- 1Covalent Bio, LLC, St. Louis, MO, United States
- 2Section of Minimally Invasive Surgery, David Geffen School of Medicine at University of California, Los Angeles, Santa Monica, CA, United States
- 3Hospital Universitario Vall d'Hebrón, Universidad Autónoma de Barcelona, Barcelona, Spain
- 4Becton, Dickinson and Company, Warwick, RI, United States
Background: Poly-4-hydroxybutyrate (P4HB) is a fully resorbable, biologically-produced polymer with a strength and flexibility comparable to permanent synthetic polymers. The objective was to identify/summarize all peer-reviewed publications involving P4HB mesh.
Methods: A scoping review was conducted within PubMed and included articles published through October 2022.
Results: A total of n = 79 studies were identified (n = 12 in vitro/bench; n = 14 preclinical; n = 6 commentaries; n = 50 clinical). Of the clinical studies, n = 40 reported results applicable to hernia and n = 10 to plastic/reconstructive surgery and involved patients of all Centers for Disease Control (CDC) wound classes and Ventral Hernia Working Group (VHWG) grades.
Conclusion: P4HB mesh provides long-term hernia repair strength and exhibits promising clinical outcomes beyond its resorption period. Future studies should include randomized controlled trials comparing P4HB to other biomaterials, as well as optimal patient selection, operative technique, long-term outcomes, minimization of potential mesh-related complications, and potential contraindications/complications for P4HB in hernia/abdominal wall reconstruction.
1. Introduction
The development of a unique, fully resorbable, biologically-produced, thermoplastic polyester called poly-4-hydroxybutyrate (P4HB) was first reported in 2003 for implantable medical device applications (TephaFLEX®, Tepha Inc., Lexington, Massachusetts) (1). While the chemical synthesis of P4HB is possible (2–4), the molecular weight needed for practical applications as an implantable fiber or mesh can presently only be achieved by fermentation, and there are currently no chemically synthesized P4HB-based products used in commercial medical products or devices. Rather, P4HB is typically produced through a biologic recombinant fermentation process using Escherichia coli K12, a microorganism widely utilized in the biopharmaceutical industry to develop other products for human use (1, 5–7). Thus, P4HB is free of the residual metal catalysts that are common in chemically-derived polymers (1, 7). The resulting P4HB is extracted from the fermented cells, purified and processed into fibers using established plastics processing techniques (1, 5, 8).
P4HB is a strong and flexible material, with a tensile strength comparable to permanent synthetic polymers such as polypropylene and ultrahigh molecular weight polyethylene and can be stretched up to 10× its initial length prior to failure (1, 6, 7). P4HB can also be tailored to achieve a wide range of physical and mechanical properties as desired for various applications (1). P4HB has a long-term degradation profile of 12–18 months (5, 9) and degrades into 4HB fragments through bulk hydrolysis, as well as surface erosion (5, 7). With a half-life of approximately 30 min, 4HB degradation products are quickly metabolized through the Krebs cycle, and eliminated as carbon dioxide and water (1, 5, 7). The gradual resorption process results in a steady decline in mechanical strength for a P4HB mesh as the load is progressively transferred back to the repaired tissue (1, 6). This is a major benefit compared to many other resorbable polymers that degrade rapidly through bulk hydrolysis, resulting in a steep decline in mechanical strength before the wound has had time to sufficiently remodel (1, 7).
The United States Food and Drug Administration (FDA) cleared monofilament P4HB suture and monofilament P4HB surgical mesh for human use in 2007, followed by regulatory clearance in Europe in 2009 (5). P4HB has subsequently been utilized to develop a variety of commercially-available devices, including: sutures for tissue approximation (MonoMax® Suture, Aesculap AG, Tuttlingen, Germany), scaffolds to support tendon and ligament repair (BioFiber™ Scaffold, Tornier, Inc., Edina, MN), biomaterials for plastic and reconstructive surgery (GalaFLEX® Scaffold, Galatea Surgical, Inc., Lexington, MA), and meshes for hernia repair (Phasix™ Mesh, Becton, Dickinson, and Company, Franklin Lakes, NJ) (1, 5–7). Currently, P4HB mesh is available in two configurations for hernia repair: as a bare, macroporous mesh (Phasix™ Mesh) or combined with a resorbable hydrogel layer (Phasix™ ST Mesh). The hydrogel layer, comprised of sodium hyaluronate (HA), carboxymethylcellulose (CMC), and polyethylene glycol (PEG) (9), serves as a barrier that minimizes tissue adherence of the bowels to the underlying mesh by separating the abdominal viscera from the P4HB mesh structure (10). The uncoated side of the mesh is porous to allow tissue ingrowth the abdominal wall. The hydrogel is fully resorbed in approximately 30 days (10). The addition of this hydrogel barrier layer to P4HB mesh provides a fully resorbable, barrier mesh option appropriate for intraperitoneal placement (11, 12).
In the last decade, P4HB mesh has been clinically studied in the scientific literature across several surgical specialties (7). In the area of plastic and reconstructive surgery, P4HB mesh has been used primarily in breast surgery (13–21), with some early work in rhytidectomy (16, 22). P4HB mesh has also been evaluated as a potential biomaterial for the repair of pelvic organ prolapse in urogynecological surgery (23–26). The majority of data and clinical research using P4HB mesh has focused on general surgery applications, particularly in the area of hernia repair (i.e., ventral/incisional hernia, incisional hernia prophylaxis, inguinal hernia, and hiatal/paraesophageal hernia) (27–31). The objective of the current study was to review the results of all peer-reviewed studies involving P4HB mesh, guided by the following research questions:
(1) How has P4HB mesh been evaluated to date (i.e., benchtop, preclinical, and clinical peer-reviewed studies)?
(2) What is the clinical role of P4HB mesh in hernia repair, abdominal wall surgery, and plastic/reconstructive surgery?
(3) What are the knowledge gaps and opportunities for future research for P4HB?
2. Methods
A scoping review of the published literature was conducted according to the PRISMA-ScR Guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses—extension for Scoping Reviews) (32). A search string with Boolean operators was utilized to retrieve results within the PubMed database, along with a hand search of references from eligible articles, reviews on the topic that were not identified in the literature search, and personal reference collections. Search terms included: “poly-4-hydroxybutyrate” OR “4-hydroxybutyrate mesh” OR “P4HB” OR “Phasix” + “hernia” and included articles published through October 2022. Inclusion criteria included: all peer-reviewed papers involving P4HB mesh, that were published in the English language, and which could be obtained as a full-text. Abstracts, posters, slide presentations, and Letters to the Editor were excluded, as well as articles in which 4HB was combined with other monomers to form a copolymer; P4HB was used as a coating material rather than as a mesh construct; or those articles in which it was determined to be a polymer other than P4HB after review of the full-text.
Outcomes of interest were recorded in a tabular format. Studies were grouped according to category (i.e., bench, preclinical, clinical, commentary). Publication date, mesh type, summary of results, and citation were recorded for all study categories. For bench studies, the type of study and its objective, the technique used, and the results were collected. For preclinical studies in animals, the species used, the surgical technique, the mesh evaluated, and the implantation time were recorded. For clinical studies, the number of patients in the trial, as well as details of the surgical technique and study design were recorded. Additionally, follow-up period and clinical outcomes such as quality of life metrics and rate of hernia recurrence, surgical site infection (SSI), and reoperation were captured and tabulated. Centers for Disease Control (CDC) wound classification (33) and Ventral Hernia Working Group (VHWG) grades (34, 35) were likewise reported. Studies were classified as “restricted” if the study design limited the inclusion of patients with specific CDC wound classification or VHWG grades based on either the original (31) or modified (32) grading systems. Similarly, studies were classified as “unrestricted” if the study design permitted the inclusion of all CDC wound classes and VHWG grades.
3. Results
Following the PRISMA-ScR schematic (Figure 1), a total of n = 79 full-text, peer-reviewed publications were identified that involved P4HB mesh and were published in the English language. The articles were subdivided into type of study—bench (n = 12), preclinical (n = 14), commentaries (n = 6), and clinical (n = 50) in Figure 2 and Table 1. The clinical studies were further subdivided into plastic and reconstructive surgery (n = 10) and general surgery (n = 40). The general surgery population was then divided into incisional hernia prophylaxis (n = 2) and hernia repair based on type: inguinal hernia (n = 2), hiatal/paraesophageal hernia (n = 5), and ventral/incisional hernia (n = 31). Lastly, all ventral/incisional hernias were stratified, when possible, by CDC wound class, VHWG grade, and/or follow-up period. All published studies are summarized in Table 1, including commentaries comprised of plastic and reconstructive surgery (16, 17, 87), general surgery (86, 88), and urogynecology (25). Table 2 summarizes the clinical outcomes associated with P4HB mesh in incisional hernia prophylaxis, inguinal hernia repair, and hiatal/paraesophageal hernia repair. Table 3 summarizes the clinical outcomes associated with ventral/incisional hernia repair. Studies without specified follow-up time, case reports, and protocols without outcomes data were not included in Tables 3 or 4, but are reported in Table 1 for completeness.
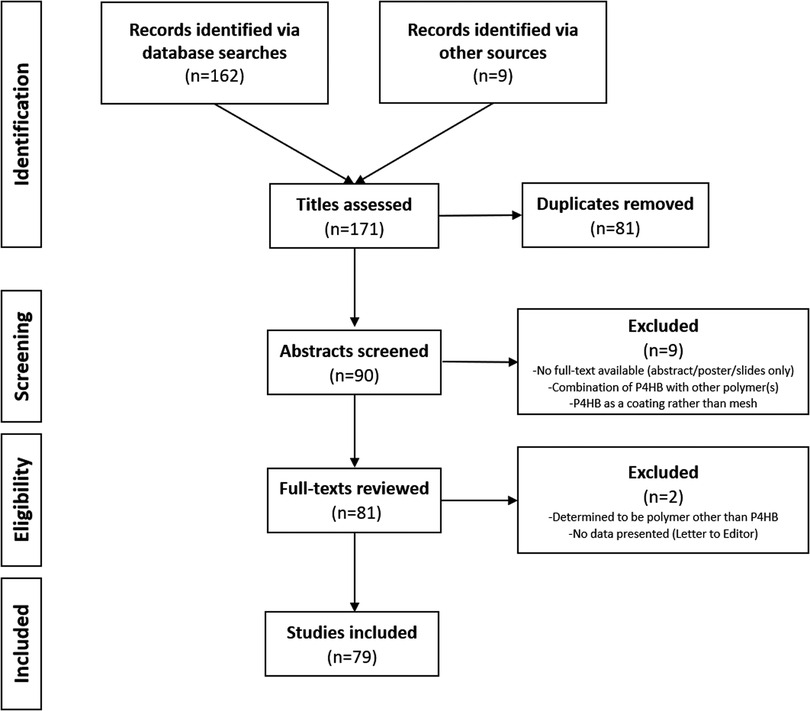
Figure 1. PRISMA-ScR diagram—preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews.
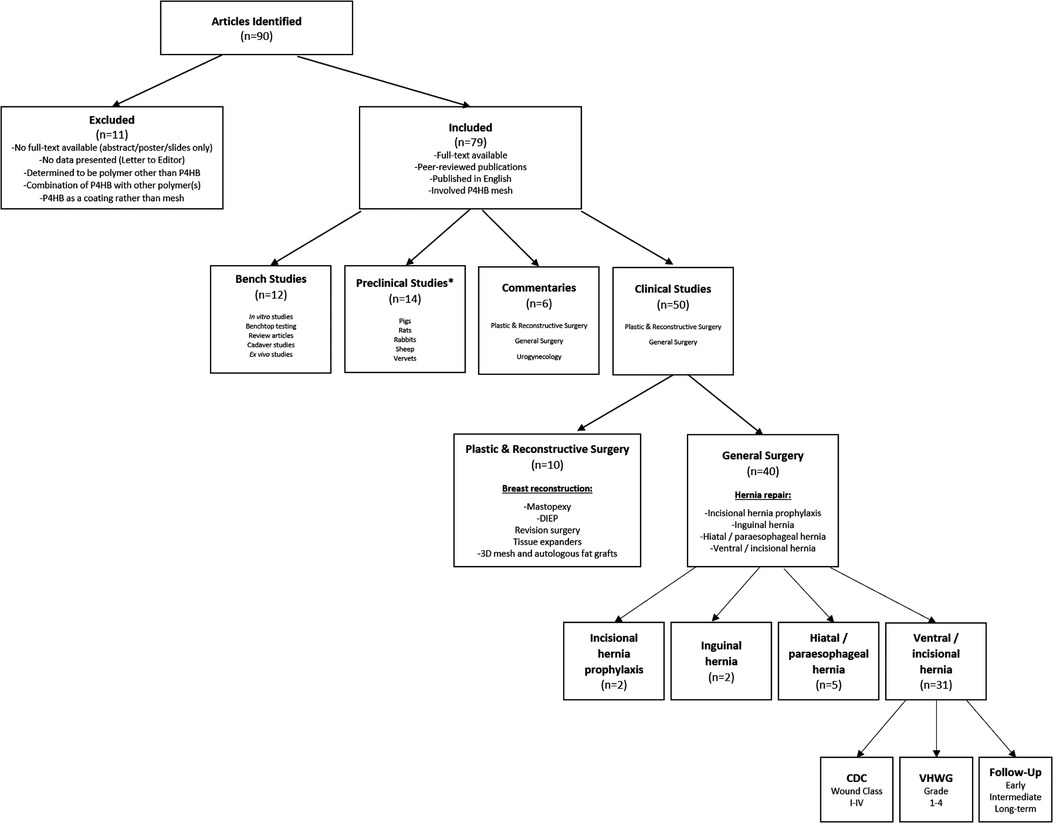
Figure 2. Flowchart depicting depth and breadth of studies that have evaluated P4HB mesh. *n = 3 articles with both Bench and Preclinical components. DIEP, deep inferior epigastric perforator flap.

Table 1. Study design parameters and outcomes associated with all published studies involving P4HB mesh.
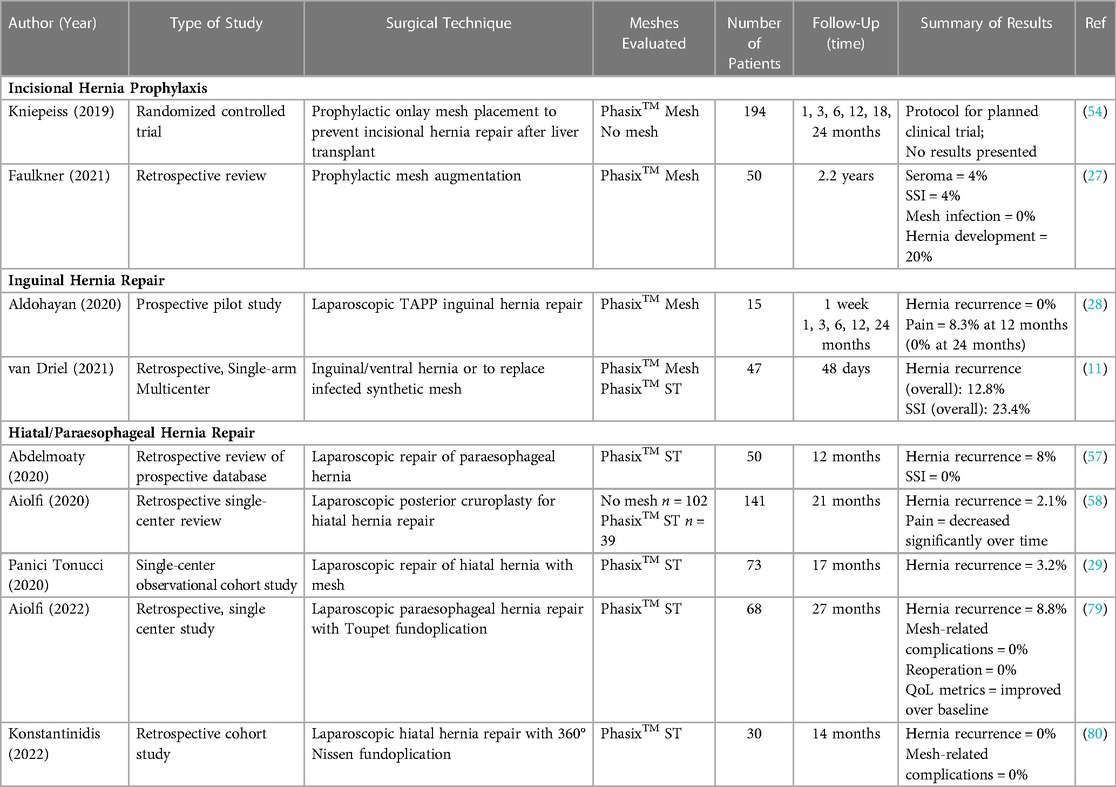
Table 2. Clinical outcomes associated with P4HB mesh in incisional hernia prophylaxis, inguinal hernia repair, and hiatal/paraesophageal hernia repair.
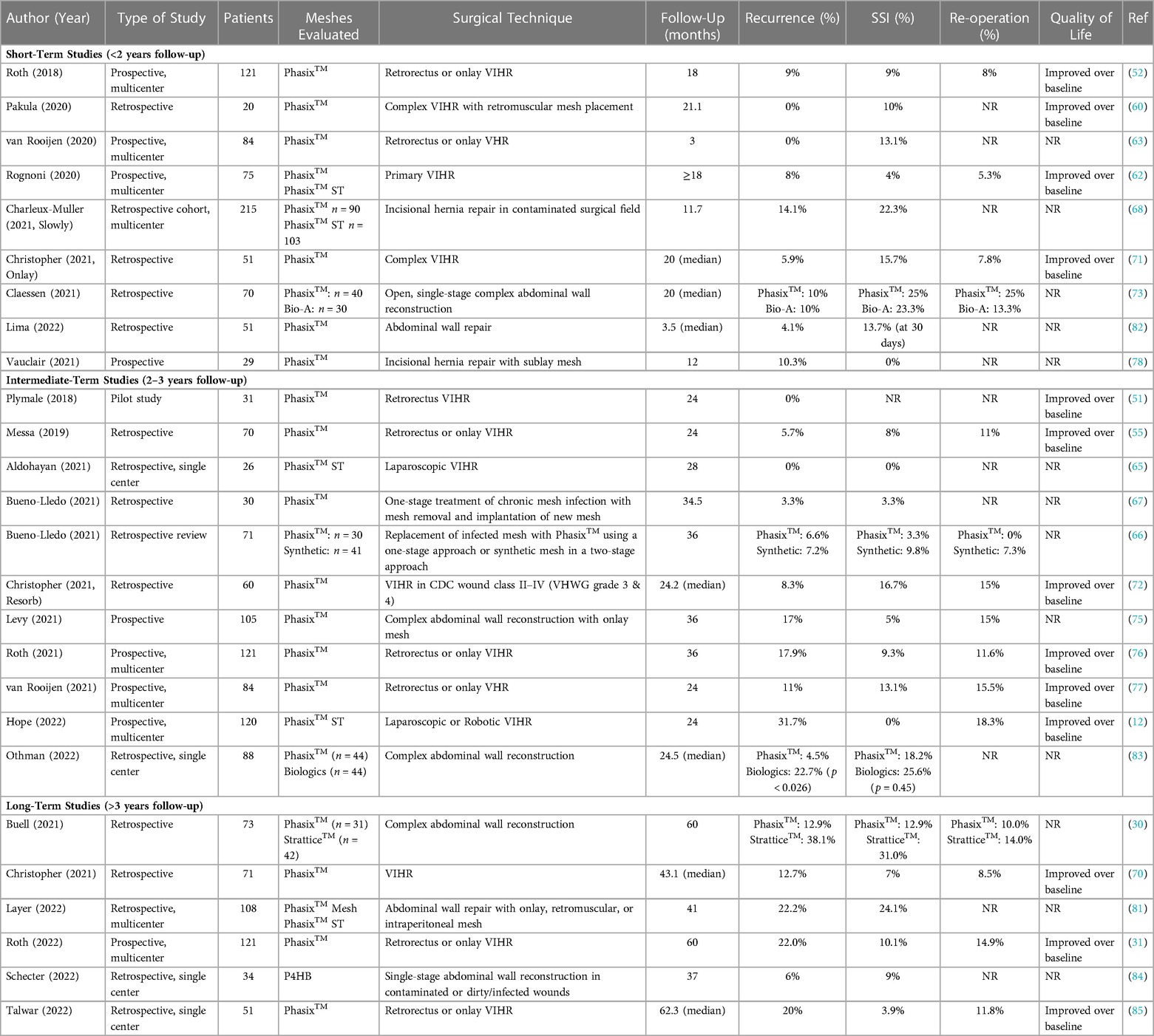
Table 3. Clinical outcomes associated with P4HB mesh in ventral/incisional hernia repair; categorized by follow-up time.
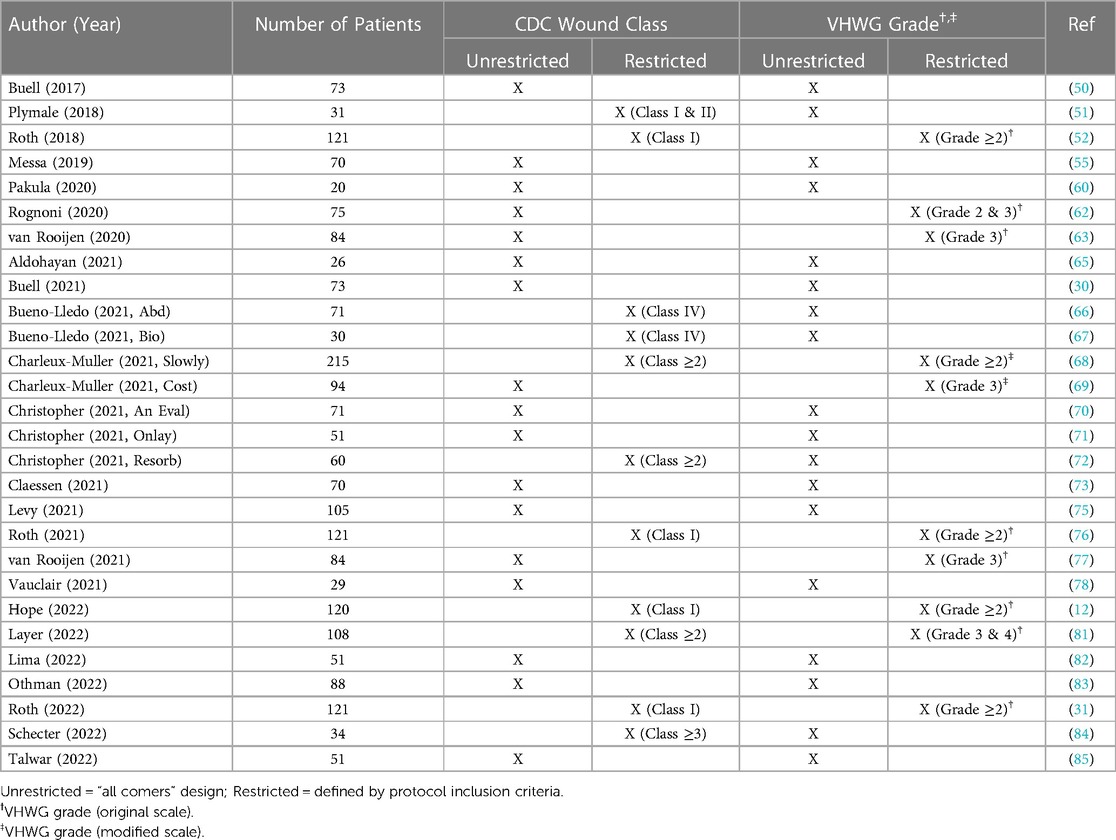
Table 4. CDC wound class and ventral hernia working group (VHWG) grade associated with clinical studies involving P4HB mesh in ventral/incisional hernia repair.
A subset of the identified publications (n = 12) involved in vitro studies, benchtop testing, cadaver studies, review articles, and ex vivo studies that were broadly categorized as “bench studies” as shown in Figure 2, and Table 1 (5–8, 22, 36–42). Several major themes were identified within these studies, namely a correlation between the mechanical strength of P4HB meshes and their remaining molecular weight during degradation studies, as well as the cellular response to P4HB mesh constructs and their degradation products. In an in vitro degradation test performed by Martin et al. (6), P4HB mesh exhibited a significant reduction in mechanical strength and molecular weight over time, which correlated with the integrity of the individual fibers comprising the mesh. They found that when changes to the structure of the mesh could first be appreciated visually through scanning electron microscopy, the mechanical strength and molecular weight of the P4HB mesh had already been reduced by approximately 90% (6). Martin et al., utilized this mechanical strength and molecular weight data to construct a standard curve that can be used to predict the ball burst strength of P4HB mesh when molecular weight is known. Care must be used, however, before applying such a correlation to different forms of the polymer, as the degree of orientation imparted during processing affects the susceptibility of P4HB to enzymatic degradation and surface erosion in vivo.
A number of bench studies also investigated the degradation products of P4HB mesh (i.e., 4HB monomer) using murine bone marrow-derived macrophages (36, 37, 42). These studies found that the degradation products are not cytotoxic, and in fact, may help promote bacterial clearance by macrophages through an upregulation of antimicrobial peptides (AMPs) (36, 37, 42). The degradation products have also been shown to influence the cellular expression of macrophage phenotypes of murine bone marrow-derived macrophages (37) and potentially provoke a unique polarization state in human peripheral blood-derived macrophages (41). Cellular response to P4HB meshes was favorable compared to polypropylene meshes, including higher collagen I : III ratio, as well as improved cellular attachment and proliferation (38, 40). Additionally, despite greater surface area relative to polypropylene mesh, P4HB meshes were not associated with greater bacterial adhesion or biofilm formation (39).
Fourteen publications identified by this scoping review involved preclinical studies in a variety of animal models and species, including pigs, rats, rabbits, sheep, mice, and vervets (Figure 2, and Table 1) (6, 23, 24, 26, 36, 37, 43–49, 89). Three of these studies (n = 3) contained both a benchtop component and a preclinical component (6, 36, 37). These studies are reported in both categories and included in Figure 2, and Table 1. Several outcomes were identified within these preclinical studies. First, P4HB meshes provided a durable and long-lasting hernia repair with mechanical strength of the mesh-repaired site equal to or greater than the native porcine abdominal wall in studies of 24-, 52- and 72-week duration (i.e., 6-, 12- and 18-month duration) (6, 43, 44). Second, P4HB meshes activated macrophages from a pro-inflammatory phenotype (M1) to a pro-remodeling phenotype (M2) earlier in the postoperative period than all of the other meshes evaluated (i.e., Bard™ Mesh, TIGR®, Bio-A®, and Strattice™ meshes). Finally, in numerous studies involving subcutaneous dorsal implantation of P4HB mesh with deliberate bacterial contamination, monofilament P4HB meshes exhibited significantly reduced bacterial colonization relative to other mesh types (36, 45, 89).
The majority of the identified publications (n = 50/79; 63%) involved clinical studies describing the use of P4HB mesh in plastic and reconstructive surgery (n = 10) (13–15, 18–21, 56, 61, 64) or general surgery applications (n = 40) (11, 12, 27–31, 50–55, 57–60, 62, 63, 65–85) as shown in Figure 2, and Table 1. In the area of plastic and reconstructive surgery, P4HB meshes have been utilized primarily in cosmetic breast applications (21) including mastopexy/reduction mammaplasty (18, 19), prevention of bulge at the donor site on the abdominal wall (14, 64), support of the lower pole (13, 20, 56), tissue expander-based reconstruction (15), and 3-D mesh breast reconstruction with autologous fat grafting (61).
P4HB meshes have been utilized extensively in general surgery for a wide variety of hernia repair and abdominal wall reconstruction applications (11, 12, 27–31, 50–55, 57–60, 62, 63, 65–85). A small number of studies (n = 2 in each group) evaluated P4HB mesh for incisional hernia prophylaxis (27, 54) and inguinal hernia repair (Table 1, and Table 2) (11, 28). Several studies (n = 5) also explored the use of P4HB mesh in hiatal/paraesophageal hernia repair (Table 1, and Table 2) (29, 57, 58, 79, 80). These studies reported favorable clinical outcomes, including low hernia recurrence rates (range: 0%–8%) and no surgical site infections (SSI), mesh-related complications, or reoperations with follow-up periods of at least 1 year.
Most of the general surgery studies identified by this literature review (n = 31/40; 78%) utilized P4HB mesh in the repair of ventral/incisional hernias, primarily at the midline (Table 1 and Table 3) (12, 30, 31, 50–53, 55, 59, 60, 62, 63, 65–78, 81–85). Primary fascial closure was planned in all cases, with definitive repair as the goal. In contaminated cases, bridging repair with P4HB was performed as an initial stage operation, with a future, planned definitive repair. Twenty-five (n = 25) studies utilized retrorectus/retromuscular, n = 11 underlay, n = 18 onlay, and n = 2 inlay mesh repair, with many studies incorporating a variety of surgical approaches and tissue planes. These studies documented a wide range of follow-up periods, including short-term (<2 years), intermediate (2–3 years), and long-term (>3 years) studies (Table 3), with P4HB meshes implanted in several different tissue planes, through a variety of surgical techniques. It should be noted that clinical outcomes were evaluated in patients along the entire continuum of Centers for Disease Control (CDC) wound classes (33) and Ventral Hernia Working Group (VHWG) grades (34, 35), ranging from clean cases at low risk of postoperative complications to contaminated/dirty cases at high risk of postoperative complications (Table 4). Systematic reviews (59), commentaries (86), and clinical studies of CDC Class I (clean) cases only (51, 65) comprised a small number of articles, with the majority of the identified clinical studies describing complex cases with elevated CDC wound class or comorbidities placing patients at high risk of postoperative complications (n = 13) (12, 30, 31, 50, 52, 55, 60, 62, 71, 75, 76, 83, 85) or “off-label” use of P4HB mesh to repair ventral/incisional hernias in potentially contaminated or contaminated fields (n = 15) (11, 53, 63, 66–70, 72–74, 77, 78, 81, 82, 84). After a thorough evaluation of the clinical studies identified by this scoping review, several major themes emerged, namely: (1) P4HB mesh provides long-term strength at the repair site, leading to acceptable rates of recurrence as compared to higher-risk cohorts and those repaired with non-synthetic biomaterials (30, 31, 66, 70, 75, 76); (2) P4HB mesh performs favorably in contaminated settings where permanent synthetic mesh use may be higher risk or contraindicated, resulting in low incidence of surgical site infection (SSI) (70, 76, 90); and (3) P4HB mesh represents an alternative for ventral/incisional hernia repair relative to biologic meshes and when permanent synthetic mesh complications are taken into consideration (30, 69).
P4HB mesh provides a long-term resorption profile with an initial mechanical strength similar to permanent synthetic mesh (6). However, once the P4HB polymer has been fully resorbed, the mesh contributes negligible strength to the overall repair, allowing for the potential for hernia recurrence. The current scoping review documented comparable recurrence rates for P4HB mesh at 3 and 5 years postimplantation [6.6%–17.9% (66, 70, 75, 76) and 12.9%–22.0% (30, 31), respectively] as permanent synthetic mesh at 5 years postimplantation (12.7%) (91) in study populations involving a variety CDC wound classes and VHWG grades.
The clinical literature also revealed that P4HB mesh performs favorably in the setting of contamination, evidenced by low rates of SSI at 3 years postimplantation. Similar rates were reported in studies involving all CDC wound classes and VHWG grades (i.e., 5%–7%) (70, 90). These outcomes are supported by the bench and preclinical studies in which P4HB mesh degradation products were shown to promote bacterial resistance and tissue remodeling through upregulation of AMPs and influence over macrophage phenotype (36, 37). In CDC class I (clean) wounds, P4HB was associated with 9.3% SSI, a rate higher than seen in mesh based cohorts (76). However, permanent synthetic meshes have been associated with higher rates of SSI in studies involving CDC wound classes II–IV [e.g., 7%–19% SSI at 30 days postimplantation in studies involving CDC wound classes II–III (92) and 14% SSI at a median follow-up of 24 months in studies involving CDC wound classes II–IV (93)].
Studies demonstrated a cost savings associated with P4HB mesh relative to biological tissue-based materials such as porcine dermal matrix (Strattice™, Allergen Aesthetics, Madison, NJ) (30, 69). In a study by Buell et al., P4HB mesh exhibited improved clinical outcomes including hernia recurrence and was associated with a cost savings of $10,595 compared to porcine dermal matrix (Strattice™, p = 0.005) (30). In a cost-effectiveness model, Charleux-Muller et al., showed that P4HB mesh repairs are associated with fewer serious complications (21% vs. 33%) and are less costly than biological tissue-derived materials (cost savings of $42,883) in VHWG Grade 3 hernia repairs (69).
4. Discussion
Poly-4-hydroxybutyrate (P4HB) mesh has been studied for more than a decade, including a broad collection of in vitro, preclinical, and clinical studies. To date, this biomaterial has been utilized in patients along the entire spectrum of CDC wound classes and VHWG grades, ranging from patients with clean wounds at low risk of postoperative complications to patients with contaminated/dirty wounds at high risk of postoperative complications. Short-term, intermediate, and long-term clinical studies have been performed, including implantation in many different tissue planes, using a wide variety of surgical techniques. This comprehensive literature review yielded 79 peer-reviewed, published studies evaluating P4HB mesh in a wide variety of bench, preclinical, and clinical study designs. Importantly, 40 clinical studies provide a current understanding of the performance of P4HB mesh in hernia repair and abdominal wall reconstruction. In the scope of hernia repair, P4HB remains a novel material. These early studies suggest that P4HB mesh provides strength at the repair site beyond its resorption profile, leading to reasonable rates of recurrence especially in settings where use of permanent synthetic mesh is avoided or contraindicated. P4HB performed favorably in the setting of contamination, resulting in low incidence of surgical site infection (SSI). Recent long-term studies, including Roth (31) and Talwar (85), also demonstrated improvement in long-term quality of life metrics.
Preclinical studies demonstrate long-term resorption of P4HB over a period of approximately 12–18 months, during which the mesh loses all of its mechanical strength (6, 43, 44, 47). The clinical studies identified in this literature review provided insight into the performance of P4HB in a variety of patient populations and clinical scenarios. Once the P4HB polymer has been fully resorbed, the mesh no longer contributes to the mechanical strength of the repair. This property has important implications in hernia repair, stressing the importance of operative technique and primary fascial closure to achieve durable long-term results. Two studies utilized P4HB mesh in the inlay position, serving primarily as tissue coverage and to prevent fascial retraction as a salvage or staged operation for future definitive repair. In all clinical studies where definitive hernia repair was desired, the defect was closed with mesh either in the underlay, retrorectus/retromuscular plane, or onlay position. These findings compliment the premise that the long-term resorbable matrix confers mechanical strength during the vulnerable phases of wound healing, shifting the curve to favorable mature scar formation with the potential for a durable repair beyond the resorption profile of the P4HB.
Several clinical studies warrant in-depth discussion. First, in a study restricted to patients with CDC class I (clean) wounds at high risk of developing postoperative complications (i.e., VHWG grade ≥2) Roth et al. evaluated clinical outcomes including SSI, hernia recurrence, and quality of life over a period of 5 years (31, 52, 76). Incidence of SSI remained consistent over time with 9.0%, 9.3%, and 10.1% SSI reported at 18, 36, and 60 months, respectively (31, 52, 76). Throughout the follow-up period, recurrence rates rose from 9% at 18 months to 17.9% at 36 months, and ultimately, 22% at 60 months postimplantation (31, 52, 76). However, these reported recurrence rates are interesting given that at this long-term time point, it is expected that there is no residual material and can be viewed as acceptable given a patient population at high risk of postoperative complications such as recurrence (i.e., VHWG grade ≥2). In this context, P4HB is a viable option, especially where avoidance of a permanent synthetic is desired based upon patient factors, operative conditions, and both patient and surgeon preference. Kanters et al. have demonstrated that the risk of hernia recurrence increases significantly in patient populations with elevated VHWG grade (35). In this context, long-term recurrence rates for P4HB mesh are comparable to those reported for permanent synthetic meshes at 5 and 10 years [12.7% (91) and 32% (94), respectively].
In a study involving patients across all CDC wound classes and all VHWG grades, Buell et al. evaluated long-term clinical outcomes associated with P4HB mesh compared to a porcine dermal matrix (Strattice™) (30). At 60-months postimplantation, Strattice™ was associated with approximately 3× greater incidence of recurrence (38.1%) and SSI (31.0%) compared to P4HB (12.9% recurrence, p = 0.017; 12.9% SSI, p = 0.071) (30). Hernia recurrence and SSI rates associated with P4HB were comparable to the published literature.
In another study, P4HB mesh was utilized “off-label” in contaminated hernia repairs, Christopher et al. reported hernia recurrence of 8.3% and SSI of 16.7% at 24 months postimplantation in a patient population with CDC wound class ≥2 and VHWG grade ≥3 hernia repairs (72). These represent favorable results compared to similar studies involving other types of hernia repair materials. In the COBRA trial, Rosen et al. reported comparable SSI (18%) and 2× higher recurrence rates (17%) for another fully resorbable polymer mesh material (Bio-A®, W.L. Gore & Associates, Inc., Flagstaff, AZ) implanted in clean-contaminated and contaminated wounds in a population with multiple comorbidities predictive of postoperative complications (95). In the RICH trial, Itani et al. reported rates of recurrence of 28% and SSI of 35% for a biological tissue-derived material (Strattice™) in patients with CDC class II, III, or IV wounds (96).
Finally, when P4HB mesh was utilized to replace infected mesh in a single-stage approach, Bueno-Lledo et al., reported a recurrence rate of 6.6% and SSI of 3.3% (mean follow-up of 36.5 months) (66). They compared these results to a second cohort in which permanent synthetic mesh was used to replace infected mesh in a two-stage approach. The permanent synthetic mesh cohort exhibited a recurrence rate comparable to P4HB mesh (7.2%; p = 0.101), but 3× greater incidence of SSI (9.8%, p = 0.002) (66).
Previous studies have shown that the risk of surgical site occurrences (SSOs) such as SSI increases with increasing CDC wound class (35). However, the current literature review showed that P4HB mesh is associated with relatively low rates of SSI compared to other resorbable materials or biological tissue-derived matrices, including in patient populations with elevated CDC wound class. These outcomes are supported by bench and preclinical studies in which P4HB mesh degradation products were shown to promote bacterial resistance and tissue remodeling through upregulation of AMPs and influence over macrophage phenotype (36, 37). Based on the current literature, P4HB provides a viable option for repair in this challenging patient population at higher risk for infection and recurrence.
As a scoping review, the current study is not without limitations. First, the quality of the studies included in this review was not formally assessed as would be completed in a systematic review or meta-analysis. Nor, were the potential biases of any of the included studies discussed. The majority of the studies in this review are observational with the corresponding limitations. Roth et al. provides the longest-term follow-up to 60 months, but is a single-arm, observational trial with follow-up capturing only 44.6% of the cohort (31). Due to the limited follow-up, the actual recurrence and SSI rates would be expected to be higher, and the risk of bias is greater than data derived from a randomized controlled trial. This is an inherent limitation and challenge of large-scale clinical research and provides insight to guide future research.
Several knowledge gaps in the existing literature were identified by the current scoping review, generating a number of research questions to be addressed. Future studies should continue to evaluate P4HB mesh for a variety of clinical applications in the field of hernia repair. Inguinal hernia repair and incisional hernia prophylaxis are particularly promising areas of research that warrant additional studies with long-term follow-up and comprehensive evaluation of clinical outcomes. Future studies are also needed to compare outcomes associated with P4HB mesh to those of permanent synthetic meshes, particularly in non-complicated cases (i.e., CDC class I, VHWG grade 1). Permanent synthetic meshes have traditionally been utilized in these cases, and data is currently lacking for P4HB mesh in this patient population. The operative technique and mesh position are key to obtaining a durable repair with a completely resorbable mesh. Dedicated studies and a systematic review are needed to clearly define the optimal surgery, tissue plane, and the significance of fascial closure. One of the advantages of a fully resorbable material such as P4HB is to address increasing public sentiment and medicolegal implications regarding the use of permanent prosthetics in surgery and provide durable alternatives. The ongoing desire to identify the optimal prosthetic in hernia repair remains one of the central research themes in the field of hernia and abdominal wall reconstruction. Finally, randomized controlled trials (RCT) evaluating P4HB mesh against other biomaterials are absent in the scientific literature and are needed to properly delineate the appropriate and ideal role of P4HB in hernia repair.
5. Conclusions
The use of P4HB is safe and effective in hernia repair, but more research is needed. Repair with P4HB provides strength beyond the scope of its resorption profile with promising results in the context of contamination and demonstrates a role where the avoidance of a permanent mesh prosthesis is desired. Future studies are warranted to evaluate the appropriate and optimal role for P4HB in hernia and abdominal wall surgery, including hernia prophylaxis, inguinal hernia, and as an alternative to permanent synthetic mesh in routine hernia repair.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Study conception and design: AB, CD. Data collection: AB, CD. Data analysis and interpretation: AB, DC, CD, ML, DM. Drafting of manuscript: AB, DC, CD, ML, DM. Critical revision: AB, DC, CD, ML, DM. Final approval of version submitted for publication: AB, DC, CD, ML, DM. All authors contributed to the article and approved the submitted version.
Funding
This project was sponsored by Becton, Dickinson and Company (BD) of Warwick, Rhode Island (USA). CD is the owner of Covalent Bio, LLC, which received consulting fees from BD for this project, as well as other, unrelated projects. DM and AB are employees of BD. DC and ML are consultants for BD.
Conflict of interest
This project was sponsored by Becton, Dickinson and Company (BD) of Warwick, Rhode Island (USA). CD is the owner of Covalent Bio, LLC, which received consulting fees from BD for this project, as well as other, unrelated projects. DM and AB are employees of BD. DC and ML are consultants for BD. CD reports consulting fees from C.R. Bard, Inc./Davol/Becton Dickinson (BD) during the conduct of the study. DC also reports consulting fees from C.R. Bard, Inc./Davol/Becton Dickinson (BD), Johnson & Johnson, Medtronic, SurgiMatrix, Tissium, Surgical Innovation Associates, Americas Hernia Society Quality Collaborative, Colorado Therapeutics, TelaBio, Osteogenics, Polynovo, MedSkin Solutions, and Aran Biomedical outside the submitted work. In addition, CD is the owner of Covalent Bio, LLC and holds the following issued patents: 2009293001, 2334257, 2,334,257UK, 602009046407.8, 2,334,257FR, 16/043,849 and 2,737,542. DC reports consulting fees from Becton Dickinson (BD) during the conduct of this study. ML reports consulting fees from Becton Dickinson (BD) during the conduct of this study. DM reports employment by Becton Dickinson (BD) during the conduct of this study, as well as outside of the current work. AB reports employment by Becton Dickinson (BD) during the conduct of this study, as well as outside of the current work.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Martin D, Williams S. Medical applications of poly-4-hydroxybutyrate: a strong flexible absorbable biomaterial. Biochem Engr J. (2003) 16:97–105. doi: 10.1089/107632703322066660
2. Shen Y, Zhao Z, Li Y, Liu S, Liu F, Li Z. A facile method to prepare high molecular weight bio-renewable poly(γ-butyrolactone) using a strong base/urea binary synergistic catalytic system. Polym Chem. (2019) 10:1231–7. doi: 10.1039/c8py01812j
3. Yuan S, Sun X, Shen Y, Li Z. Bioabsorbable poly(4-hydroxybutyrate) (P4HB) fibrous membranes as a potential dermal substitute. J Mater Chem B. (2021) 9(38):8074–80. doi: 10.1039/d1tb01271a
4. Hong M, Chen EY. Towards truly sustainable polymers: a metal-free recyclable polyester from biorenewable non-strained gamma-butyrolactone. Angew Chem Int Ed Engl. (2016) 55(13):4188–93. doi: 10.1002/anie.201601092
5. Williams SF, Rizk S, Martin DP. Poly-4-hydroxybutyrate (P4HB): a new generation of resorbable medical devices for tissue repair and regeneration. Biomed Tech (Berl). (2013) 58(5):439–52. doi: 10.1515/bmt-2013-0009
6. Martin DP, Badhwar A, Shah DV, Rizk S, Eldridge SN, Gagne DH, et al. Characterization of poly-4-hydroxybutyrate mesh for hernia repair applications. J Surg Res. (2013) 184(2):766–73. doi: 10.1016/j.jss.2013.03.044
7. Williams SF, Martin DP, Moses AC. The history of GalaFLEX P4HB scaffold. Aesthet Surg J. (2016) 36(suppl 2):S33–42. doi: 10.1093/asj/sjw141
8. Singhi B, Ford EN, King MW. The effect of wet spinning conditions on the structure and properties of poly-4-hydroxybutyrate fibers. J Biomed Mater Res Part B Appl Biomater. (2021) 109(7):982–9. doi: 10.1002/jbm.b.34763
10. Instructions for Use—Phasix ST Mesh, C. R. Bard, Inc. (Warwick, RI), accessed electronically 2017.
11. van Driel LJ, Miserez M, Aarts F, Tollens T. Observational cohort study on the use of a slowly fully resorbable synthetic mesh (phasix™) in the treatment of complex abdominal wall pathology with different grades of contamination. Surg Technol Int. (2021) 38:186–92. doi: 10.52198/21.sti.38.hr1418
12. Hope WW, El-Ghazzawy AG, Winterstein BA, Blatnik JA, Davis SS, Greenberg JA, et al. A prospective, multicenter trial of a long-term bioabsorbable mesh with sepra technology in cohort of challenging laparoscopic ventral or incisional hernia repairs (ATLAS trial). Ann Med Surg (Lond). (2022) 73:103156. doi: 10.1016/j.amsu.2021.103156
13. Adams WP J, Moses AC. Use of poly-4-hydroxybutyrate mesh to optimize soft-tissue support in mastopexy: a single-site study. Plast Reconstr Surg. (2017) 139(1):67–75. doi: 10.1097/PRS.0000000000002922
14. Wormer BA, Clavin NW, Lefaivre JF, Korn JM, Teng E, Aukskalnis AS, et al. Reducing postoperative abdominal bulge following deep inferior epigastric perforator flap breast reconstruction with onlay monofilament poly-4-hydroxybutyrate biosynthetic mesh. J Reconstr Microsurg. (2017) 33(1):8–18. doi: 10.1055/s-0036-1586495
15. Levy AS, Bernstein JL, Xia JJ, Otterburn DM. Poly-4-hydroxybutyric acid mesh compares favorably with acellular dermal matrix in tissue expander-based breast reconstruction. Ann Plast Surg. (2020) 85(S1):S2–7. doi: 10.1097/SAP.0000000000002339
16. Adams WP J, Toriumi DM, Van Natta BW. Clinical use of GalaFLEX in facial and breast cosmetic plastic surgery. Aesthet Surg J. (2016) 36(Suppl 2):S23–32. doi: 10.1093/asj/sjw142
17. Baxter R. Internal bra: a unifying solution for reconstructive and aesthetic breast surgery issues. Plast Aesthet Res. (2016) 3:3–7. doi: 10.20517/2347-9264.2015.68
18. Adams WP J, Baxter R, Glicksman C, Mast BA, Tantillo M, Van Natta BW. The use of poly-4-hydroxybutyrate (P4HB) scaffold in the ptotic breast: a multicenter clinical study. Aesthet Surg J. (2018) 38(5):502–18. doi: 10.1093/asj/sjy022
19. Calobrace MB, Mays C, Wilson R, Wermeling R. Popcorn capsulorrhaphy in revision aesthetic breast surgery. Aesthet Surg J. (2020) 40(1):63–74. doi: 10.1093/asj/sjy324
20. Mallucci P, Bistoni G. Experience and indications for the use of the P4HB scaffold (galaflex) in aesthetic breast surgery: 100 case experience. Aesthet Surg J. (2022) 42(12):1394–405. doi: 10.1093/asj/sjac198
21. Sigalove S, O'Rorke E, Maxwell GP, Gabriel A. Evaluation of the safety of a GalaFLEX-AlloDerm construct in prepectoral breast reconstruction. Plast Reconstr Surg. (2022) 150:75S–81S. doi: 10.1097/PRS.0000000000009520
22. Angelos PC, Brennan TE, Toriumi DM. Biomechanical properties of superficial musculoaponeurotic system tissue with vs without reinforcement with poly-4-hydroxybutyric acid absorbable mesh. JAMA Facial Plast Surg. (2014) 16(3):199–205. doi: 10.1001/jamafacial.2013.2738
23. Diedrich CM, Guler Z, Hympanova L, Vodegel E, Zundel M, Mazza E, et al. Evaluation of the short-term host response and biomechanics of an absorbable poly-4-hydroxybutyrate scaffold in a sheep model following vaginal implantation. BJOG. (2022) 129:1039–49. doi: 10.1111/1471-0528.17040
24. O'Shaughnessy D, Grande D, El-Neemany D, Sajjan S, Pillalamarri N, Shalom D, et al. Evaluation of the histological and biomechanical properties of poly-4-hydroxybutyrate scaffold for pelvic organ prolapse, compared with polypropylene mesh in a rabbit model. Int Urogynecol J. (2021) 33(8):2213–20. doi: 10.1007/s00192-021-04851-6
25. Rostaminia G. A commentary on “evaluation of the histological and biomechanical properties of poly-4-hydroxybutyrate (P4HB) scaffold for pelvic organ prolapse, as compared to polypropylene mesh in a rabbit model”. Int Urogynecol J. (2021) 33(8):2221. doi: 10.1007/s00192-021-04944-2
26. Verhorstert KWJ, Guler Z, de Boer L, Riool M, Roovers JWR, Zaat SAJ, et al. The impact of bacterial contamination on the host response towards fully absorbable poly-4-hydroxybutyrate and nonabsorbable polypropylene pelvic floor implants. Mater Today Bio. (2022) 15:100268. doi: 10.1021/acsami.0c14668
27. Faulkner JD, Bilezikian JA, Beeson ST, Jernigan R, Fox SS, Hope WW. Evaluation of absorbable mesh for prophylactic mesh augmentation in high-risk patients. Surg Technol Int. (2021) 38:213–7. doi: 10.52198/21.STI.38.HR1430
28. Aldohayan A, Bamehriz F, Khalid Alghamdi G, Ahmed AlJunidel R, AlBalawi M, Zakaria Aldhayan A, et al. A novel use of fully absorbable phasix(TM) mesh for laparoscopic inguinal hernia repair. J Soc Laparoendosc Surg/Soc Laparoendosc Surg. (2020) 24(3):e2020.00041. doi: 10.04293/JSLS.02020.00041
29. Panici Tonucci T, Asti E, Sironi A, Ferrari D, Bonavina L. Safety and efficacy of crura augmentation with phasix ST mesh for large hiatal hernia: 3-year single-center experience. J Laparoendosc Adv Surg Tech A. (2020) 30(4):369–72. doi: 10.1089/lap.2019.0726
30. Buell J, Flaris A, Raju S, Hauch A, Darden M, Parker G. Long-term outcomes in complex abdominal wall reconstruction repaired with absorbable biologic polymer scaffold (poly-4-hydroxybutyrate). Ann Surg. (2021) 1(e032):1–7. doi: 10.1097/as9.0000000000000032
31. Roth JS, Anthone GJ, Selzer DJ, Poulose BK, Pierce RA, Bittner JG, et al. Long-term, prospective, multicenter study of P4HB (phasix™) mesh for hernia repair in cohort at risk for complications: 60-months follow-up. J Am Coll Surg. (2022) 1:894–904. doi: 10.1097/XCS.0000000000000363
32. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169(7):467–73. doi: 10.7326/M18-0850
33. Surgical Site Infection Event Protocol, Centers for Disease Control and Prevention (CDC). https://wwwcdcgov/nhsn/pdfs/pscmanual/9pscssicurrentpdf (Accessed February 15, 2017). 1–31.
34. Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. (2010) 148(3):544–58. doi: 10.1016/j.surg.2010.01.008
35. Kanters AE, Krpata DM, Blatnik JA, Novitsky YM, Rosen MJ. Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg. (2012) 215(6):787–93. doi: 10.1016/j.jamcollsurg.2012.08.012
36. Pineda Molina C, Hussey GS, Eriksson J, Shulock MA, Cardenas Bonilla LL, Giglio RM, et al. 4-Hydroxybutyrate promotes endogenous antimicrobial peptide expression in macrophages. Tissue Engr Part A. (2019) 25:693–706. doi: 10.1089/ten.tea.2018.0377
37. Pineda Molina C, Giglio R, Gandhi RM, Sicari BM, Londono R, Hussey GS, et al. Comparison of the host macrophage response to synthetic and biologic surgical meshes used for ventral hernia repair. J Immunol Regen Med. (2019) 3:13–25. doi: 10.1016/j.regen.2018.12.002
38. Trapani V, Bagni G, Piccoli M, Roli I, Di Patti F, Arcangeli A. Analysis of resorbable mesh implants in short-term human muscular fascia cultures: a pilot study. Hernia. (2020) 24(6):1283–91. doi: 10.1007/s10029-020-02271-x
39. Verhorstert KWJ, Guler Z, de Boer L, Riool M, Roovers JWR, Zaat SAJ. In vitro bacterial adhesion and biofilm formation on fully absorbable poly-4-hydroxybutyrate and nonabsorbable polypropylene pelvic floor implants. ACS Appl Mater Interfaces. (2020) 12(48):53646–53. doi: 10.1021/acsami.0c14668
40. Diedrich CM, Roovers JP, Smit TH, Guler Z. Fully absorbable poly-4-hydroxybutyrate implants exhibit more favorable cell-matrix interactions than polypropylene. Mater Sci Eng C Mater Biol Appl. (2021) 120:111702. doi: 10.1016/j.msec.2020.111702
41. Mora-Navarro C, Ozpinar EW, Sze D, Martin DP, Freytes DO. Transcriptome-targeted analysis of human peripheral blood-derived macrophages when cultured on biomaterial meshes. Biomed Mater. (2021) 16(2):025006. doi: 10.1088/1748-605X/abdbdb
42. Pineda Molina C, Hussey GS, Liu A, Eriksson J, D'Angelo WA, Badylak SF. Role of 4-hydroxybutyrate in increased resistance to surgical site infections associated with surgical meshes. Biomaterials. (2021) 267:120493. doi: 10.1016/j.biomaterials.2020.120493
43. Deeken CR, Matthews BD. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (poly-4-hydroxybutyrate-PHASIX mesh) in a porcine model of hernia repair. ISRN Surg. (2013) 2013:238067. doi: 10.1155/2013/238067
44. Scott JR, Deeken CR, Martindale RG, Rosen MJ. Evaluation of a fully absorbable poly-4-hydroxybutyrate/absorbable barrier composite mesh in a porcine model of ventral hernia repair. Surg Endosc. (2016) 30(9):3691–701. doi: 10.1007/s00464-016-5057-9
45. Stoikes NFN, Scott JR, Badhwar A, Deeken CR, Voeller GR. Characterization of host response, resorption, and strength properties, and performance in the presence of bacteria for fully absorbable biomaterials for soft tissue repair. Hernia. (2017) 21(5):771–82. doi: 10.1007/s10029-017-1638-3
46. Miserez M, Jairam AP, Boersema GSA, Bayon Y, Jeekel J, Lange JF. Resorbable synthetic meshes for abdominal wall defects in preclinical setting: a literature review. J Surg Res. (2019) 237:67–75. doi: 10.1016/j.jss.2018.11.054
47. Deeken CR, Gagne DH, Badhwar A. Mechanical and histological characteristics of phasix™ ST mesh in a porcine model of hernia repair. J Invest Surg. (2020) 35:1–9. doi: 10.1080/08941939.2020.1830318
48. Pascual G, Rodríguez M, Pérez-Köhler B, Benito-Martínez S, Calvo B, García-Moreno F, et al. Long term comparative evaluation of two types of absorbable meshes in partial abdominal wall defects: an experimental study in rabbits. Hernia. (2020) 24(6):1159–73. doi: 10.1007/s10029-020-02201-x
49. Pascual G, Benito-Martínez S, Rodríguez M, Pérez-Köhler B, García-Moreno F, Bellón JM. Behaviour at the peritoneal interface of next-generation prosthetic materials for hernia repair. Surg Endosc. (2022) 36(1):579–90. doi: 10.1007/s00464-021-08320-5
50. Buell JF, Sigmon D, Ducoin C, Shapiro M, Teja N, Wynter E, et al. Initial experience with biologic polymer scaffold (poly-4-hydroxybuturate) in complex abdominal wall reconstruction. Ann Surg. (2017) 266(1):185–8. doi: 10.1097/SLA.0000000000001916
51. Plymale MA, Davenport DL, Dugan A, Zachem A, Roth JS. Ventral hernia repair with poly-4-hydroxybutyrate mesh. Surg Endosc. (2018) 32(4):1689–94. doi: 10.1007/s00464-017-5848-7
52. Roth JS, Anthone GJ, Selzer DJ, Poulose BK, Bittner JG, Hope WW, et al. Prospective evaluation of poly-4-hydroxybutyrate mesh in CDC class I/high-risk ventral and incisional hernia repair: 18-month follow-up. Surg Endosc. (2018) 32(4):1929–36. doi: 10.1007/s00464-017-5886-1
53. van Rooijen MMJ, Jairam AP, Tollens T, Jorgensen LN, de Vries Reilingh TS, Piessen G, et al. A post-market, prospective, multi-center, single-arm clinical investigation of phasix mesh for VHWG grade 3 midline incisional hernia repair: a research protocol. BMC Surg. (2018) 18(1):104. doi: 10.1186/s12893-018-0439-7
54. Kniepeiss D, Waha JE, Auer T, Berghold A, Schemmer P. PRevention of INCisional hernia after liver transplantation (PRINC trial): study protocol for a randomized controlled trial. Trials. (2019) 20(1):371. doi: 10.1186/s13063-019-3477-2
55. Messa CA, Kozak G, Broach RB, Fischer JP. When the mesh goes away: an analysis of poly-4-hydroxybutyrate mesh for complex hernia repair. Plast Reconstr Surg Glob Open. (2019) 7(11):e2576. doi: 10.1097/GOX.0000000000002576
56. Nair NM, Mills DC. Poly-4-hydroxybutyrate (P4HB) scaffold internal support: preliminary experience with direct implant opposition during complex breast revisions. Aesthet Surg J. (2019) 39(11):1203–13. doi: 10.1093/asj/sjy276
57. Abdelmoaty WF, Dunst CM, Filicori F, Zihni AM, Davila-Bradley D, Reavis KM, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastroint Surg. (2020) 24(7):1477–81. doi: 10.1007/s11605-019-04358-y
58. Aiolfi A, Cavalli M, Saino G, Sozzi A, Bonitta G, Micheletto G, et al. Laparoscopic posterior cruroplasty: a patient tailored approach. Hernia. (2022) 26(2):619–26. doi: 10.1007/s10029-020-02188-5
59. Mellia JA, Othman S, Naga HI, Messa CA, 4th, Elfanagely O, Byrnes YM, et al. Outcomes of poly-4-hydroxybutyrate mesh in ventral hernia repair: a systematic review and pooled analysis. Plast Reconstr Surg Glob Open. (2020) 8(12):e3158. doi: 10.1097/GOX.0000000000003158
60. Pakula A, Skinner R. Outcomes of open complex ventral hernia repairs with retromuscular placement of poly-4-hydroxybutyrate bioabsorbable mesh. Surg Innov. (2020) 27(1):32–7. doi: 10.1177/1553350619881066
61. Rehnke RD, Schusterman MA 2nd, Clarke JM, Price BC, Waheed U, Debski RE, et al. Breast reconstruction using a three-dimensional absorbable mesh scaffold and autologous fat grafting: a composite strategy based on tissue-engineering principles. Plast Reconstr Surg. (2020) 146(4):409e–13e. doi: 10.1097/PRS.0000000000007172
62. Rognoni C, Cuccurullo D, Borsoi L, Bonavina L, Asti E, Crovella F, et al. Clinical outcomes and quality of life associated with the use of a biosynthetic mesh for complex ventral hernia repair: analysis of the “Italian hernia club” registry. Sci Rep. (2020) 10(1):10706. doi: 10.1038/s41598-020-67821-w
63. van Rooijen MMJ, Jairam AP, Tollens T, Jorgensen LN, de Vries Reilingh TS, Piessen G, et al. Outcomes of a new slowly resorbable biosynthetic mesh (phasix™) in potentially contaminated incisional hernias: a prospective, multi-center, single-arm trial. Int J Surg. (2020) 83:31–6. doi: 10.1016/j.ijsu.2020.08.053
64. Yu D, Patel AT, Rossi K, Topham NS, Chang EI. Comparison of phasix, polypropylene, and primary closure of the abdominal donor site after bilateral free flap breast reconstruction: long-term evaluation of abdominal hernia and bulge formation. Microsurgery. (2020) 40(4):434–9. doi: 10.1002/micr.30541
65. Aldohayan A, Alamri H, Aljunidel R, Alotaibi A, Alosaimi M, Alburakan A, et al. Laparoscopic ventral hernia repair with poly-4-hydroxybutyrate absorbable barrier composite mesh. J Soc Laparoendosc Surg/Soc Laparoendosc Surg. (2021) 25(1):1–5. doi: 10.4293/JSLS.2020.00105
66. Bueno-Lledó J, Ceno M, Pérez-Alonso C, Martinez-Hoed J, Pous-Serrano S. Abdominal wall reconstruction with biosynthetic absorbable mesh after infected prosthesis explantation: single stage is better than two-stage approach of chronic mesh infection. Hernia. (2020) 25(4):1005–12. doi: 10.1007/s10029-020-02309-0
67. Bueno-Lledó J, Ceno M, Perez-Alonso C, Martinez-Hoed J, Torregrosa-Gallud A, Pous-Serrano S. Biosynthetic resorbable prosthesis is useful in single-stage management of chronic mesh infection after abdominal wall hernia repair. World J Surg. (2021) 45(2):443–50. doi: 10.1007/s00268-020-05808-4
68. Charleux-Muller D, Hurel R, Fabacher T, Brigand C, Rohr S, Manfredelli S, et al. Slowly absorbable mesh in contaminated incisional hernia repair: results of a French multicenter study. Hernia. (2021) 25(4):1051–9. doi: 10.1007/s10029-020-02366-5
69. Charleux-Muller D, Romain B, Boisson C, Velten M, Brigand C, Lejeune C. Cost-effectiveness analysis of resorbable biosynthetic mesh in contaminated ventral hernia repair. J Visc Surg. (2021) 159(4):29–295. doi: 10.1016/j.jviscsurg.2021.06.001
70. Christopher AN, Morris MP, Patel V, Mellia JA, Fowler C, Messa CA 4th, et al. An evaluation of clinical and quality of life outcomes after ventral hernia repair with poly-4-hydroxybutyrate mesh. Hernia. (2021) 25(3):717–26. doi: 10.1007/s10029-021-02394-9
71. Christopher AN, Patel V, Othman S, Jia H, Mellia JA, Broach RB, et al. Onlay poly-4-hydroxybutyrate (P4HB) mesh for complex hernia: early clinical and patient reported outcomes. J Surg Res. (2021) 264:199–207. doi: 10.1016/j.jss.2021.02.012
72. Christopher AN, Morris MP, Jia H, Broach R, Fischer JP. Resorbable synthetic ventral hernia repair in contaminated fields: outcomes with poly-4-hydroxybutyrate mesh. Plast Reconstr Surg. (2021) 148(6):1367–75. doi: 10.1097/PRS.0000000000008579
73. Claessen JJM, Timmer AS, Atema JJ, Boermeester MA. Outcomes of mid-term and long-term degradable biosynthetic meshes in single-stage open complex abdominal wall reconstruction. Hernia. (2021) 26:1–11. doi: 10.1007/s10029-021-02415-7
74. Lambrecht M, Tollens T. Successful conservative treatment of a poly-4-hydroxybutyrate mesh infection: a case report. Ann Med Surg (Lond). (2021) 63:102162. doi: 10.1016/j.amsu.2021.02.008
75. Levy AS, Bernstein JL, Premaratne ID, Rohde CH, Otterburn DM, Morrison KA, et al. Poly-4-hydroxybutyrate (phasix™) mesh onlay in complex abdominal wall repair. Surg Endosc. (2021) 35(5):2049–58. doi: 10.1007/s00464-020-07601-9
76. Roth JS, Anthone GJ, Selzer DJ, Poulose BK, Pierce RA, Bittner JG, Hope WW, et al. Prospective, multicenter study of P4HB (phasix) mesh for hernia repair in cohort at risk for complications: 3-year follow-up. Ann Med Surg (Lond). (2020) 61:1–7. doi: 10.1016/j.amsu.2020.12.002
77. van Rooijen MMJ, Tollens T, Jorgensen LN, de Vries Reilingh TS, Piessen G, Kockerling F, et al. Slowly resorbable biosynthetic mesh: 2-year results in VHWG grade 3 hernia repair. Hernia. (2021) 26(1):131–8. doi: 10.1007/s10029-021-02453-1
78. Vauclair E, Bert M, Facy O, Cheynel N, Rat P, Ortega-Deballon P. What results can be expected one year after complex incisional hernia repair with biosynthetic mesh? J Visc Surg. (2021) 158(2):111–7. doi: 10.1016/j.jviscsurg.2020.07.008
79. Aiolfi A, Cavalli M, Sozzi A, Lombardo F, Lanzaro A, Panizzo V, et al. Medium-term safety and efficacy profile of paraesophageal hernia repair with phasix-ST((R)) mesh: a single-institution experience. Hernia. (2022) 26(1):279–86. doi: 10.1007/s10029-021-02528-z
80. Konstantinidis H, Charisis C. Surgical treatment of large and complicated hiatal hernias with the new resorbable mesh with hydrogel barrier (phasix ST): a preliminary study. J Robot Surg. (2022) 17:141–6. doi: 10.1007/s11701-022-01406-9
81. Layer T, Benammi S, Dubuisson V, Manfredelli S, Passot G, Charleux-Muller D, et al. Incisional hernia repair with a slowly absorbable P4HB mesh: what happens after the mesh disappears? A retrospective longitudinal clinical study. Hernia. (2022). (Online only) doi: 10.1007/s10029-022-02616-8
82. Lima DL, Estrada A, Pereira X, Alcabes A, Sreeramoju P, Malcher F. Versatility of poly-4-hydroxybutyrate (phasix) mesh in abdominal wall surgery. Arq Gastroenterol. (2022) 59(2):226–30. doi: 10.1590/S0004-2803.202202000-41
83. Othman S, Christopher A, Patel V, Jia H, Mellia JA, Kovach SJ, et al. Comparative effectiveness analysis of resorbable synthetic onlay and biologic intraperitoneal mesh for abdominal wall reconstruction: a 2-year match-paired analysis. Plast Reconstr Surg. (2022) 149(5):1204–13. doi: 10.1097/PRS.0000000000009021
84. Schecter SC, Imhoff L, Lasker MV, Hornbeck S, Flores HC. Single-stage abdominal wall reconstruction in contaminated and dirty wounds is safe: a single center experience. Surg Endosc. (2022) 36(8):5766–71. doi: 10.1007/s00464-022-09058-4
85. Talwar AA, Perry NJ, McAuliffe PB, Desai AA, Thrippleton S, Broach RB, et al. Shifting the goalpost in ventral hernia care: 5-year outcomes after ventral hernia repair with poly-4-hydroxybutyrate mesh. Hernia. (2022) 26:1635–43. doi: 10.1007/s10029-022-02674-y
86. Berrevoet F. The transition from experimental data to clinical practice using P4HB mesh in ventral hernia repair. J Invest Surg. (2020) 35:1–2. doi: 10.1080/08941939.2020.1849467
87. Haddock NT, Suszynski TM, Teotia SS. Discussion: breast reconstruction using a three-dimensional absorbable mesh scaffold and autologous fat grafting: a composite strategy based on tissue-engineering principles. Plast Reconstr Surg. (2020) 146(4):414e–5e. doi: 10.1097/PRS.0000000000007175
88. Morales-Conde S, Berrevoet F, Jorgensen LN, Marchi D, Ortega-Deballon P, Windsor A. Establishing peer consensus about the use of long-term biosynthetic absorbable mesh for hernia (grades 2–3) as the standard of care. World J Surg. (2022) 46:2996–3004. doi: 10.1007/s00268-022-06743-2
89. Lake SP, Stoikes NFN, Badhwar A, Deeken CR. Contamination of hybrid hernia meshes compared to bioresorbable phasix mesh in a rabbit subcutaneous implant inoculation model. Ann Med Surg (Lond). (2019) 46:12–6. doi: 10.1016/j.amsu.2019.08.004
90. Levy AS, Bernstein JL, Premaratne ID, Rohde CH, Otterburn DM, Morrison KA, et al. Poly-4-hydroxybutyrate (phasix) mesh onlay in complex abdominal wall repair. Surg Endosc. (2020) 35(5):2049–58. doi: 10.1007/s00464-020-07601-9
91. Kokotovic D, Bisgaard T, Helgstrand F. Long-term recurrence and complications associated with elective incisional hernia repair. JAMA. (2016) 316(15):1575–82. doi: 10.1001/jama.2016.15217
92. Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg. (2013) 217(6):991–8. doi: 10.1016/j.jamcollsurg.2013.07.382
93. Warren J, Desai SS, Boswell ND, Hancock BH, Abbad H, Ewing JA, et al. Safety and efficacy of synthetic mesh for ventral hernia repair in a contaminated field. J Am Coll Surg. (2020) 230(4):405–13. doi: 10.1016/j.jamcollsurg.2019.12.008
94. Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. (2004) 240(4):578–83. doi: 10.1097/01.sla.0000141193.08524.e7
95. Rosen MJ, Bauer JJ, Harmaty M, Carbonell AM, Cobb WS, Matthews BD, et al. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg. (2017) 265:205–11. doi: 10.1097/SLA.0000000000001601
Keywords: hernia recurrence, P4HB, Phasix Mesh, poly-4-hydroxybutyrate (P4HB), resorbable, surgical site infection (SSI)
Citation: Deeken CR, Chen DC, Lopez-Cano M, Martin DP and Badhwar A (2023) Fully resorbable poly-4-hydroxybutyrate (P4HB) mesh for soft tissue repair and reconstruction: A scoping review. Front. Surg. 10:1157661. doi: 10.3389/fsurg.2023.1157661
Received: 2 February 2023; Accepted: 22 March 2023;
Published: 12 April 2023.
Edited by:
J. Scott Roth, University of Kentucky, United StatesReviewed by:
Anita Jacombs, Macquarie University Hospital, AustraliaShuaishuai Yuan, Qingdao University of Science and Technology, China
© 2023 Deeken, Chen, López-Cano, Martin and Badhwar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amit Badhwar YW1pdC5iYWRod2FyQGJkLmNvbQ==
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Corey R. Deeken
Corey R. Deeken David C. Chen2
David C. Chen2 Manuel Lopez-Cano
Manuel Lopez-Cano