosis was consistent with adrenal schwannoma. Needle-shaped cells without atypia were seen under a microscope, and diffuse positive reactivity with S100 protein was seen in immunohistochemistry. There was no staining with CD34, Desmin, and SMA. The Ki-67 index was less than 1%.
A 63-year-old man patient was admitted to the general surgery clinic because of a left upper quadrant pain. The patient had no history of medical illnesses and no history of abdominal surgery. After an unremarkable routine physical examination, abdominal ultrasound imaging was ordered. Ultrasound imaging revealed a 38-mm × 36-mm mass in the left adrenal gland with a cystic component surrounded by a thick wall. A biochemical investigation was planned, and the patient was evaluated for excess hormone production with the 1-mg dexamethasone suppression test, serum and urine metanephrines, plasma renin activity, and serum aldosterone. There was no hormonal excess, and no hormone production by the adrenal mass was shown. The patient underwent an abdominal MRI scan, and a thick-walled adrenal mass with a cystic component was revealed. The size of the mass was 33 mm × 37 mm, and the mass was described as isohyperintense in T1-weighted images, hypo-hyperintense in T2-weighted images, and contrast enhancement at the postcontrast series. Due to its heterogeneous imaging characteristics, laparoscopic left transabdominal adrenalectomy was performed. The patient had an unremarkable hospital stay and was discharged on the third day postoperatively. The final diagnosis of the adrenal mass was concluded as schwannomas with degeneration in the pathology report. The patient continued to follow-up examinations for 12 years and had nothing remarkable in imaging studies and biochemical evaluations.
A 72-year-old woman patient was presented to the outpatient clinic for left adrenal incidentaloma. This mass appeared in a CT scan during the investigation for hydrocephalus. The patient has a past medical history of high blood pressure, and vertigo, and laparoscopic cholecystectomy. In routine laboratory tests, no excess hormone production was shown. In the CT scan, the size of the left adrenal mass was 125 mm × 102 mm × 123 mm, and heterogeneous contrast enhancement with cystic-necrotic components was observed (Figures 4A,B). This mass was pushing the pancreas and splenic vein to the anterior, and it was in close contact with the stomach; however, there was no sign of invasion. In whole-body FDG-PET/CT, FDG uptake was observed on the adrenal mass, and SUVmax was 7.1. This finding increased suspicion of malignancy. Because of its size and suspicion of malignancy, conventional adrenalectomy was applied. The operation was uneventful, and the patient was discharged on the 8th day postoperatively without any complications. The final pathology result was consistent with schwannomas, and staining with immunohistochemistry was positive for S100 and negative for SMA, CD34, Desmin, and CD117. The Ki-67 score was 1%−2%. Ten months after the operation, the patient has no complaint in the follow-up examinations, and no recurrence has been observed.
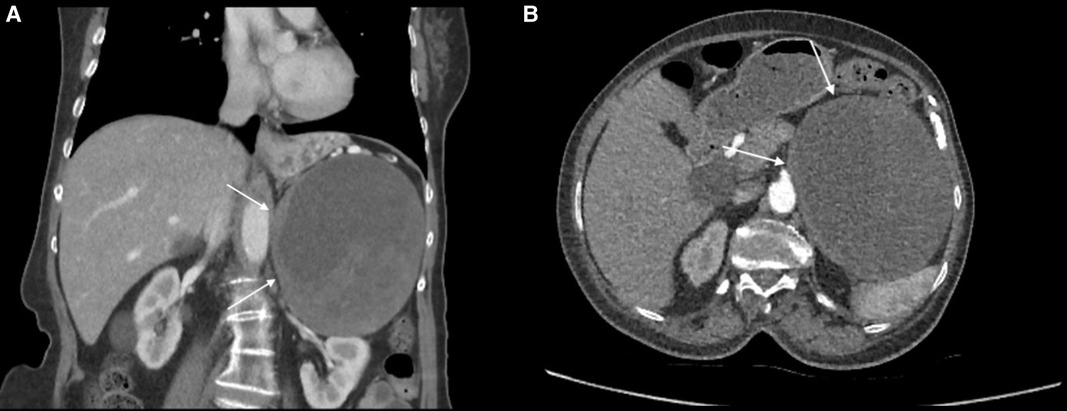
Figure 4. (A) left adrenal schwannoma in the coronal plane of the CT scan. The cystic necrotic component can be seen in this imaging study. The schwannoma was shown by a white arrow. (B) Left adrenal schwannoma in the axial plane of the CT scan. The cystic necrotic component can be seen in this imaging study. The schwannoma was shown by a white arrow.
Schwannomas are frequently seen in the head, neck, and extremities; however, adrenal schwannomas are a rare clinical entity and constitute only 0.7% of benign adrenal tumors (4). Schwannomas were mostly seen as sporadic cases (almost 90%); on the other hand, several cases associated with neurofibromatosis type 2, Carney complex, and schwannomatosis were reported (1, 6). Nevertheless, none of the adrenal schwannomas described to date have been associated with genetic syndromes (7).
Adrenal schwannoma cases are seen more frequently at ages 39 and 59 years and more frequently in women (1.17 times more than men) (1, 6). These masses were mostly seen in the 5th and 6th decades and more often in women. However, two of our cases were in their 7th decade and one of them was in her 8th decade. In a literature review by Incampo and colleagues, 169 patients with adrenal schwannomas were reviewed, and approximately 25% of patients were in their 7th decade or older (7).
The largest diameters of the previously reported adrenal schwannomas varied between 1 and 15 cm, and 64% of these masses were between 4 and 10 cm (7). However, in our study, the largest diameters of Cases 1 and 2 were less than 4 cm and the largest diameter of Case 3 was 12.5 cm. In nonhormone-secreting masses of the adrenal gland, the possibility of malignancy is the main indication for surgery, and suspicious imaging findings guided the surgeons for adrenalectomy in Cases 1 and 2. In Case 3, the largest diameter was more than 6 cm, and consequently, higher suspicion for malignancy was raised due to the increased size. After the operation, none of the masses were concluded as malignant tumors.
Patients with adrenal schwannomas mostly have complaints of vague pain on the left upper quadrant or have no complaint and incidentally detected a mass on adrenal glands. Due to the COVID-19 pandemic, many patients underwent thorax CT scans, which may increase the possibility of finding more incidentalomas. However, adrenal schwannomas cannot be diagnosed from imaging studies and biochemical tests. Hormonal inactivity of these masses may not be helpful to clinicians for their differential diagnosis; nevertheless, imaging findings may confuse clinicians for preoperative diagnosis. Heterogeneous imaging findings such as thick walls, mixed cystic solid components, calcification, and necrosis may cause suspicion of pheochromocytoma and adrenocortical carcinoma (5). In MRI studies, intensity characteristics of adrenal schwannomas may be low in T1-weighted images and high in T2-weighted images. However, these lesions may have heterogeneous findings as in CT scans (8) (Table 1).
In addition to the other imaging techniques, FDG-PET/CT scans may give information about the metabolic activity of adrenal lesions (11). Adrenal schwannomas may show increased FDG uptake, and evaluation of this imaging study may increase suspicion of malignancy. In previous reports, only seven cases of adrenal schwannoma were reported with the results of the PET scan, and they were presented with increased FDG uptake (7). In this study, two of our patients were presented with increased FDG uptake. In Case 1, the adrenal mass had increased FDG uptake and cystic necrotic component, which caused increased suspicion and led the endocrinologist and surgeons to decide on an operation. Also, the size and high FDG uptake of the mass increased suspicion of malignancy in Case 3. Other than a previously reported case of a microcystic reticular adrenal schwannoma in which the SUVmax was 71.7, the SUVmax of reported adrenal schwannomas was between 2.8 and 10.3 (1, 7, 12–14). Similar to these numbers, also in our cases, SUVmax values in Cases 1 and 3 were 9.94 and 7.1, respectively. These numbers showed high metabolic activity in these masses. The possible reasons for increased uptake are hypercellular areas in the adrenal schwannomas and peritumoral lymphoid cuffs (11). With cystic and necrotic components in the adrenal masses, positive FDG uptake may increase the suspicion of malignancy. However, at the end of the pathological evaluation, a benign result such as an adrenal schwannoma may be seen. These results show that an FDG-PET scan cannot be helpful for the differential diagnosis of schwannomas. As another whole-body screening test, MIBG scintigraphy results were rarely discussed in adrenal schwannomas. Only two cases of adrenal schwannomas previously underwent MIBG scintigraphy in the literature, and these cases had no increased uptake. Compared to the previous studies, Case 1 had an MIBG scintigraphy and had no increased uptake. Therefore, in patients with suspicious imaging findings for pheochromocytoma and without positive laboratory findings, MIBG scintigraphy can be a possible whole-body screening test to exclude pheochromocytoma (15, 16).
The main treatment modality of schwannomas is surgical excision; however, there is no certain adrenalectomy technique for schwannomas. An impression of malignancy or invasion may direct surgeons to an open adrenalectomy; therefore, excellent preoperative imaging is crucial for choosing the right surgical technique. Masses with smooth margins are suitable for laparoscopic adrenalectomy. An appropriate size of the masses for laparoscopic surgery is debatable; however, in case reports from several centers, patients with masses larger than 10 cm were operated without complications (2, 12). Pathological evaluation reveals the final diagnosis (6). Schwannomas are not sensitive to radiotherapy and chemotherapy; hence, complete excision of the mass is critical (17). Therefore, conventional adrenalectomy was chosen for Case 3. Increased possibility for malignancy in adrenal masses larger than 6 cm and increased FDG uptake played a role in this decision.
Schwannomas originate from nerve sheaths and have hypocellular and hypercellular areas of spindle cells. Immunohistochemistry results show strongly positive staining of spindle cells with S100, and this result verifies their neural origin (18). These masses are not expected to be stained by HMB45, EMA, Desmin, and SMA (2, 8). Staining with CD34 was reported in one-third of the patients (18). The Ki-67 proliferation index was reported to be less than 5% in the literature (2, 19). Due to the hypocellular and hypercellular areas of spindle cells, contrast enhancement patterns show heterogeneity, and radiological findings may be suspicious for malignancy (7). In the pathology results of these lesions, findings were concordant with the radiology reports, such as cystic changes and necrosis. However, radiological findings were suspicious of malignancy, and pathology results were consistent with increased cellularity. In all three cases, cystic changes were observed in radiological and pathological data. Although cystic and/or necrotic changes are reported in 43% of cases in the literature, focal necrotic areas were also reported in pathology results in Cases 1 and 3 (7). These results are coherent with the radiology reports.
Recurrence or metastasis is not expected after complete resection in adrenal schwannomas. There is no consensus on the follow-up period in these patients; however, long-term follow-up data are limited (2, 7, 19). There is no recurrence and distant metastasis in the cases presented in this paper, and follow-up periods are 8 months for Case 1, 12 years for Case 2, and 10 months for Case 3.
Adrenal schwannomas are rare tumors that are mostly diagnosed after surgical resection. Imaging findings are confounding, and these masses are nonfunctional tumors. Radiologic findings cause suspicion of malignancy, and there are no specific preoperative diagnostic criteria. The final diagnosis can be made in pathological evaluation after resection of the mass. The main treatment is surgery, and after complete resection, recurrence and metastasis are infrequent. There is limited data in the literature, and more case reports and case series are needed for better patient care.
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MK, MU, NA, FT, YS, and MU contributed to the conception and design of the study. MK, OC, YI, and FT acquired the data of cases. MK, MU, and YI wrote the first draft of the manuscript. NA, AD, IS, FT, YS, and MU wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Case 1 was presented previously at the 9th Biennial Congress of the European Society of Endocrine Surgeons on May 26−28, 2022, in Athens, Greece.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Maciel JM, Pereira DV, Simões HF, Leite VA. Adrenal microcystic reticular schwannoma. AACE Clin Case Rep. (2019) 13:250–4. doi: 10.4158/ACCR-2018-0621
2. Zhou J, Zhang D, Li W, Zhou L, Xu H, Zheng S, et al. Primary adrenal schwannoma: a series of 31 cases emphasizing their clinicopathologic features and favorable prognosis. Endocrine. (2019) 65:662–74. doi: 10.1007/s12020-019-01992-z
3. Goh BK, Tan YM, Chung YF, Chow PK, Ooi LL, Wong WK. Retroperitoneal schwannoma. Am J Surg. (2006) 192:14–8. doi: 10.1016/j.amjsurg.2005.12.010
4. Gong X, Yu Y, Zhan W. Ultrasonographic findings of 1385 adrenal masses: a retrospective study of 1319 benign and 66 malignant masses. J Ultrasound Med. (2019) 38:2249–57. doi: 10.1002/jum.14471
5. Wilson MP, Katlariwala P, Huang J, Low G, Wiebe E. Benign adrenal and suprarenal retroperitoneal schwannomas can mimic aggressive adrenal malignancies: case report and review of the literature. Intractable Rare Dis Res. (2020) 9:156–62. doi: 10.5582/irdr.2020.01027
6. Huang H, Ding Q, Lin X, Li D, Zeng J, Fu W. Clinical features and outcomes of adrenal schwannoma: a study of 13 cases from a single centre. Endocr Connect. (2021) 10:543–9. doi: 10.1530/EC-21-0062
7. Incampo G, Di Filippo L, Grossrubatscher EM, Dalino Ciaramella P, Frara S, Giustina A, et al. Adrenal schwannoma: why should endocrinologists be aware of this uncommon tumour? Endocrine. (2022) 75:684–97. doi: 10.1007/s12020-022-02997-x
8. AlMalki MH, Alotaibi M, Ahmad MM, Rahman MAU, Alharthi T. Schwannoma misdiagnosed as adrenal adenoma: a case report and review of the literature. Case Rep Endocrinol. (2020) 2020:8020761. doi: 10.1155/2020/8020761
9. Jason DS, Oltmann SC. Evaluation of an adrenal incidentaloma. Surg Clin North Am. (2019) 99(4):721–9. doi: 10.1016/j.suc.2019.04.009
10. Kebebew E. Adrenal incidentaloma. N Engl J Med. (2021) 384(16):1542–51. doi: 10.1056/NEJMcp2031112
11. Miyake KK, Nakamoto Y, Kataoka TR, Ueshima C, Higashi T, Terashima T, et al. Clinical, morphologic, and pathologic features associated with increased FDG uptake in schwannoma. AJR Am J Roentgenol. (2016) 207:1288–96. doi: 10.2214/AJR.15.14964
12. Li SQ, Zhang YS, Shi J, Li HZ. Clinical features and retroperitoneal laparoscopic resection of adrenal schwannoma in 19 patients. Endocr Pract. (2015) 21:323–9. doi: 10.4158/EP14453.OR
13. Yorita K, Naroda T, Tamura M, Ito S, Nakatani K. Adrenal schwannoma in an elderly man: a case report and literature review. Intern Med. (2022) 61:65–9. doi: 10.2169/internalmedicine.7026-21
14. Adas M, Ozulker F, Adas G, Koc B, Ozulker T, Mansuroglu Sahin I. A rare adrenal incidentaloma: adrenal schwannoma. Case Rep Gastroenterol. (2013) 7:420–7. doi: 10.1159/000355871
15. Suzuki K, Nakanishi A, Kurosaki Y, Nogaki J, Takaba E. Adrenal schwannoma: CT and MRI findings. Radiat Med. (2007) 25:299–302. doi: 10.1007/s11604-007-0136-4
16. Oberoi A, Kataria K, Prakash O, Yadav R, Goyal A. Management of giant adrenal schwannoma. Int Surg J. (2019) 6:2605–8. doi: 10.18203/2349-2902.isj20193003
17. Richter KK, Premkumar R, Yoon HS, Mercer P. Laparoscopic adrenalectomy for a rare 14-cm adrenal schwannoma. Surg Laparosc Endosc Percutan Tech. (2011) 21:339–43. doi: 10.1097/SLE.0b013e31823ac4d4
18. Abdessater M, El Mokdad M, Gas J, Sleiman W, Coloby P, Bart S. Juxta-adrenal schwannoma presenting as a giant adrenal tumor: a case report and a literature review. Int J Surg Case Rep. (2018) 53:132–6. doi: 10.1016/j.ijscr.2018.10.017
Keywords: adrenal glands, schwannoma, adrenalectomy, adrenocortical carcinoma, adrenal schwannoma
Citation: Kostek M, Unlu MT, Caliskan O, Aygun N, Iscan Y, Dural AC, Sormaz IC, Tunca F, Giles Senyurek Y and Uludag M (2023) An unusual finding after adrenal surgery: a case series of adrenal schwannomas. Front. Surg. 10:1175633. doi: 10.3389/fsurg.2023.1175633
Received: 27 February 2023; Accepted: 4 May 2023;
Published: 24 May 2023.
Edited by:
Paolo Bernante, University of Bologna, ItalyReviewed by:
Ilze Strumfa, Riga Stradiņš University, Latvia© 2023 Kostek, Unlu, Caliskan, Aygun, Iscan, Dural, Sormaz, Tunca, Giles Senyurek and Uludag. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehmet Kostek ZHIubWtvc3Rla0BnbWFpbC5jb20=
†ORCID Mehmet Kostek orcid.org/0000-0001-7259-2461
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.