- 1IRCCS Istituto Ortopedico Galeazzi, Milan, Italy
- 2Spine Center, Schulthess Clinic, Zurich, Switzerland
- 3Department of Health Sciences and Technologies, ETH Zurich, Zurich, Switzerland
- 4Dipartimento di Scienze Biomediche per la Salute, Università Degli Studi di Milano, Milan, Italy
- 5Complex Unit of Radiology, Department of Diagnostic and Interventional Radiology, Azienda Socio Sanitaria Territoriale (ASST) Lodi, Lodi, Italy
Introduction: A novel classification scheme for endplate lesions, based on T2-weighted images from magnetic resonance imaging (MRI) scan, has been recently introduced and validated. The scheme categorizes intervertebral spaces as “normal,” “wavy/irregular,” “notched,” and “Schmorl's node.” These lesions have been associated with spinal pathologies, including disc degeneration and low back pain. The exploitation of an automatic tool for the detection of the lesions would facilitate clinical practice by reducing the workload and the diagnosis time. The present work exploits a deep learning application based on convolutional neural networks to automatically classify the type of lesion.
Methods: T2-weighted MRI scans of the sagittal lumbosacral spine of consecutive patients were retrospectively collected. The middle slice of each scan was manually processed to identify the intervertebral spaces from L1L2 to L5S1, and the corresponding lesion type was labeled. A total of 1,559 gradable discs were obtained, with the following types of distribution: “normal” (567 discs), “wavy/irregular” (485), “notched” (362), and “Schmorl's node” (145). The dataset was divided randomly into a training set and a validation set while preserving the original distribution of lesion types in each set. A pretrained network for image classification was utilized, and fine-tuning was performed using the training set. The retrained net was then applied to the validation set to evaluate the overall accuracy and accuracy for each specific lesion type.
Results: The overall rate of accuracy was found equal to 88%. The accuracy for the specific lesion type was found as follows: 91% (normal), 82% (wavy/irregular), 93% (notched), and 83% (Schmorl's node).
Discussion: The results indicate that the deep learning approach achieved high accuracy for both overall classification and individual lesion types. In clinical applications, this implementation could be employed as part of an automatic detection tool for pathological conditions characterized by the presence of endplate lesions, such as spinal osteochondrosis.
1. Introduction
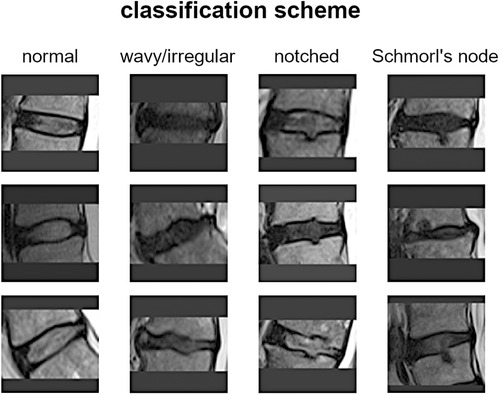
The vertebral endplate is a crucial structure situated between the vertebral body and the intervertebral disc. It serves as a mechanical interface between the rigid bone and the flexible disc, playing a vital role in maintaining the morphological integrity and physiological function of the disc. Traditionally, most endplate pathologies or lesions were classified as Schmorl's nodes, which refer to the protrusion of disc tissue through the endplate into the vertebral marrow (1). Schmorl's nodes are often asymptomatic and are frequently discovered incidentally during clinical practice through magnetic resonance imaging (MRI) scans. However, in recent years, several studies have aimed to differentiate Schmorl's nodes from other morphological abnormalities such as indentations or defects on the endplate (2). For instance, Feng et al. distinguished among focal, corners, and erosive endplate lesions (3). Another study employed a more comprehensive endplate scoring system based on T1-weighted MRI scans to correlate lesion presence with the transport of a contrast agent into the intervertebral disc (4). More recently, Brayda-Bruno et al. proposed a novel classification scheme for endplate lesions based on T2-weighted MRI scans, which simplified and adapted the aforementioned scoring system (5). In this scheme, the intervertebral spaces are classified as “normal,” “wavy/irregular,” “notched,” and “Schmorl's node” (Figure 1). This classification scheme has been validated in terms of reliability between different observers and within the same observer, and has been evaluated for potential associations with disc degeneration, disc herniation, and low back pain (5–7).
The presence of endplate lesions and their relation to spinal pathologies, such as disc degeneration, disc herniation, and juvenile vertebral osteochondrosis (Scheuermann's disease), has been extensively investigated and confirmed in numerous studies (3, 8–17). The development of an automatic tool for classifying the type of endplate lesion would offer advantages in clinical practice by reducing the workload for operators and the time required for diagnosis. In addition, such a tool would enable the rapid and accurate processing of large datasets for research purposes. In this regard, artificial intelligence techniques have been increasingly employed in spine research to predict parameters from radiological images, aiming to provide reproducible and reliable evaluations (18, 19). Deep learning methods utilizing convolutional neural networks have been applied to recognize vertebral landmarks, reconstruct spinal alignment from radiographic images (20–23), and automatically segment and identify vertebrae in computed tomography and MRI scans (24). Moreover, specific applications, like the “SpineNet” computer vision-based system, have been developed to extract relevant measurements, such as Pfirrmann grade, Modic changes, spinal stenosis, and disc herniation, from MRI scans (23, 25–28). As a novel contribution, the present work exploits a deep learning model based on convolutional neural networks to automatically classify the type of endplate lesion according to the scheme proposed by Brayda-Bruno et al. (5), in a dataset of 1,559 intervertebral spaces obtained from retrospectively collected subjects.
2. Materials and methods
2.1. Classification of the endplate lesions
The scoring system for classifying intervertebral spaces based on endplate lesions, as described in reference (5), is summarized as follows: (i) “normal” classification is assigned when no lesions are visually identified in the sagittal MRI slices that encompass the intervertebral space. The endplates appear structurally intact without any noticeable abnormalities; (ii) “wavy/irregular” classification is given when there are no specific lesions detectable in the intervertebral space, but the shape of at least one of the endplates exhibits alterations compared with the typical curvature seen in a healthy intervertebral space. The endplate may appear wavy or irregular in shape; (iii) “notched” classification is assigned if a small lesion is visible in at least one slice of the sagittal MRI. The lesion has a V-shaped or circular appearance and is present on one or both of the endplates. These notches may indicate small defects or indentations on the endplates; (iv) “Schmorl's node” classification is used when a deep focal defect is observed in the vertebral endplate. The lesion has a smooth margin and a rounded appearance. Schmorl's nodes are characterized by disc tissue protruding through the endplate and into the vertebral marrow.
It is important to note that the additional class “fracture” mentioned in the reference study was excluded in the present work due to its limited representation in the considered group of subjects. Therefore, the focus of the study was on classifying intervertebral spaces into normal, wavy/irregular, notched, and Schmorl's node categories based on the identified lesions in the MRI scans (Figure 1). For more detailed information on the scoring system and its validation, please refer to the original reference (5).
2.2. Dataset and image processing
In this study conducted at the IRCCS Istituto Ortopedico Galeazzi in Milan, Italy, a retrospective search was performed using the Picture Archiving and Communication System (PACS) to identify anonymized subjects who had undergone lumbosacral MRI scans between June 2016 and January 2018 (Figure 2). The search included a Caucasian population ranging in age from 10 to 90 years. The MRI scans were performed using either 1.5 T scanners (Avanto and Espree, Siemens AG, Erlangen, Germany) or a 1.0 T scanner (Harmony, Siemens AG, Erlangen, Germany). The resulting dataset partially integrates that exploited in our previous study by Brayda-Bruno et al. (5). T2-weighted sagittal MRI scans of the lumbosacral spine were collected for consecutive patients. Only subjects with at least one spinal level showing the presence of endplate lesions were included in the study. An experienced radiologist reviewed the MRI scans and identified the lesion type for each intervertebral space from levels L1L2 to L5S1, according to the classification scheme proposed by Brayda-Bruno et al. (Figure 1).
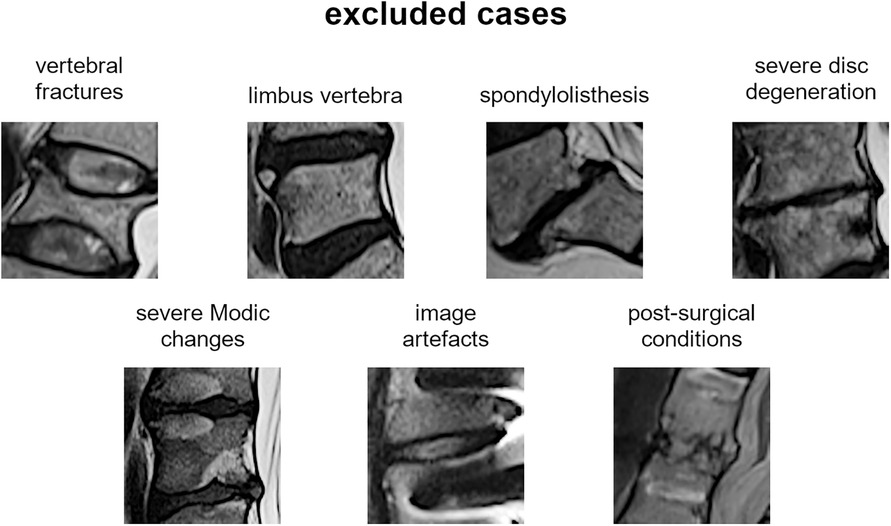
The most representative slice image, typically the middle slice, was selected for each patient. Spinal levels with certain conditions, such as vertebral fractures, limbus vertebra, spondylolisthesis, severe disc degeneration [graded Pfirrmann 5 (29)], severe Modic changes (30) (extending over 25% or more of the vertebral height), as well as image artifacts and postsurgical conditions (e.g., interbody fusion), were excluded from the analysis (Figure 3). The selected slice images were imported into a custom tool developed using MATLAB software (MathWorks Inc., Natick, MA, USA). The tool allowed the manual identification of the region of interest (ROI) for the intervertebral spaces by selecting the corners of the corresponding upper and lower vertebral endplates (Figure 4, left panel). The images were preprocessed by contrast enhancement (saturation of the bottom 1% and the top 1% of all pixel values), and the ROIs encapsulating the disc and approximately 30% of the upper and lower vertebrae, along with some spinal canal cerebrospinal fluid, were resized to 128 × 128 pixels. Gray padding was applied to fill the image height (Figure 4, right panel). A total of 1,559 images of intervertebral spaces were obtained, distributed among the following lesion types: “normal” (567 discs), “wavy/irregular” (485), “notched” (362), and “Schmorl's node” (145).
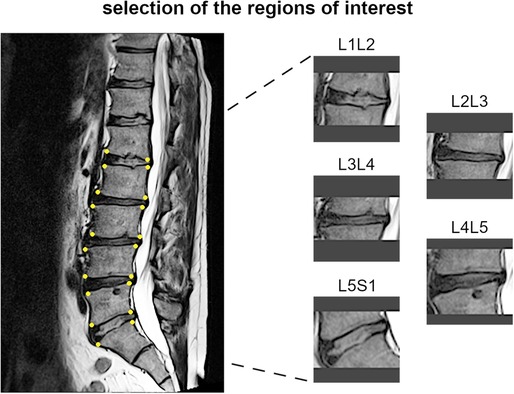
Figure 4. Example of identification of the region of interests for one subject, with the manually handpicked vertebral corners of the upper and lower endplates depicted as yellow dots (left panel) and the obtained images for the corresponding spinal levels (right panel).
The dataset was divided randomly into a training set (1,247 discs, 80%) and a validation set (312 discs, 20%), while maintaining the original distribution of the four lesion types in each set. A pretrained deep learning model based on the ResNet18 architecture, originally trained on the ImageNet dataset, was used for image classification. The model was fine-tuned using the training set by progressively unfreezing the weights of the deeper layers in multiple steps with increasing numbers of epochs. The network was trained for 100 epochs, with a learning rate of 0.001 that was reduced by a factor of 10 if the loss did not improve for 10 epochs. To address the class imbalance issue in the dataset, sample weighting correction was applied during the training process (31, 32). This correction involved assigning different weights to individual samples, which influenced their probability of being selected during the sampling process. The goal was to give more relevance to the underrepresented classes, ensuring that they had a higher chance of being chosen during training. This approach helps to balance the training process and prevent the model from favoring the majority class. A weighted random sampler function was used with a batch size of 32. This allowed for resampling the images from the underrepresented classes after each batch set was preprocessed using augmentation techniques such as small shifts, rotations, and flips, in order to increase the variety of the dataset and the robustness of the model.
The validation set was used to evaluate the performance of the trained model. The overall accuracy was calculated by dividing the number of correct predictions by the total number of samples in the validation set. In addition, the accuracy level for each specific lesion type, referred to as class sensitivity, was determined by dividing the number of correct predictions for that type by the number of samples in that specific class. Model calibration was performed using the Platt scaling method (33). This approach rescaled the predicted probabilities of the model to make them more representative of the true likelihood of occurrences of the classes present in the training data. It helped to mitigate potential overconfidence of the model, especially in the presence of unbalanced classes (34). To provide accurate estimations of the performance metrics, bootstrap resampling was carried out using a 1-vs.-all approach. A total of 500 iterations were run on the validation set to calculate the mean values of metrics such as area under the curve (AUC), accuracy, precision, recall, specificity, and F1-score. In addition, 95% confidence intervals were computed for these metrics to provide a measure of their variability and reliability. The image resizing and the implementation of the deep learning model were performed using Python, utilizing the open-cv library for image processing and the PyTorch framework (35) for the deep learning model. These tools and libraries are commonly used in computer vision and deep learning applications.
3. Results
The final dataset included subjects with an age range of 10–88 years, with a mean age of 52 ± 15 years. The gender distribution was equal, with 50% males and 50% females. The presence of endplate lesions generally increased with age (Table 1 and Figure 5A). The distribution of lesion types across age groups showed that normal cases were more common in subjects younger than 70 years, while Schmorl's node cases were predominantly found in subjects older than 40 years. The distribution of lesion types along the spinal levels was similar for normal and wavy/irregular types. Notched endplates were more prevalent at higher lumbar levels, and Schmorl's node cases were less represented only at the L5S1 level (Table 2 and Figure 5B).
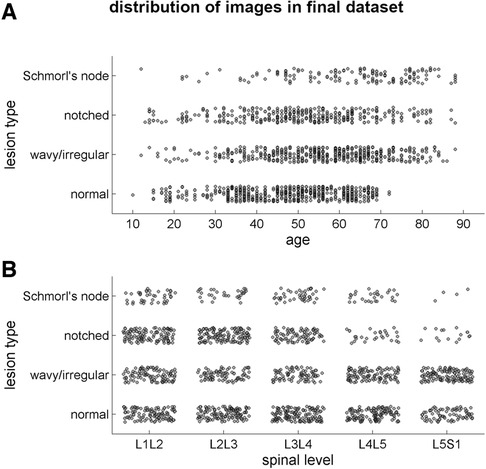
Figure 5. Scatter plots illustrating the distribution of the lesion types in the final image dataset (1,559 samples) depending on the age of the subjects (A) and on the spinal level (B).
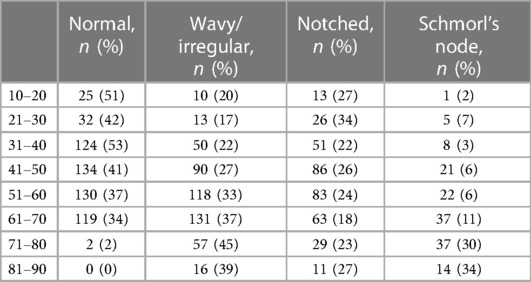
Table 1. Distribution of the endplate lesion types in the age range (indicated as the number of cases and corresponding percentage in the considered age).
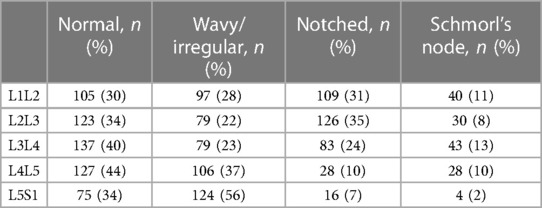
Table 2. Distribution of the endplate lesion types in the spinal levels (indicated as the number of cases and corresponding percentage in the considered level).
The deep learning model achieved an overall accuracy of 88% when processing the validation set (Figure 6). Out of 312 samples, 275 were correctly classified by the model. The accuracy rates for the specific lesion type were found as follows: 91% for normal type (104 in 114 samples), 82% for wavy/irregular (80 in 97), 93% for notched (67 in 72), and 83% for Schmorl's node (24 in 29). The model performance metrics, calculated using bootstrap resampling, showed average values of 96% for AUC, 88% for accuracy, 87% for precision, recall, and F1-score, and 96% for specificity (Table 3).
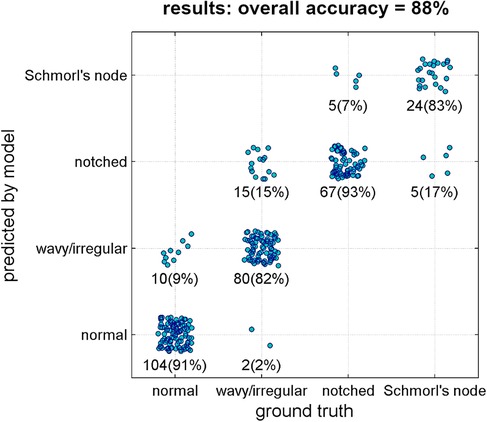
Figure 6. Results of the classification model (ground truth type vs. predicted type). Each point represents one single validation sample. Number of samples and corresponding percentage in the considered ground truth type are reported as well.
4. Discussion
The presence of endplate lesions in the lumbosacral spine was found in 35% of the subjects included in the study (580 out of 1,647 cases, Figure 2), which is consistent with the prevalence observed in a previous study (37%) using a similar methodology in a dataset of Caucasian subjects from the same institute but with a narrower time period (5). However, studies involving Chinese populations and random selection of subjects, including both back pain patients and healthy volunteers, have reported higher prevalence rates of approximately 60% (7, 12, 36). These variations in prevalence could be attributed to differences in assessment methodologies, subject inclusion criteria, and the presence of other spinal pathologies. Unfortunately, other studies assessing Caucasian populations did not provide the prevalence inside subjects but only in terms of evaluated discs, thus preventing a direct and consistent comparison with the prevalence findings of the current study (9, 37).
The final dataset obtained after applying exclusion criteria for intervertebral spaces (Figure 3) showed a similar distribution of lesion types across age ranges and spinal levels (Figure 5 and Tables 1, 2). However, there was a tendency for higher lesion prevalence with increasing age, which is in line with previous studies (3, 11). Schmorl's node was the least prevalent type (145 out of 1,559 discs), while wavy/irregular and notched types were similarly represented (485 and 362, respectively). In this regard, the sample weighting correction procedure used in training the deep learning model helped mitigate the effects of imbalance among the lesion types by adjusting their proportional contribution.
The validation of the model demonstrated a strong overall accuracy rate (88%, Figure 6), with similarly high percentages of correct predictions for each lesion type (ranging from 82% to 93%). The model performance metrics, including accuracy, precision, recall, specificity, and F1-score, ranged from 87% to 96% (Table 3), indicating the model's reliability and potential utility for research and clinical purposes. Specifically, the implementation of the model could facilitate the efficient processing of large image datasets in descriptive studies reducing the workload for operators, and potentially be integrated into existing systems for automatic detection of pathological conditions characterized by endplate lesions such as spinal osteochondrosis. In this regard, the potential integration with existing systems such as “SpineNet” (23, 25–28) is a valuable consideration. “SpineNet” is indeed currently capable of automatically recognizing the presence of endplate lesions, although as a binary outcome (presence or absence). In contrast, the present model developed in the study focuses on classifying the specific types of endplate lesions. By integrating the two approaches, it would be possible to enhance the capabilities of the system and provide more comprehensive information.
The present study has the following limitations. The number of images in the dataset is moderate for a deep learning approach, and the imbalance among the lesion types, particularly for Schmorl's node, poses a challenge. Although the trained model demonstrated high accuracy levels for each lesion type, larger and more balanced datasets, along with cross-validation analyses, would be beneficial for further model refinement. Another limitation is the arrangement of the training and validation sets, which were randomly selected at the image level rather than the subject level. The final dataset was indeed characterized by 1,559 images from 580 subjects, implying a variable number of spinal levels from each single subject. Such an approach was required to guarantee the fundamental aspect of preserving in each subset the original distribution of the four lesion types. This could result in data from the same individual being present in both subsets, although this should not significantly affect the results. Lastly, with regard to the evaluated subjects, no clinical data or relevant information regarding comorbidities or specific reasons for undergoing MRI scans could be retrieved from the retrospective search because they were not stored in the PACS. Although this information would be useful in characterizing the evaluated population and understanding the context of the observed endplate lesions, its absence does not directly impact the classification of lesion types by the developed model.
In conclusion, although further validation and refinement are necessary, the deep learning model demonstrated promising performance levels for the automatic detection and classification of endplate lesions. The model has potential applications in both research and clinical settings, but larger datasets and external validation (exploiting data from other populations, study groups, and scanning devices) are needed to establish its robustness and generalizability.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with national legislation and institutional requirements.
Author contributions
TB wrote the manuscript and implemented the software procedures in MATLAB. ACi and FG made substantial contributions to the development and application of the deep learning procedures. LMS, FB, and DA provided the image dataset and the lesion labeling from radiological examination. ACo and AL made substantial contributions to the interpretation of the results. MB-B made substantial contributions to the conception and design of the work. All authors critically revised the manuscript and approved the version to be published. All authors contributed to the article and approved the submitted version.
Funding
The study was fully supported by the Italian Ministry of Health (Ricerca Corrente).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor, EG, declared a past collaboration with the author(s).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Wang Y, Videman T, Battié MC. ISSLS prize winner: lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine. (2012) 37(17):1490–6. doi: 10.1097/BRS.0b013e3182608ac4
3. Feng Z, Liu Y, Yang G, Battié MC, Wang Y. Lumbar vertebral endplate defects on magnetic resonance images: classification, distribution patterns, and associations with Modic changes and disc degeneration. Spine. (2018) 43(13):919–27. doi: 10.1097/BRS.0000000000002450
4. Rajasekaran S, Venkatadass K, Naresh Babu J, Ganesh K, Shetty AP. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs: results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. Eur Spine J. (2008) 17(5):626–43. doi: 10.1007/s00586-008-0645-6
5. Brayda-Bruno M, Albano D, Cannella G, Galbusera F, Zerbi A. Endplate lesions in the lumbar spine: a novel MRI-based classification scheme and epidemiology in low back pain patients. Eur Spine J. (2018) 27(11):2854–61. doi: 10.1007/s00586-018-5787-6
6. Colombini A, Galbusera F, Cortese MC, Gallazzi E, Viganò M, Albano D, et al. Classification of endplate lesions in the lumbar spine and association with risk factors, biochemistry, and genetics. Eur Spine J. (2021) 30(8):2231–7. doi: 10.1007/s00586-021-06719-1
7. Pei J, Yu A, Geng J, Liu Y, Wang L, Shi J, et al. The lumbar spinal endplate lesions grades and association with lumbar disc disorders, and lumbar bone mineral density in a middle-young general Chinese population. BMC Musculoskelet Disord. (2023) 3;24(1):258. doi: 10.1186/s12891-023-06379-w
8. Mok FPS, Samartzis D, Karppinen J, Luk KDK, Fong DYT, Cheung KMC. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine. (2010) 35(21):1944–52. doi: 10.1097/BRS.0b013e3181d534f3
9. Munir S, Freidin MB, Rade M, Määttä J, Livshits G, Williams FMK. Endplate defect is heritable, associated with low back pain and triggers intervertebral disc degeneration: a longitudinal study from TwinsUK.. Spine. (2018) 43(21):1496–501. doi: 10.1097/BRS.0000000000002721
10. Rade M, Määttä JH, Freidin MB, Airaksinen O, Karppinen J, Williams FMK. Vertebral endplate defect as initiating factor in intervertebral disc degeneration: strong association between endplate defect and disc degeneration in the general population. Spine. (2018) 43(6):412–9. doi: 10.1097/BRS.0000000000002352
11. Wang Y, Videman T, Battié MC. Lumbar vertebral endplate lesions: prevalence, classification, and association with age. Spine. 2012;37(17):1432–9. doi: 10.1097/BRS.0b013e31824dd20a
12. Chen L, Battié MC, Yuan Y, Yang G, Chen Z, Wang Y. Lumbar vertebral endplate defects on magnetic resonance images: prevalence, distribution patterns, and associations with back pain. Spine J. (2020) 20(3):352–60. doi: 10.1016/j.spinee.2019.10.015
13. Din RU, Cheng X, Yang H. Diagnostic role of magnetic resonance imaging in low back pain caused by vertebral endplate degeneration. J Magn Reson Imaging. (2022) 55(3):755–71. doi: 10.1002/jmri.27858
14. Luoma K, Vehmas T, Kerttula L, Grönblad M, Rinne E. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J. (2016) 25(9):2873–81. doi: 10.1007/s00586-016-4715-x
15. Minetama M, Kawakami M, Teraguchi M, Matsuo S, Sumiya T, Nakagawa M, et al. Endplate defects, not the severity of spinal stenosis, contribute to low back pain in patients with lumbar spinal stenosis. Spine J. (2022) 22(3):370–8. doi: 10.1016/j.spinee.2021.09.008
16. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. (2014) 81(3):209–14. doi: 10.1016/j.jbspin.2013.11.012
17. Aufdermaur M. Juvenile kyphosis (Scheuermann’s disease): radiography, histology, and pathogenesis. Clin Orthop Relat Res. (1981) (154):166–74.7471550
18. Galbusera F, Casaroli G, Bassani T. Artificial intelligence and machine learning in spine research. JOR Spine. (2019) 2(1):e1044. doi: 10.1002/jsp2.1044
19. Galbusera F, Niemeyer F, Wilke H, Bassani T, Casaroli G, Anania C, et al. Fully automated radiological analysis of spinal disorders and deformities: a deep learning approach. Eur Spine J. (2019) 28(5):951–60. doi: 10.1007/s00586-019-05944-z
20. Cai Y, Landis M, Laidley DT, Kornecki A, Lum A, Li S. Multi-modal vertebrae recognition using transformed deep convolution network. Comput Med Imaging Graph. (2016) 51:11–9. doi: 10.1016/j.compmedimag.2016.02.002
21. Cina A, Bassani T, Panico M, Luca A, Masharawi Y, Brayda-Bruno M, et al. 2-Step deep learning model for landmarks localization in spine radiographs. Sci Rep. (2021) 11(1):9482. doi: 10.1038/s41598-021-89102-w
22. Galbusera F, Bassani T, Panico M, Sconfienza LM, Cina A. A fresh look at spinal alignment and deformities: automated analysis of a large database of 9832 biplanar radiographs. Front Bioeng Biotechnol. (2022) 10:863054. doi: 10.3389/fbioe.2022.863054
23. Huang J, Shen H, Wu J, Hu X, Zhu Z, Lv X, et al. Spine explorer: a deep learning based fully automated program for efficient and reliable quantifications of the vertebrae and discs on sagittal lumbar spine MR images. Spine J. (2020) 20(4):590–9. doi: 10.1016/j.spinee.2019.11.010
24. Lessmann N, van Ginneken B, de Jong PA, Išgum I. Iterative fully convolutional neural networks for automatic vertebra segmentation and identification. Med Image Anal. (2019) 53:142–55. doi: 10.1016/j.media.2019.02.005
25. Grob A, Loibl M, Jamaludin A, Winklhofer S, Fairbank JCT, Fekete T, et al. External validation of the deep learning system “SpineNet” for grading radiological features of degeneration on MRIs of the lumbar spine. Eur Spine J. (2022) 31(8):2137–48. doi: 10.1007/s00586-022-07311-x
26. Ishimoto Y, Jamaludin A, Cooper C, Walker-Bone K, Yamada H, Hashizume H, et al. Could automated machine-learned MRI grading aid epidemiological studies of lumbar spinal stenosis? Validation within the Wakayama spine study. BMC Musculoskelet Disord. (2020) 21(1):158–1. doi: 10.1186/s12891-020-3164-1
27. Jamaludin A, Kadir T, Zisserman A. SpineNet: automated classification and evidence visualization in spinal MRIs. Med Image Anal. (2017) 41:63–73. doi: 10.1016/j.media.2017.07.002
28. Jamaludin A, Lootus M, Kadir T, Zisserman A, Urban J, Battié MC, et al. ISSLS Prize in Bioengineering Science 2017: automation of Reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. Eur Spine J. (2017) 26(5):1374–83. doi: 10.1007/s00586-017-4956-3
29. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. (2001) 26(17):1873–8. doi: 10.1097/00007632-200109010-00011
30. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. (1988):166–1(Pt 1):193–199. doi: 10.1148/radiology.166.1.3336678
31. He K, Gkioxari G, Dollar P, Girshick R. Mask R-CNN. IEEE Trans Pattern Anal Mach Intell. (2020) 42(2):386–97. doi: 10.1109/TPAMI.2018.2844175
32. Lin T, Goyal P, Girshick R, He K, Dollar P. Focal loss for dense object detection. IEEE Trans Pattern Anal Mach Intell. (2020) 42(2):318–27. doi: 10.1109/TPAMI.2018.2858826
33. Lin H, Lin C, Weng RC. A note on Platt’s probabilistic outputs for support vector machines. Mach Learning. (2007) 68(3):267–76. doi: 10.1007/s10994-007-5018-6
34. Rajaraman S, Ganesan P, Antani S. Deep learning model calibration for improving performance in class-imbalanced medical image classification tasks. PLoS One. (2022) 17(1):e0262838. doi: 10.1371/journal.pone.0262838
35. Paszke A, Gross S, Massa F, Lerer A, Bradbury J, Chanan G, et al. PyTorch: an imperative style, high-performance deep learning library. Adv Neural Inf Process Syst. (2019) 32. doi: 10.48550/arXiv.1912.01703
36. Zehra U, Cheung JPY, Bow C, Lu W, Samartzis D. Multidimensional vertebral endplate defects are associated with disc degeneration, Modic changes, facet joint abnormalities, and pain. J Orthop Res. (2019) 37(5):1080–9. doi: 10.1002/jor.24195
Keywords: spine, endplate lesions, osteochondrosis, artificial intelligence, deep learning, automatic classification, convolutional neural networks
Citation: Bassani T, Cina A, Galbusera F, Sconfienza LM, Albano D, Barcellona F, Colombini A, Luca A and Brayda-Bruno M (2023) Automatic classification of the vertebral endplate lesions in magnetic resonance imaging by deep learning model. Front. Surg. 10:1172313. doi: 10.3389/fsurg.2023.1172313
Received: 23 February 2023; Accepted: 8 June 2023;
Published: 22 June 2023.
Edited by:
Enrico Gallazzi, Istituto Ortopedico Gaetano Pini, ItalyReviewed by:
Simone Faenza, Istituto Ortopedico Gaetano Pini, ItalyValerio Maria Caccavella, Istituto Ortopedico Gaetano Pini, Italy
© 2023 Bassani, Cina, Galbusera, Sconfienza, Albano, Barcellona, Colombini, Luca and Brayda-Bruno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tito Bassani dGl0by5iYXNzYW5pQGdydXBwb3NhbmRvbmF0by5pdA==
 Tito Bassani
Tito Bassani Andrea Cina
Andrea Cina Fabio Galbusera
Fabio Galbusera Luca Maria Sconfienza
Luca Maria Sconfienza Domenico Albano1
Domenico Albano1 Alessandra Colombini
Alessandra Colombini Andrea Luca
Andrea Luca