
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 03 May 2023
Sec. Orthopedic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1152995
This article is part of the Research TopicComplications and Revision Surgery in Orthopedics Focusing on TraumatologyView all 8 articles
 Fulvio Tartara1,2
Fulvio Tartara1,2 Daniele Armocida3,4*
Daniele Armocida3,4* Diego Garbossa5
Diego Garbossa5 Francesco Meli6
Francesco Meli6 Gabriele Costantino6
Gabriele Costantino6 Fabio Cofano5
Fabio Cofano5 Natale Francaviglia6
Natale Francaviglia6
Background: Vertebral augmentation procedures (VAPs) are used in cases of persistent and unresponsive pain in patients with vertebral compression fractures (VCFs). Although VAPs are considered a safe procedure providing quick pain relief and improved physical function, some postoperative complications can occur, for example, bone cement leakage. The material used in this procedure is almost exclusively polymethyl methacrylate (PMMA), which appears to lack biological activity and osteointegration capabilities. In this study, we introduce a new filling system consisting of cannulas preloaded with titanium microspheres, which stabilizes and consolidates the structure of the vertebral body in treating VCFs after the performance of the kyphoplasty procedure.
Methods: We report a retrospective case series of six patients affected by osteoporotic vertebral fractures with worsening back pain, neurologic impairment, and failed conservative treatment who underwent the VAP at our institute, for which the SPHEROPLAST [MT ORTHO s.r.l., Aci Sant’Antonio (CT), Italy] system was used.
Results: The patients had failed an average conservative trial of 3.9 weeks before they presented to us with neurodeficit. There were two men and four women with a mean age of 74.5 years. The average hospital stay was 2 days. There were no reported perioperative complications related to cement injection, such as intraoperative hypoxia, hypotension, pulmonary embolization, myocardial infarction, neurovascular or viscera injury, or death. The VAS score significantly decreased from a mean preoperative of 7.5 (range 6–19) to 3.8 (range 3–5) immediately after surgery and 1.8 (range 1–3).
Conclusion: We report the first clinical results in a series of six patients treated for VCF using the microsphere system after analyzing the clinical results produced by, and the complications that arose from, this new device. In patients with VCF, the VAP using titanium microspheres appears to be a feasible and safe procedure with a low risk of material leakage.
Osteoporosis is the most frequently occurring bone disease with high incidence in a population of over 55 years of age. The occurrence of vertebral compression fractures (VCFs) is very common in patients with osteoporosis either in association with minor trauma or as a spontaneous event, whereas any sudden force of axial compression, distraction, or rotation on brittle bone could lead to osteoporotic fractures (1). Clinically, VCFs may be associated with invalidation of pain and disability, leading to significant limitations in the physical activity and quality of life of patients. In addition, fracture progression with progressive crushing and wedge deformation of the vertebral soma may contribute to the generation of segmental and overall kyphotic deformity of the spine. The initial treatment of VCFs is conservative, with the adoption of rigid orthoses and pain-drug therapy (2). Although conservative treatments can help manage most patients with osteoporotic vertebral fractures, debilitating pain and substantial functional limitations often continue to impact their quality of life (3). Vertebral augmentation procedures (VAPs) such as vertebroplasty and kyphoplasty are used in persistent and unresponsive cases of pain. These treatments consist of an injection into the vertebral body, through percutaneous needles (trokars), of material (cement) that can consolidate within the vertebra, stabilizing the fracture and imparting solidity to the vertebral soma itself. Although VAPs are considered a safe procedure providing quick pain relief and improved physical function, some postoperative complications can still occur, such as bone cement leakage, embolism, bone cement implantation syndrome, infection, thermal damage to surrounding soft tissue, and adjacent vertebral fracture (4, 5).
The material that is used almost exclusively in this procedure is polymethyl methacrylate (PMMA), which is available with different viscosities.
This type of material lacks complete biological activity and osteointegration capabilities. To date, only cases of bone cement displacement after the performance of the VAP have been reported. The factors closely related to the occurrence of bone cement displacement have not been determined. There are no systematic studies on bone cement displacement after the VAP, and a quantitative definition of bone cement displacement is lacking (5). In this preliminary study, we introduce a new augmentation system consisting of porous titanium microspheres that are able to stabilize and consolidate VCFs after a kyphoplasty procedure was performed. We report the first clinical results in a series of patients treated for VCF using the microsphere system after analyzing the clinical results produced by, and the complications arising from, this new device.
The present study included patients with osteoporotic vertebral fractures and worsening back pain related to failed conservative treatment. These patients underwent the VAP at our institute between March and September 2022. In accordance with the World Health Organization’s (WHO) diagnostic criteria, osteoporosis in these patients was diagnosed with a T score of 2.5 by a dual-energy x-ray absorptiometry of the lumbar spine. The included patients had a bone mineral density (BMD) of more than 2.5 SD below the young adult mean (−2.5 SD or less). Patients younger than 65 years, patients with pathological fracture and osteomyelitis, and patients with a near-normal T score for BMD were excluded from the study. All patients provided personalized informed consent.
Clinical evaluation was done using the visual analog scale (VAS) for pain intensity, the severity of physical impairment was assessed using the Roland–Morris Low Back Pain and Disability Questionnaire (RMQ) impairment scale, and the severity of disability was assessed using the Oswestry Disability Index (ODI).
All patients had restrictions in standing and walking because of severe pain. For each patient, demographic data, the time between the clinical debut and the procedure, and potential complications were recorded. All patients who underwent the VAP were included for analysis regardless of surgical indication. Patients required a minimum of 3 months of follow-up for inclusion. This time point was chosen to optimize the number of patients evaluated for immediate postoperative complications.
All patients were evaluated by using plain radiographs, computed tomography scan (CT), and magnetic resonance imaging (MRI) to determine the morphology of the fracture.
We used SPHEROPLAST [MT ORTHO s.r.l., Aci Sant’Antonio (CT), Italy], a filling system consisting of cannulas preloaded with spheres and a dedicated introducer with the aid of a compactor that was able to create a solid cast within the vertebral body for VCF augmentation. The surgical technique is illustrated in Figure 1. The patients were positioned prone on bolsters. This led to a partial correction of kyphosis and restoration of the anterior vertebral height (AVH) with the balloon compression (Figure 2). First, the pedicles of the affected segment were identified and the skin was marked. Trokar needles were passed into the pedicles together with a cutter to reach optimal position with image intensifier guidance. Then, 15 ml of balloon kyphoplasty were applied on both sides to create space for housing microspheres. Microspheres were then prepared and loaded into the introducer and progressively pushed through the needle. The procedure involves inserting the largest number of spheres to generate a solid cast covering the entire fracture surface. A compactor was used to facilitate microsphere progression.
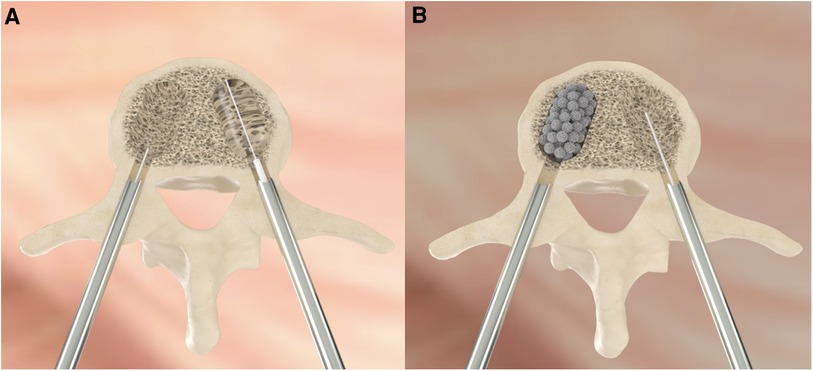
Figure 1. The figure shows the bilateral percutaneous approach to perform VAP introducing the ballon to restore the height (A) and the injection of the solution containing the titanium microspheres (B).
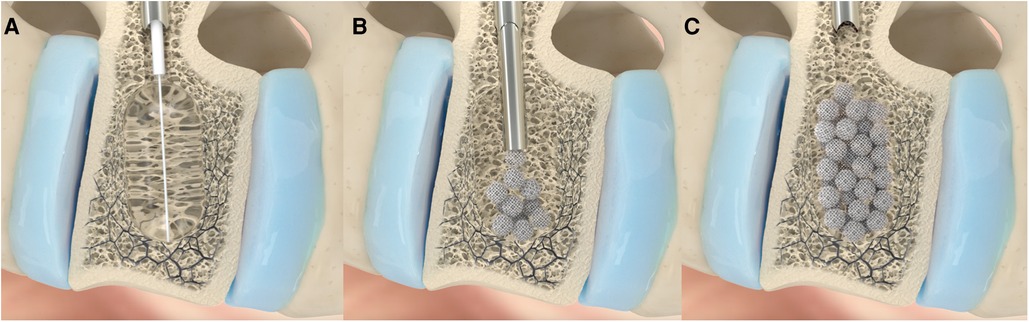
Figure 2. The main part of VAP treatment with spheroplast is schematized in this image. Through a percutaneous biportal access using trokar, the balloon is insufflated, restoring, as much as possible, the height of the fractured vertebra and creating a cavity (A). Then, the solution containing the titanium microspheres is injected in multiple jets; this step is performed under radioscopy (B). The injected material is then compacted until the cavity is fully occupied (C).
An x-ray of the lateral and anterior views of the vertebrae was done during the microsphere introduction to observe the distribution pattern. The patients were mobilized after 3 h from the end of the procedure with braces. Clinical and radiological evaluations were done preoperatively, immediately postoperatively, and at 3 months after procedure.
We report six patients who underwent the minimally invasive percutaneous technique VAP with spheroplasts included in the study. Electronic medical records of these patients were evaluated clinically, as shown in Table 1. These patients had failed an average conservative trial of 3.9 weeks (range 2–6 weeks) before they presented to us with invalidated worsening pain.
There were two men and four women with a mean age of 74.5 years (range 66–82 years). The average hospital stay was 1.5 days (1–3 days).
No perioperative complications related to microsphere injection were reported. In particular, a non-displacement of microspheres outside the vertebral body was observed. At 3-month follow-up, x-ray control revealed that the microsphere cast remained largely unchanged in its position with partial signs of fusion (Figure 3).
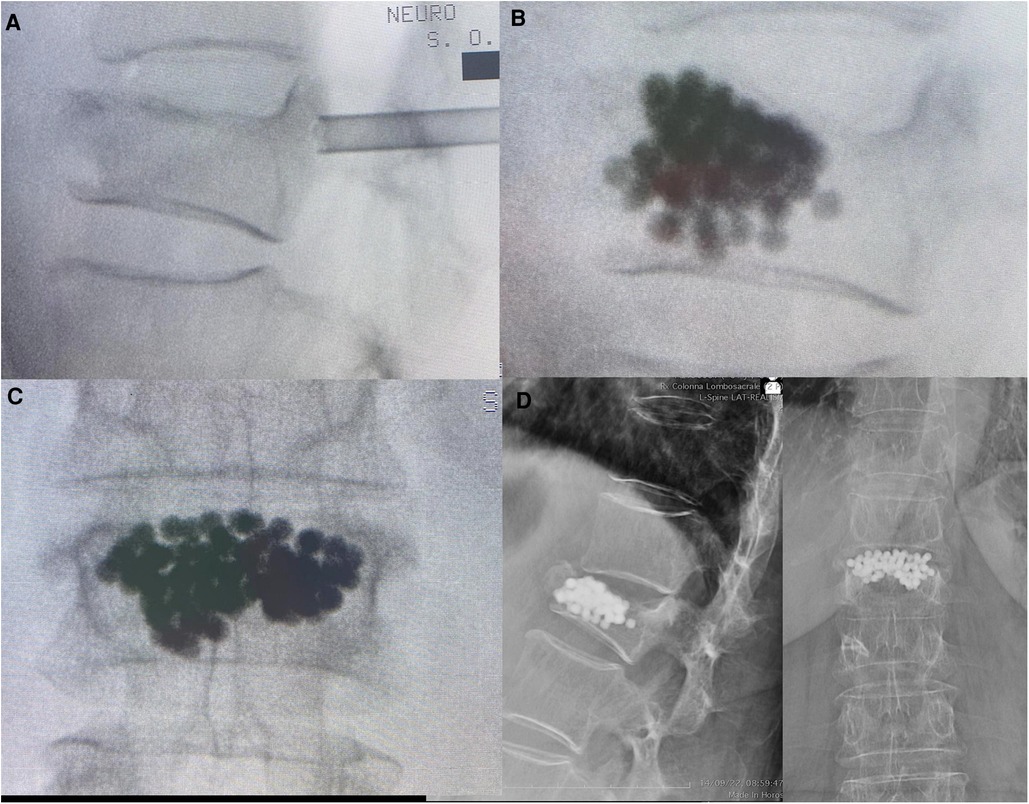
Figure 3. A representative case of an 82-year-old woman with vertebral osteoporotic compression L1 fracture without posterior wall involvement, unresponsive to pain drugs. (A) Cannula insertion and (B,C) intraoperative view after the titanium microsphere injection. (D) Radiographic follow-up control at 3 months with signs of partial ossification and fusion.
The VAS score significantly decreased from a mean preoperative 7.5 (range 6–9) to 3.8 (range 3–5) immediately after surgery and to 1.8 (range 1–3) at the 3-month follow-up. Statistical analysis could not be carried out because of the low number of patients involved in the study.
The RMQ and ODI scores decreased from a mean preoperative RMQ = 19.1 (range 16–22) and ODI = 53.3 (range 42–68) to RMQ = 6 (range 5–8) and ODI = 12.7 (range 8–18), respectively, at last evaluation at follow-up.
The VAP is a type of surgery that is used to restore the strength and stiffness of the vertebra after fractures caused by tumors, trauma, or osteoporosis. From the time this technique was introduced in 1987 by Galibert et al. (6), it has been used extensively to treat several traumas and diseases, particularly VCFs.
The VAP includes two kinds of surgeries: percutaneous vertebroplasty and percutaneous kyphoplasty. The core purpose of both is to insert bone cement into the fractured vertebral body (7). The treatment present optimal results only when it was used for “the most suitable patients” (8). VAPs have shown good uptake, and subsequent studies have shown the effectiveness of vertebroplasty procedures in controlling pain secondary to fracture conversely (9). Equally, a trend of kyphoplasty procedures scoring over vertebroplasty procedures has emerged (10). These methods have been widely used and remain viable in treating refractory pain secondary to osteoporotic compression fractures. Several trials have analyzed the effectiveness of vertebroplasty and kyphoplasty procedures although with discordant results (11–16).
One of the main criticisms advanced against traditional VAPs is the permanence of PMMA as a foreign body within the vertebral soma without any evidence of osteoinductive or reparative activity. The second critical aspect of PMMA is the risk of displacement and extravasation (leakage), which is the most frequent complication, especially in osteoporotic fractures (17).
Although bone cement displacement is a rare complication, some scholars believed that it could cause vertebral collapse, local instability of the spine, and pseudojoint formation, which may lead to intractable pain, aggravation of kyphosis, and even neurological impairment (18).
Leakage of cement from the vertebral body is relatively frequent and can occur either through bony continuum solutions or through veins departing from the vertebral soma. Cement leakage can affect the vertebral canal, leading to neurological problems. Extravasation into venous structures can lead to the phenomenon of embolism, especially at the pulmonary level, with severe consequences until death (19–21).
The use of a titanium microsphere system can plug cement leakage and simultaneously introduce, in the vertebral bone, consolidation material with documented biological activity.
Titanium microspheres are a filling material that offers immediate remedy for fracture stabilization and vertebral consolidation and at the same time is able to induce and initiate bone healing in the treatment of compression fractures. This aspect may represent a new concept and method in the vertebral body augmentation procedure.
The spherical-shaped trabecular structure, made using EBM—electron-beam melting technology, offers a scaffold for cell colonization mimicking the structure of the cancellous bone. Porous trabecular titanium, in fact, shows in vitro-marked osteoinductive and osteoconductive activity (22). The rough surface of 3D-printed EBM titanium trabeculae is prone to induce mesenchymal stem cell colonization and osteoblastic differentiation with bone matrix production (23), as demonstrated by electron microscopy, increased alkaline phosphatase activity, calcium deposition, and finally, a marked increase in the expression of genes typical of osteoblastic activity (24). Also, an in vivo study with 18-NAF PET documented the rapid induction of osteoblastic activity in the vertebral endplate by cages for interbody fusion built with the same trabecular titanium.
Porous microspheres are placed inside the vertebral soma after kyphoplasty balloon dilation, and they tend to form a stable cast with little likelihood of migration outside the created cavity and the surrounding bony gaps. This aspect may avoid the risk of embolism and neurological deficits related to cement overflow and leakage.
This system creates a compact agglomerate (inside the vertebrae) related to the “Velcro” effect generated between the trabeculae, guaranteeing stability to dynamic loads. A combination of the structure and the material used for production makes it possible to sustain the loads envisaged, with an elasticity module very similar to that of the bone tissue. The trabecular structure of the porous titanium spheres is similar to the spongy bone structure. It will be interesting to evaluate whether kyphoplasty with microspheres can permanently reduce and restore segmental kyphosis post-VCF (25).
The osteoinductive capacity of the spheres can lead to a complete rehabilitation of the trabecular structure by osteoblastic cell and integration with the surrounding bone tissue. This aspect is likely related to the stability of the microsphere cast and fracture at 3 months’ follow-up (Figure 4).
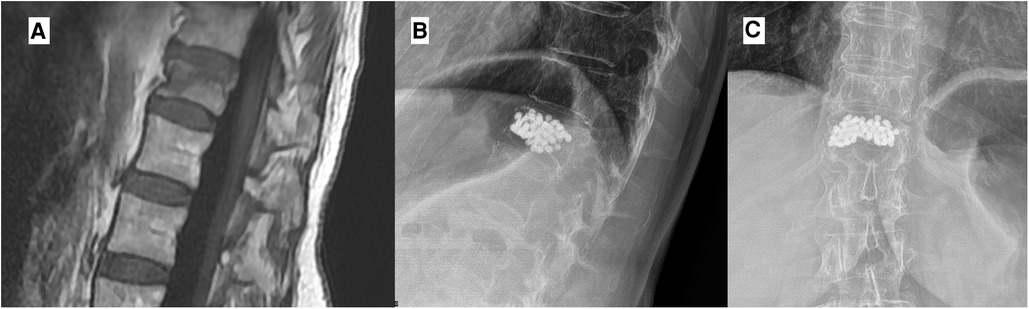
Figure 4. In this case, a 76-year-old woman with vertebral compression active fracture visible in MRI with STIR sequences (A) was treated with D11 vertebroplasty with the injection of titanium microspheres. The follow-up radiographic control shows the stability of the implant without any sign of leakage (B,C).
An evaluation of the use of this type of material for “vertebral augmentation” may lead to a potential increase in the safety and efficacy of kyphoplasty procedures. The learning curve for this innovative system is not very steep since the instrumentation, introducer, and trokar are quite similar to those normally used for PMMA injection. Nevertheless, we suggest prospective studies to validate our preliminary results and suggestions.
The main limitation of this study was the low number of patients included in the sample, which did not allow us to assess statistical significance. In addition, it was not possible at this time to make an assessment of the recovery of lordosis loss or curvature [measurement of SL and others (22)], since some patients with very high VAS scores were not found suitable for preoperative standing imaging. We suggest that a larger study with a prospective arm can provide further validity for the introduction of alternate methods for the use of PMMA in VAPs. The protocol of the prospective observational study has been submitted on the Clinicaltrials.gov platform and is now in the stage of patient recruitment.
This is the first clinical report describing the treatment of osteoporotic fractures by kyphoplasty with porous titanium microspheres. The procedure can be performed feasibly and stably using titanium microspheres with the goal of promoting better osteointegration and avoiding the risks of material leakage.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Informed consent was obtained from all individual participants included in the study. No patients under the age of 18 were included in this article. Any potentially identifiable images or data included in this article. The patient has consented to the submission of this review article to the journal. Written consent for publication was obtained from all patients.
FT and NF performed the surgical procedure and conceptualized the study. DA drafted the manuscript and made the submission. DG performed supervision. FM and GC collected data and performed the surgical procedure. FC collected data and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
VCFs, vertebral compression fractures; VAPs, vertebral augmentation procedures; PMMA, polymethyl methacrylate; WHO, World Health Organization; BMD, bone mineral density; VAS, visual analog scale; RMQ, Roland–Morris Low Back Pain and Disability Questionnaire; ODI, Oswestry Disability Index; CT, computed tomography scan; MRI, magnetic resonance imaging; AVH, anterior vertebral height
1. Caliogna L, Bina V, Botta L, Benazzo FM, Medetti M, Maestretti G, et al. Clinical outcomes after acute osteoporotic vertebral fractures: a 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med J Aust. (2006) 184:113–7. doi: 10.5694/j.1326-5377.2006.tb00148.x
2. McGuire R. AAOS clinical practice guideline: the treatment of symptomatic osteoporotic spinal compression fractures. J Am Acad Orthop Surg. (2011) 19:183–4. doi: 10.5435/00124635-201103000-00008
3. Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev. (2018) 4(4):Cd006349. doi: 10.1002/14651858.CD006349.pub3
4. Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine. (2009) 34:1349–54. doi: 10.1097/BRS.0b013e3181a4e628
5. Gao X, Du J, Gao L, Hao D, Hui H, He B, et al. Risk factors for bone cement displacement after percutaneous vertebral augmentation for osteoporotic vertebral compression fractures. Front Surg. (2022) 9:947212. doi: 10.3389/fsurg.2022.947212
6. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. (2013) 68:1243–51. doi: 10.1093/gerona/glt093
7. Clark W, Bird P, Gonski P, Diamond TH, Smerdely P, McNeil HP, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. (2016) 388(10052):1408–16. doi: 10.1016/S0140-6736(16)31341-1
8. Wang S, Zheng L, Ma JX, Wang H, Sun ST, Zhang BH, et al. Analysis of the most influential publications on vertebral augmentation for treating osteoporotic vertebral compression fracture: a review. Medicine. (2022) 101(31):e30023. doi: 10.1097/MD.0000000000030023
9. Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine. (2006) 31:2747–55. doi: 10.1097/01.brs.0000244639.71656.7d
10. Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. (2009) 361:569–79. doi: 10.1056/NEJMoa0900563
11. Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. (2006) 6:479–87. doi: 10.1016/j.spinee.2006.04.013
12. Wong CC, McGirt MJ. Vertebral compression fractures: a review of current management and multimodal therapy. J Multi-Discip Healthc. (2013) 6:205–14. doi: 10.2147/JMDH.S31659
13. Wang H, Sribastav SS, Ye F, Yang C, Wang J, Liu H, Zheng Z. Comparison of percutaneous vertebroplasty and balloon kyphoplasty for the treatment of single level vertebral compression fractures: a meta-analysis of the literature. Pain Physician. (2015) 18:209–22. PMID: 26000665
14. Tsai TT, Chen WJ, Lai PL, Chen LH, Niu CC, Fu TS, et al. Polymethylmethacrylate cement dislodgment following percutaneous vertebroplasty: a case report. Spine. (2003) 28(22):E457–60. doi: 10.1097/01.Brs.0000096668.54378.25
15. Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. (1987) 33:166–8. PMID: 3600949
16. Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. Am J Neuroradiol. (1997) 18:1897–904. PMID: 9403451; PMCID: PMC83373809403451
17. Armocida D, Pesce A, Cimatti M, Proietti L, Santoro A, Frati A. Minimally invasive transforaminal lumbar interbody fusion using expandable cages: increased risk of late postoperative subsidence without a real improvement of perioperative outcomes: a clinical monocentric study. World Neurosurg. (2021) 156:e57–63. doi: 10.1016/j.wneu.2021.08.127
18. Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine. (2009) 34:1228–32. doi: 10.1097/BRS.0b013e3181a3c742
19. Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. (2009) 361:557–68. doi: 10.1056/NEJMoa0900429
20. Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. Am J Neuroradiol. (2007) 28:555–60. PMID: 17353335; PMCID: PMC797784217353335
21. Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. (2008) 8:488–97. doi: 10.1016/j.spinee.2007.04.004
22. Ragni E, Perucca Orfei C, Bidossi A, De Vecchi E, Francaviglia N, Romano A, et al. Superior osteo-inductive and osteo-conductive properties of trabecular titanium vs. PEEK scaffolds on human mesenchymal stem cells: a proof of concept for the use of fusion cages. Int J Mol Sci. (2021) 22(5):2379. doi: 10.3390/ijms22052379
23. Caliogna L, Bina V, Botta L, Benazzo FM, Medetti M, Maestretti G, et al. Osteogenic potential of human adipose derived stem cells (hASCs) seeded on titanium trabecular spinal cages. Sci Rep. (2020) 10(1):18284. doi: 10.1038/s41598-020-75385-y
24. Cofano F, Armocida D, Ruffini L, Scarlattei M, Baldari G, Di Perna G, et al. The efficacy of trabecular titanium cages to induce reparative bone activity after lumbar arthrodesis studied through the 18f-Naf PET/CT scan: observational clinical in-vivo study. Diagnostics (Basel). (2022) 12(10):2296. doi: 10.3390/diagnostics12102296
Keywords: vertebroplasty, kyphoplasty, poly (methyl methacrylate) (PMMA), titanium-based composite, osteoporotic age-related fractures
Citation: Tartara F, Armocida D, Garbossa D, Meli F, Costantino G, Cofano F and Francaviglia N (2023) Porous titanium microsphere kyphoplasty for augmentation treatment of osteoporotic vertebral fractures: Technical report and case series. Front. Surg. 10:1152995. doi: 10.3389/fsurg.2023.1152995
Received: 28 January 2023; Accepted: 14 March 2023;
Published: 3 May 2023.
Edited by:
Andrea Perna, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Alessandro Pesce, Hospital Santa Maria Goretti, Italy© 2023 Tartara, Armocida, Garbossa, Meli, Costantino, Cofano and Francaviglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Armocida ZGFuaWVsZWFybW9jaWRhQHlhaG9vLml0
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.