
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 12 April 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1150460
This article is part of the Research Topic Recent Advances and New Challenges in Minimally Invasive Surgery and Chemotherapy for Colorectal Cancer View all 7 articles
 Takehito Yamamoto1*
Takehito Yamamoto1* Mami Yoshitomi2
Mami Yoshitomi2 Yoshiki Oshimo1
Yoshiki Oshimo1 Yuta Nishikawa1
Yuta Nishikawa1 Koji Hisano1
Koji Hisano1 Kenzo Nakano1
Kenzo Nakano1 Takayuki Kawai1
Takayuki Kawai1 Yoshihisa Okuchi1
Yoshihisa Okuchi1 Kohta Iguchi1
Kohta Iguchi1 Eiji Tanaka1
Eiji Tanaka1 Meiki Fukuda1
Meiki Fukuda1 Kojiro Taura1
Kojiro Taura1 Hiroaki Terajima1
Hiroaki Terajima1
Background: Surgical site infection (SSI) is one of the most important complications of surgery for gastroenterological malignancies because it leads to a prolonged postoperative hospital stay and increased inpatient costs. Furthermore, SSI can delay the initiation of postoperative treatments, including adjuvant chemotherapy, negatively affecting patient prognosis. Identifying the risk factors for SSI is important to improving intra- and postoperative wound management for at-risk patients.
Methods: Patients with gastroenterological malignancies who underwent surgery at our institution were retrospectively reviewed and categorized according to the presence or absence of incisional SSI. Clinicopathological characteristics such as age, sex, body mass index, malignancy location, postoperative blood examination results, operation time, and blood loss volume were compared between groups. The same analysis was repeated of only patients with colorectal malignancies.
Results: A total of 528 patients (330 men, 198 women; mean age, 68 ± 11 years at surgery) were enrolled. The number of patients with diseases of the esophagus, stomach, small intestine, colon and rectum, liver, gallbladder, and pancreas were 25, 150, seven, 255, 51, five, and 35, respectively. Open surgery was performed in 303 patients vs. laparoscopic surgery in 225 patients. An incisional SSI occurred in 46 patients (8.7%). Multivariate logistic regression analysis showed that postoperative hyperglycemia (serum glucose level ≥140 mg/dl within 24 h after surgery), colorectal malignancy, and open surgery were independent risk factors for incisional SSI. In a subgroup analysis of patients with colorectal malignancy, incisional SSI occurred in 27 (11%) patients. Open surgery was significantly correlated with the occurrence of incisional SSI (P = 0.024).
Conclusions: Postoperative hyperglycemia and open surgery were significant risk factors for SSI in patients with gastroenterological malignancies. Minimally invasive surgery could reduce the occurrence of incisional SSI.
Surgical site infection (SSI) is one of the largest contributors to overall inpatient costs (1–5). Furthermore, especially in patients with gastroenterological malignancies, SSI can lead to delayed initiation of postoperative treatment and negatively affect patient prognosis. Determining the risk factors for SSI could potentially improve intra- and postoperative wound management in at-risk patients.
Many studies have reported different pre-, intra-, and postoperative risk factors for SSI in abdominal surgery (6–8), such as diabetes, perioperative transfusion, cirrhosis, and bowel anastomosis. Numerous studies have reported that patients with diabetes are prone to postoperative infectious diseases, including SSI (9–11). Martin et al. reported an association between diabetes and the risk of SSI in a large systematic review and meta-analysis (9). In contrast, Ata et al. revealed that a high serum glucose level was the only significant predictor of SSI in colorectal surgery patients, which means that postoperative hyperglycemia, rather than diabetes mellitus itself, affects the occurrence of SSI in known as well as unknown or non-diabetic patients (12).
Minimally invasive surgery, including laparoscopic or robotic surgery, recently became the standard treatment for many types of cancer. Minimally invasive surgery can reduce the occurrence of SSI. Kagawa et al. reported an association between increased use of the laparoscopic approach and decreased SSI rates during a 13-year study period (13).
The guidelines of the Centers for Disease Control and Prevention (CDC) divide SSI into three types: incisional, deep, and organ/space (14). Although previous studies discussed all SSI types, we believe that the risk factors and preventive measures for each of the three types differ (15). Therefore, in the present study, we focused on incisional SSI and its risk factors among patients who underwent gastroenterological surgery for malignancies.
Patients with gastroenterological malignancies who underwent surgery in our hospital between 2011 and 2013 were reviewed. The gastroenterological malignancies included malignant diseases (preoperative diagnoses of cancer, neuroendocrine tumors, and gastrointestinal stromal tumors) of the esophagus, stomach, small intestine, colon and rectum, liver, gallbladder, biliary duct, and pancreas. Based on the concept that SSI occurrence was associated with the operative procedure rather than the disease's location, patients with disease of the distal biliary duct and papilla of Vater who received pancreatic resection were classified as “pancreas.” We then divided the study patients into SSI and no-SSI groups according to the occurrence of incisional SSI and compared the clinical characteristics between them. Furthermore, we conducted the same comparison for a subgroup of only patients with colorectal malignancies.
Patients with diabetes mellitus were defined as those who received pharmacological treatment for diabetes before the operation or a higher than normal hemoglobin A1c level. Postoperative hyperglycemia was defined as a serum glucose level of >140 mg/dl within 24 h postoperative. Current smokers were defined as individuals who smoked within 1 month preoperative.
Patients with incisional SSI based on the CDC guidelines were included in the SSI group (16). The wound was examined by a doctor and nurse at least once daily until hospital discharge. After discharge, the wound was examined by an outpatient doctor for 30 days postoperative. SSI was diagnosed after discussion with surgeons, nurses, and members of the SSI surveillance team.
Continuous variables are presented as mean ± standard deviation or median [range], and categorical variables were presented as numbers and percentages. The χ2 and Wilcoxon rank sum tests were used to compare groups. Variables potentially associated with SSI in the univariate analysis (values of P < 0.1) were included in the multivariate logistic regression model. The effects of the associations are expressed as odds ratios (OR) and 95% confidence intervals (CI). All statistical analyses were conducted by a participating physician (TY) using JMP version 16 (SAS Institute, Cary, NC, United States). All reported P-values were two-sided; those <0.05 were considered statistically significant. The study design was approved by the Ethics Review Board of Kitano Hospital (no. 2201013).
A total of 528 patients were enrolled. Table 1 shows the clinical characteristics of all participants (330 men, 198 women; mean age, 68 ± 11 years at surgery). The mean body mass index (BMI) was 22.6 ± 3.7 kg/m2. The number of patients with diseases of the esophagus, stomach, small intestine, colon and rectum, liver, gallbladder, and pancreas were 25, 150, seven, 255, 51, five, and 35, respectively. Open surgery was performed in 303 patients vs. laparoscopic surgery in 225 patients. Intraoperative blood transfusions were performed in 52 patients.
An incisional SSI occurred in 46 patients (8.7%). Table 2 compares the SSI and non-SSI groups. A univariate analysis showed that significantly more patients in the SSI group had postoperative hyperglycemia (P = 0.024) and that male sex, higher BMI, and open surgery tended to affect the occurrence of incisional SSI (P = 0.094, 0.058, and 0.080, respectively). As shown in Table 3, the multivariate logistic regression analysis showed that postoperative hyperglycemia (OR, 1.94; 95% CI, 1.02–3.70; P = 0.043), colorectal malignancy (OR, 3.09; 95% CI, 1.49–6.44; P = 0.003), and open surgery (OR, 2.73; 95% CI, 1.27–5.89; P = 0.010) were independent risk factors for incisional SSI. Based on these three factors, the incisional SSI rates of the two groups are shown in Figure 1.
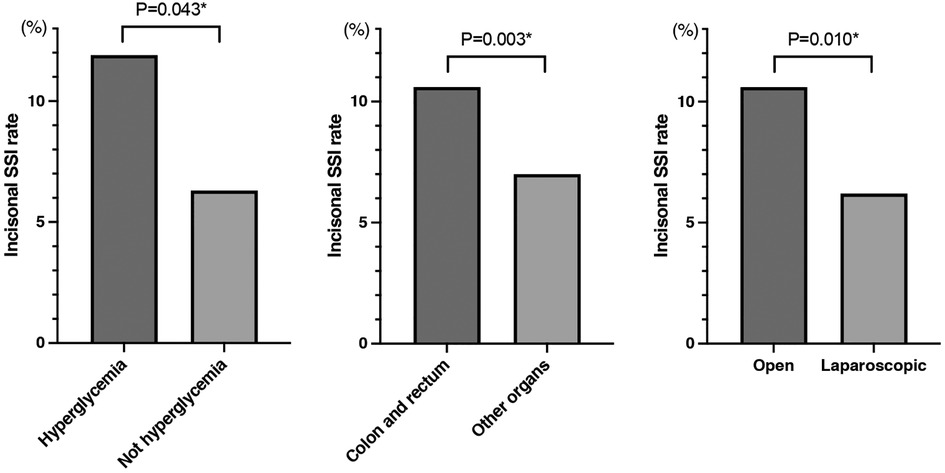
Figure 1. Comparison of the incisional SSI rate by glycemia status, affected organs, and surgical approach. SSI, surgical site infection
In a subgroup analysis of patients with colorectal malignancy, incisional SSI occurred in 27 (11%) patients. Table 4 compares the SSI and no-SSI patients. Open surgery was significantly associated with the occurrence of incisional SSI vs. laparoscopic surgery (P = 0.024), while postoperative hyperglycemia tended to affect the occurrence of incisional SSI (P = 0.051).
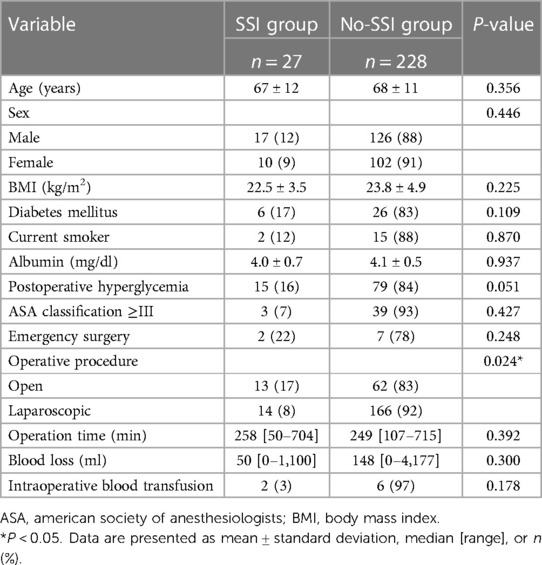
Table 4. Clinical characteristics of SSI vs. no-SSI groups among patients who underwent colorectal surgery (n = 255).
Numerous studies have analyzed the risk factors for SSI in patients with different diseases. Especially in gastroenterology, risk factors and preventive measures for SSI have been widely discussed because of the high rate of SSI.
We found that postoperative hyperglycemia independently affected the occurrence of incisional SSI. Despite ample evidence showing that perioperative hyperglycemia is associated with postoperative infective complications, these studies analyzed all infectious diseases, including SSI, pneumonia, and urinary tract infections (17–19). Previous reports that focused on the association between postoperative hyperglycemia and SSI rate highlighted specific surgical procedures, such as colorectal surgery, cardiothoracic surgery, and sleeve gastrectomy (10, 11, 20). On the other hand, Ata et al. analyzed a total of 2,090 general and vascular surgery patients in whom only the postoperative serum glucose level was associated with SSI (12). Although this result was consistent with the present findings, the study included a diverse array of surgeries for many diseases: clean or dirty, benign or malignant, and gastroenterological or cardiovascular. Our study selected patients with a common circumstance of gastroenterological malignancies, an inclusion criterion that was reasonable for improving postoperative wound management provided by medical personnel in the same department.
In addition, compared to laparoscopic surgery, open surgery was an independent risk factor for incisional SSI, consistent with the results of previous reports (21, 22). Wang et al. reported in a large systematic review and meta-analysis that the laparoscopic approach significantly reduced the SSI rate in gastrointestinal surgeries (21).
In the present study, patients who underwent colorectal surgery were exclusively analyzed as subgroups. Although the subgroup results were slightly different from those of all study patients, open surgery was also the principal risk factor for incisional SSI. Specifically, several studies have reported that use of the laparoscopic vs. open approach in colon cancer patients could reduce the SSI rate (13, 22–25). For example, a large-scale study of 229,726 colorectal cancer patients revealed that the SSI rate was significantly lower in laparoscopic than open surgery, with an OR of 0.43 (26). Similarly, Kulkarni et al. discussed incisional SSI as being separate from other types of SSI and showed that laparoscopic surgery could reduce the rate of all SSI types (25). One could theorize that, especially in open colorectal surgery, a longer incision on the skin will be more prone to intraoperative contamination by fecal ascites than laparoscopic surgery. Therefore, minimally invasive surgery, including laparoscopic surgery, decreases the incisional SSI rate, especially among patients with colorectal cancer.
In this subgroup analysis, postoperative hyperglycemia tended to be associated with incisional SSI. Although several previous studies have analyzed patients who underwent elective colorectal surgery, they have not focused on postoperative serum blood glucose levels, preferring to evaluate the presence or absence of diabetes (27–29). Thus, the results of our subgroup analysis should be of further value for preventing SSI after colorectal surgery.
Unfortunately, in our retrospective study, data on intraoperative body temperature were not available and we were unable to conduct a detailed analysis of the correlation between this factor and SSI occurrence. The guidelines for safe surgery published by the World Health Organization describe that “maintenance of normothermia during surgery” reduces the SSI rate (30). Reports have shown an association between intraoperative hypothermia and SSI incidence (31, 32). Tsuchida et al. reported that severe hypothermia (<35.0°C) and late-nadir hypothermia (<36°C for >2 h after anesthesia induction) were significant risk factors for SSI in prolonged gastroenterological surgery (31). On the other hand, contrasting results were also reported by some studies (33, 34), and the appropriate intraoperative body temperature remains a controversial aspect. Further studies of the impact of intraoperative hypothermia on SSI are warranted.
One measure to reduce SSI is to build a preventive SSI care bundle (i.e., use of systematic approaches). Many reports have indicated the effectiveness of these bundles, some of which include strict glycemic control (15, 35–38). In our hospital, severely diabetic patients were admitted more than 1 week preoperatively and should receive specific management of blood glucose levels by diabetes physicians to reduce potential perioperative complications caused by hyperglycemia. The results reported herein indicate the importance of postoperative management of blood glucose levels for both patients with severe diabetes and for all patients with gastroenterological malignancies.
Our study had several limitations. First, it was conducted at a single center and included a small number of patients. Thus, a large-scale multicenter study is needed to confirm our findings. Second, operative and postoperative management involving sutures or dressings and perioperative antibiotic administration are dependent on different clinicians result in an inconsistent quality of care. Finally, other factors not included in our analysis may have influenced the outcomes.
In conclusion, postoperative hyperglycemia and open surgery are significant risk factors for incisional SSI in patients with gastroenterological malignancies. Meticulous management of postoperative serum glucose levels can positively affect outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics approval and consent to participate: All study participants provided informed consent, and the study design was approved by the ethics review board of Kitano Hospital (no. 2201013). This study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TY: designed the study. TY and MY: acquired, analyzed, and interpreted the data and drafted and revised the manuscript. YO, YN, KH, KN, TK, YO, KI, ET, MF, KT and HT: helped revise the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. (2013) 173:2039–46. doi: 10.1001/jamainternmed.2013.9763
2. Olson MM, Lee JT Jr. Continuous, 10-year wound infection surveillance. Results, advantages, and unanswered questions. Arch Surg. (1990) 125:794–803. doi: 10.1001/archsurg.1990.01410180120020
3. Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am. (1980) 60:27–40. doi: 10.1016/s0039-6109(16)42031-1
4. Mahmoud NN, Turpin RS, Yang G, Saunders WB. Impact of surgical site infections on length of stay and costs in selected colorectal procedures. Surg Infect. (2009) 10:539–44. doi: 10.1089/sur.2009.006
5. Reilly J, Twaddle S, McIntosh J, Kean L. An economic analysis of surgical wound infection. J Hosp Infect. (2001) 49:245–9. doi: 10.1053/jhin.2001.1086
6. Pessaux P, Atallah D, Lermite E, Msika S, Hay JM, Flamant Y, et al. Risk factors for prediction of surgical site infections in “clean surgery”. Am J Infect Control. (2005) 33:292–8. doi: 10.1016/j.ajic.2004.12.005
7. Pessaux P, Msika S, Atalla D, Hay JM, Flamant Y, French Association for Surgical R. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: a multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg. (2003) 138:314–24. doi: 10.1001/archsurg.138.3.314
8. Walz JM, Paterson CA, Seligowski JM, Heard SO. Surgical site infection following bowel surgery: a retrospective analysis of 1446 patients. Arch Surg. (2006) 141:1014–8; discussion 8. doi: 10.1001/archsurg.141.10.1014
9. Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. (2016) 37:88–99. doi: 10.1017/ice.2015.249
10. Nakamura T, Sato T, Takayama Y, Naito M, Yamanashi T, Miura H, et al. Risk factors for surgical site infection after laparoscopic surgery for colon cancer. Surg Infect. (2016) 17:454–8. doi: 10.1089/sur.2015.205
11. Latham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS Jr. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. (2001) 22:607–12. doi: 10.1086/501830
12. Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. (2010) 145:858–64. doi: 10.1001/archsurg.2010.179
13. Kagawa Y, Yamada D, Yamasaki M, Miyamoto A, Mizushima T, Yamabe K, et al. The association between the increased performance of laparoscopic colon surgery and a reduced risk of surgical site infection. Surg Today. (2019) 49:474–81. doi: 10.1007/s00595-019-1760-1
14. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. (1992) 13:606–8. doi: 10.2307/30148464
15. Yamamoto T, Morimoto T, Kita R, Masui H, Kinoshita H, Sakamoto Y, et al. The preventive surgical site infection bundle in patients with colorectal perforation. BMC Surg. (2015) 15:128. doi: 10.1186/s12893-015-0115-0
16. Berrios-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. (2017) 152:784–91. doi: 10.1001/jamasurg.2017.0904
17. King JT Jr, Goulet JL, Perkal MF, Rosenthal RA. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg. (2011) 253:158–65. doi: 10.1097/SLA.0b013e3181f9bb3a
18. Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. (2010) 33:1783–8. doi: 10.2337/dc10-0304
19. Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. (2011) 34:256–61. doi: 10.2337/dc10-1407
20. Ruiz-Tovar J, Oller I, Llavero C, Arroyo A, Munoz JL, Calero A, et al. Pre-operative and early post-operative factors associated with surgical site infection after laparoscopic sleeve gastrectomy. Surg Infect. (2013) 14:369–73. doi: 10.1089/sur.2012.114
21. Wang Z, Chen J, Wang P, Jie Z, Jin W, Wang G, et al. Surgical site infection after gastrointestinal surgery in China: a multicenter prospective study. J Surg Res. (2019) 240:206–18. doi: 10.1016/j.jss.2019.03.017
22. Shabanzadeh DM, Sorensen LT. Laparoscopic surgery compared with open surgery decreases surgical site infection in obese patients: a systematic review and meta-analysis. Ann Surg. (2012) 256:934–45. doi: 10.1097/SLA.0b013e318269a46b
23. Kiran RP, El-Gazzaz GH, Vogel JD, Remzi FH. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: data from national surgical quality improvement program. J Am Coll Surg. (2010) 211:232–8. doi: 10.1016/j.jamcollsurg.2010.03.028
24. Poon JT, Law WL, Wong IW, Ching PT, Wong LM, Fan JK, et al. Impact of laparoscopic colorectal resection on surgical site infection. Ann Surg. (2009) 249:77–81. doi: 10.1097/SLA.0b013e31819279e3
25. Kulkarni N, Arulampalam T. Laparoscopic surgery reduces the incidence of surgical site infections compared to the open approach for colorectal procedures: a meta-analysis. Tech Coloproctol. (2020) 24:1017–24. doi: 10.1007/s10151-020-02293-8
26. Caroff DA, Chan C, Kleinman K, Calderwood MS, Wolf R, Wick EC, et al. Association of open approach vs laparoscopic approach with risk of surgical site infection after colon surgery. JAMA Netw Open. (2019) 2:e1913570. doi: 10.1001/jamanetworkopen.2019.13570
27. Smith RL, Bohl JK, McElearney ST, Friel CM, Barclay MM, Sawyer RG, et al. Wound infection after elective colorectal resection. Ann Surg. (2004) 239:599–605; discussion -7. doi: 10.1097/01.sla.0000124292.21605.99
28. Konishi T, Watanabe T, Kishimoto J, Nagawa H. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg. (2006) 244:758–63. doi: 10.1097/01.sla.0000219017.78611.49
29. Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. (2005) 48:438–43. doi: 10.1007/s10350-004-0827-1
30. WHO Guidelines for Safe Surgery 2009: Safe Surgery Saves Lives. Geneva: World Health Organization (2009).
31. Tsuchida T, Takesue Y, Ichiki K, Uede T, Nakajima K, Ikeuchi H, et al. Influence of peri-operative hypothermia on surgical site infection in prolonged gastroenterological surgery. Surg Infect. (2016) 17:570–6. doi: 10.1089/sur.2015.182
32. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med. (1996) 334:1209–15. doi: 10.1056/NEJM199605093341901
33. Baucom RB, Phillips SE, Ehrenfeld JM, Muldoon RL, Poulose BK, Herline AJ, et al. Association of perioperative hypothermia during colectomy with surgical site infection. JAMA Surg. (2015) 150:570–5. doi: 10.1001/jamasurg.2015.77
34. Brown MJ, Curry TB, Hyder JA, Berbari EF, Truty MJ, Schroeder DR, et al. Intraoperative hypothermia and surgical site infections in patients with class I/clean wounds: a case-control study. J Am Coll Surg. (2017) 224:160–71. doi: 10.1016/j.jamcollsurg.2016.10.050
35. Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. (2014) 149:1045–52. doi: 10.1001/jamasurg.2014.346
36. Lutfiyya W, Parsons D, Breen J. A colorectal “care bundle” to reduce surgical site infections in colorectal surgeries: a single-center experience. Perm J. (2012) 16:10–6. doi: 10.7812/TPP/12.968
37. Waits SA, Fritze D, Banerjee M, Zhang W, Kubus J, Englesbe MJ, et al. Developing an argument for bundled interventions to reduce surgical site infection in colorectal surgery. Surgery. (2014) 155:602–6. doi: 10.1016/j.surg.2013.12.004
38. Tanner J, Padley W, Assadian O, Leaper D, Kiernan M, Edmiston C. Do surgical care bundles reduce the risk of surgical site infections in patients undergoing colorectal surgery? A systematic review and cohort meta-analysis of 8,515 patients. Surgery. (2015) 158:66–77. doi: 10.1016/j.surg.2015.03.009
Keywords: colorectal cancer, laparoscopic surgery, minimally invasive surgery, surgical site infection, hyperglycemia, SSI
Citation: Yamamoto T, Yoshitomi M, Oshimo Y, Nishikawa Y, Hisano K, Nakano K, Kawai T, Okuchi Y, Iguchi K, Tanaka E, Fukuda M, Taura K and Terajima H (2023) Ability of minimally invasive surgery to decrease incisional surgical site infection occurrence in patients with colorectal cancer and other gastroenterological malignancies. Front. Surg. 10:1150460. doi: 10.3389/fsurg.2023.1150460
Received: 24 January 2023; Accepted: 20 March 2023;
Published: 12 April 2023.
Edited by:
Hiroki Hashida, Kobe City Medical Center General Hospital, JapanReviewed by:
Paolo Bruzzone, Sapienza University of Rome, Italy© 2023 Yamamoto, Yoshitomi, Oshimo, Nishikawa, Hisano, Nakano, Kawai, Okuchi, Iguchi, Tanaka, Fukuda, Taura and Terajima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takehito Yamamoto dGFrZWhpdG9feUBrdWhwLmt5b3RvLXUuYWMuanA=
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.