
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 21 April 2023
Sec. Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1148274
This article is part of the Research TopicNon-Specific Symptoms of Unruptured Intracranial AneurysmsView all 8 articles
 Ashia M. Hackett
Ashia M. Hackett Stefan W. Koester
Stefan W. Koester Emmajane G. Rhodenhiser
Emmajane G. Rhodenhiser Lea Scherschinski
Lea Scherschinski Jarrod D. Rulney
Jarrod D. Rulney Anant Naik
Anant Naik Elsa Nico
Elsa Nico Adam T. Eberle
Adam T. Eberle Joelle N. Hartke
Joelle N. Hartke Brandon M. Fox
Brandon M. Fox Ethan A. Winkler
Ethan A. Winkler Joshua S. Catapano
Joshua S. Catapano Michael T. Lawton*
Michael T. Lawton*
Background: Approximately 3.2%–6% of the general population harbor an unruptured intracranial aneurysm (UIA). Ruptured aneurysms represent a significant healthcare burden, and preventing rupture relies on early detection and treatment. Most patients with UIAs are asymptomatic, and many of the symptoms associated with UIAs are nonspecific, which makes diagnosis challenging. This study explored symptoms associated with UIAs, the rate of resolution of such symptoms after microsurgical treatment, and the likely pathophysiology.
Methods: A retrospective review of patients with UIAs who underwent microsurgical treatment from January 1, 2014, to December 31, 2020, at a single quaternary center were identified. Analyses included the prevalence of nonspecific symptoms upon clinical presentation and postoperative follow-up; comparisons of symptomatology by aneurysmal location; and comparisons of patient demographics, aneurysmal characteristics, and poor neurologic outcome at postoperative follow-up stratified by symptomatic versus asymptomatic presentation.
Results: The analysis included 454 patients; 350 (77%) were symptomatic. The most common presenting symptom among all 454 patients was headache (n = 211 [46%]), followed by vertigo (n = 94 [21%]), cognitive disturbance (n = 68[15%]), and visual disturbance (n = 64 [14%]). Among 328 patients assessed for postoperative symptoms, 258 (79%) experienced symptom resolution or improvement.
Conclusion: This cohort demonstrates that the clinical presentation of patients with UIAs can be associated with vague and nonspecific symptoms. Early detection is crucial to prevent aneurysmal subarachnoid hemorrhage. It is imperative that physicians not rule out aneurysms in the setting of nonspecific neurologic symptoms.
Approximately 3.2%–6% of the general population harbor an unruptured intracranial aneurysm (UIA). UIAs are increasingly found among women and are often detected between the fourth and sixth decades of life (1, 2). More than 91% of UIAs are asymptomatic and are only incidentally discovered when imaging is performed for unrelated reasons (3). Rupture of intracranial aneurysms represents a significant healthcare burden, with nearly 50% of cases resulting in death within 3 months of presentation. Of those patients who undergo life-saving treatment, 66% will experience varying degrees of permanent neurologic disability (4). Prevention of rupture, therefore, relies on early detection and treatment of aneurysms, although the clinical presentation is often nonspecific, and UIAs are frequently misdiagnosed on initial evaluation (5, 6). As such, recognition of symptoms related to UIAs and early detection are pertinent.
The International Study of Unruptured Intracranial Aneurysms (ISUIA) trial reported that the most common presenting symptoms are headache, ischemic cerebrovascular events, and cranial nerve deficits (7). Although headache is the most common reason for diagnostic imaging that leads to the detection of UIAs, it is unclear in most cases whether a headache is directly related to the aneurysm in the absence of subarachnoid hemorrhage (SAH) (2). Aneurysmal characteristics, such as size and location, can influence symptomatology and are likely responsible for compression of adjacent structures (1, 8). The pathophysiology associated with many of the vague symptoms reported in the presence of a UIA is not well understood. Additionally, many other nonspecific symptoms are associated with UIAs, which makes their diagnosis challenging. This study explores various symptoms associated with UIAs, the rate of resolution of such symptoms after microsurgical treatment, and the likely pathophysiology.
This retrospective observational cohort study was approved by the St. Joseph's Hospital and Medical Center Institutional Review Board (Phoenix, AZ) and complied with the Health Insurance Portability and Accountability Act. The need for patient consent was waived by the institutional review board due to the retrospective nature of the study.
All patients with a UIA who underwent microsurgical treatment between January 1, 2014, and December 31, 2020, at a single quaternary center were identified using a retrospective research database. Inclusion criteria were the availability of treatment data, adequate follow-up (≥6 weeks), and adequate symptom assessment. Patients who experienced recent (<6 months before surgery) SAH of an unrelated aneurysm were excluded from the analyses. Analyses included assessment of the prevalence of nonspecific symptoms upon clinical presentation and postoperative follow-up; comparisons of symptomatology by aneurysmal location; and a comparison of patient demographics, aneurysm characteristics, and poor neurologic outcome (defined as a modified Rankin Scale score greater than 2) at follow-up, with patients stratified by symptomatic versus asymptomatic presentation. Electronic medical records were analyzed for demographic information (age, sex, comorbidities), preoperative life expectancy, and Charlson Comorbidities Index. Aneurysm characteristics from computed tomography angiography were collected to assess each aneurysm's maximum diameter, neck diameter, perpendicular height (defined as the largest perpendicular distance from the neck of the aneurysm to the dome of the aneurysm), maximum height, aneurysm calcification, aneurysm type, location on critical perforating or branch vessels, intraluminal thrombosis, and size and aspect ratios. The size ratio was calculated as the maximum height divided by the mean vessel diameter of all branches associated with the aneurysm. The aspect ratio was calculated as the maximum perpendicular height divided by the neck diameter.
Statistical analyses included data aggregation, exploratory analysis, and multivariate analysis using R, version 4.0.1 (R Foundation for Statistical Computing). Demographic and clinical characteristics of patients were analyzed using a Wilcoxon rank sum test for interval variables, Pearson chi-square test for categorical variables, or Kruskal-Wallis rank sum test for nonparametric comparisons for multiple comparators. Fisher exact test was used for categorical variables to evaluate differences in asymptomatic and symptomatic patients and to compare symptoms based on aneurysm location. Significance was defined as p less than 0.05. Results are reported as mean (standard deviation [SD]) or as number (percentage) of patients.
A total of 454 patients treated during the 7-year study period were included in the final analyses (Table 1). The mean (SD) age of all patients was 58 (12) years; 335 (74%) were women, and 119 (26%) were men. Most patients had a preoperative life expectancy of greater than 10 years (298 of 416 [72%]). The vascular categories involved included 152 (33%) middle cerebral artery, 116 (26%) anterior cerebral artery, 97 (21%) internal carotid artery (ICA), and 89 (20%) posterior circulation aneurysms. The mean (SD) aspect ratio was 1.72 (1.53) for 419 aneurysms, and 32 (7%) of 434 aneurysms had calcification. Overall, 90% (n = 408) aneurysms were saccular and the remaining 10% (n = 46) were nonsaccular. Additionally, 126 (29%) of 431 UIAs were found on critical perforating vessels or vessel branches.
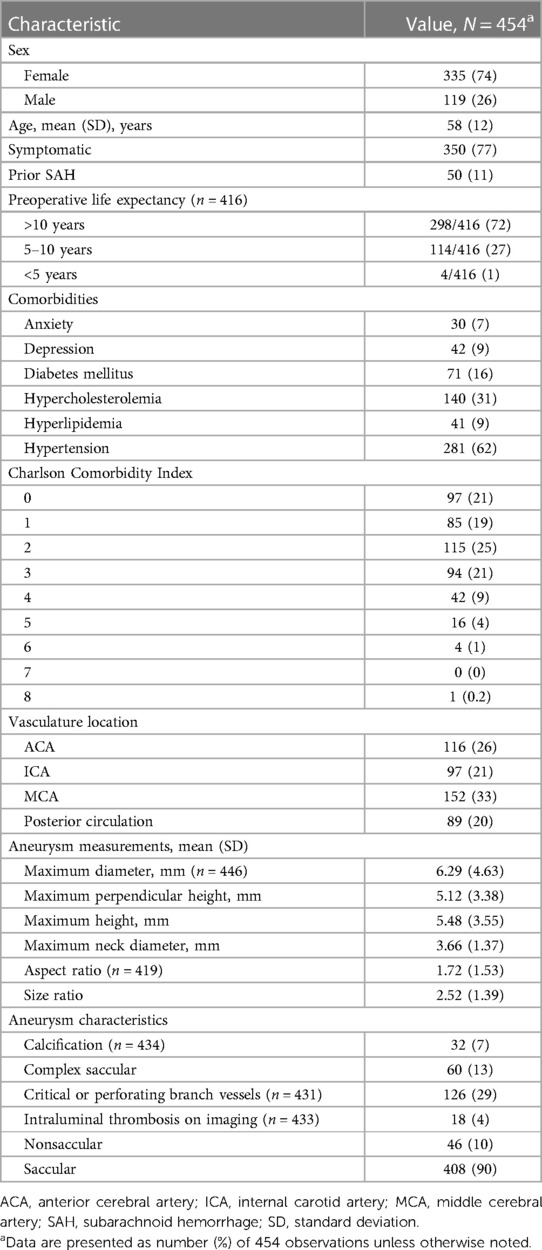
Table 1. Patient demographics and aneurysm characteristics among 454 patients with unruptured intracranial aneurysms.
Overall, 350 (77%) of the 454 patients were symptomatic; some patients had more than 1 symptom. The most common presenting symptom was headache, occurring in 211 (46%) patients, followed by vertigo (94 [21%]), cognitive disturbance (68 [15%]), and visual disturbance (64 [14%]) (Table 2). Of 328 patients for whom symptom resolution or improvement was assessed, 258 (79%) experienced full symptom resolution or improvement (Table 3). Of 204 patients assessed for headache resolution, 58 (28%) experienced residual headache at follow-up. Of these 58 patients, 32 (55%) reported an ability to control headache with over-the-counter pain medication. Of 23 patients assessed for cranial nerve recovery, 4 (17%) experienced residual cranial nerve deficits.
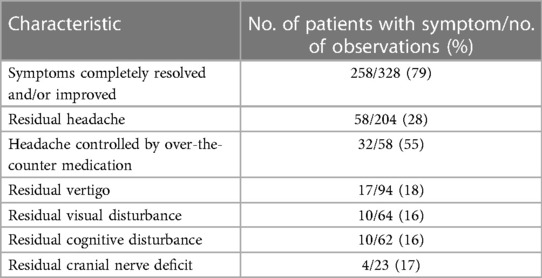
Table 3. Symptoms reported at postoperative follow-up among 454 patients with microsurgical treatment of unruptured intracranial aneurysms.
Overall, 104 (23%) patients were asymptomatic and 350 (77%) were symptomatic at presentation (Table 4). The proportion of patients with symptomatic presentation was greater among women (266/335 [79%]) than among men (84/119 [71%]). Compared with asymptomatic presentation, symptomatic presentation was associated with a greater prevalence of hypertension (65% [228/350] vs. 51% [53/104]; p = 0.009), clinically diagnosed anxiety (8% [29/350] vs. 1% [1/104]; p = 0.008], and diabetes mellitus (18% [62/350] vs. 9% [9/104]; p = 0.03). A significantly greater proportion of patients with asymptomatic presentations than patients with symptomatic presentations had an SAH >6 months before microsurgical treatment of a UIA (21% [22/104] vs. 8% [28/350]; p ≤ 0.001). The mean (SD) maximum aneurysm diameter was significantly greater in the symptomatic cohort than in the asymptomatic cohort (6.54 [4.98] mm vs. 5.46 [3.06] mm, p = 0.004). The proportion of patients with poor neurologic outcome (modified Rankin Scale score >2) at postoperative follow-up was not significantly different between the symptomatic and asymptomatic cohorts (14% [50/350] vs. 10% [10/104]; p = 0.15).
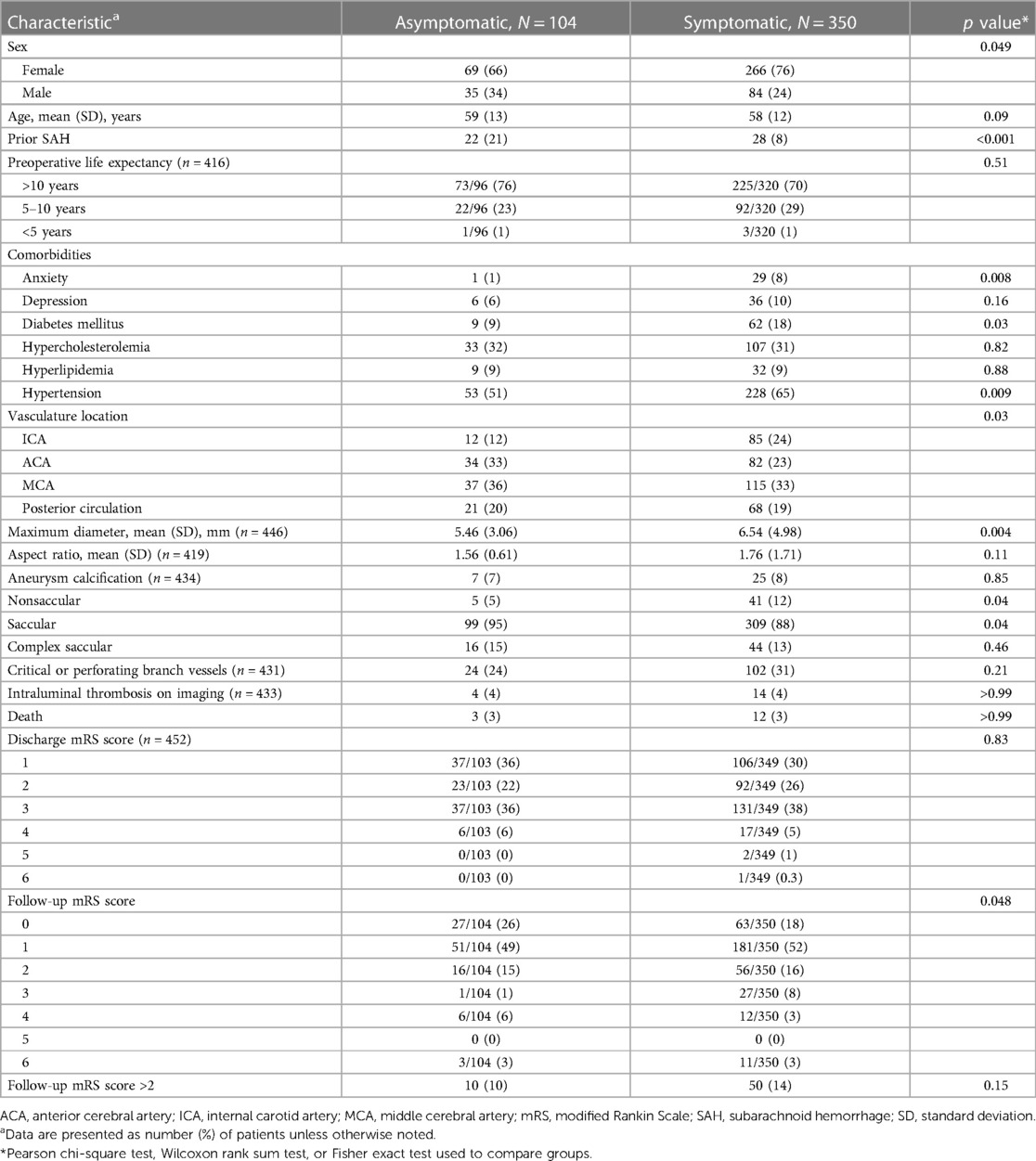
Table 4. Comparison of demographic and clinical characteristics of 104 asymptomatic and 350 symptomatic patients with unruptured intracranial aneurysms.
Table 5 reports symptoms by vascular territory. The proportion of patients with intracranial internal carotid artery aneurysms who were symptomatic (85 of 97 [88%]) was significantly greater than the proportion of patients with aneurysms in other locations who were symptomatic (p = 0.03). Gait imbalance (19 of 97 [20%]; p = 0.04) and limb weakness (13 of 97 [13%]; p = 0.01) were both significantly more prevalent in patients harboring an intracranial ICA aneurysm, compared with patients with aneurysms in other vascular locations. The prevalence of cranial nerve deficit was comparable in patients with ICA aneurysms (11 of 97 [11%]) and patients with posterior circulation aneurysms (11 of 97 [11%]); the prevalence of cranial nerve deficit among these patients was significantly greater than the prevalence among patients with aneurysms at other locations (p ≤ 0.001). The highest burden of cognitive changes was found among patients with aneurysms in the middle cerebral artery territory (29 of 152 [19%]; p = 0.04).
In our retrospective cohort, 77% (350/454) of patients presented with symptoms; often, aneurysms were discovered during evaluation for unrelated issues. It is unclear whether symptoms were a direct result of the aneurysms, yet 79% of the patients who had follow-up of 6 weeks or greater reported either improvement or complete resolution of presenting symptoms after microsurgical treatment. Although the general population of patients with UIAs tends to be asymptomatic, our institution often receives referrals of patients with complex or atypical presentations, which may account for the high percentage of patients who presented with symptoms. Patients with prior SAH (>6 months prior) were significantly more likely than other patients to have an asymptomatic presentation associated with their unruptured aneurysm diagnosis, likely due to recommended follow-up imaging after experiencing an aneurysmal SAH (aSAH). Interestingly, the presence of a clinically diagnosed anxiety disorder, prior to aneurysm diagnosis, was associated with a symptomatic UIA. Although the relationship between anxiety and aneurysms after SAH and treatment has been explored, the relationship between anxiety and symptomatic UIAs has not been discussed in literature (9). A possible explanation for this association is that patients with nonspecific symptoms who experience anxiety may be more persistent with follow-up and request further investigation, leading to higher rates of incidental UIA findings.
Headaches are the most frequently reported symptom of UIAs in the literature (10, 11) and were reported in 46% (211/454) of patients in our cohort. In these patients, the presence of an aneurysm is often missed due to nonspecificity and the varied characterization of headaches associated with UIA. Headaches can present as chronic with variable characterization in two-thirds of cases, but they may also present acutely as a sudden onset headache in one-third of cases (11). Although acute “thunderclap” headaches are typical of aSAH, less severe and more chronic headaches may be associated with UIAs. For example, a “sentinel headache” is well-described in the literature and is thought to be associated with local thrombosis (12). Chronic headaches may have variable presentations but most frequently resemble migraines (13). In 2013, Lebedeva et al. (14) conducted a prospective case-control study of 199 patients and found that migraine-like headaches without aura were significantly associated with saccular aneurysms up to 1 year before rupture (odds ratio [OR], 6.7; 95% confidence interval [CI], 3.8–11.9; p ≤ 0.001). Another examination of 172 patients found that a history of migraines was significantly associated with UIAs (OR, 1.9; 95% CI, 1.1–3.5) but no other headache types (15). Although the pathophysiology of headaches among patients with UIAs is a topic of ongoing research, headaches most likely arise from local inflammatory processes involving the meninges or cranial nerves, pulsation of the aneurysm itself, or local thrombosis (11). Intriguingly, headaches have been found to resolve in the majority of patients after treatment (11, 16, 17). Although headache was the most common residual symptom at follow-up in our analysis, 55% (32/58) of those patients reported an ability to control the pain with over-the-counter medications.
Only patients with a clear diagnosis of a cranial nerve palsy were categorized into the cranial nerve deficit cohort in this analysis. However, patients reporting hearing or visual disturbances could be experiencing a cranial nerve deficit as well. Specifically, aneurysms of the anterior inferior cerebellar artery have been implicated in patients presenting with symptoms of hearing loss and tinnitus, likely due to the close approximation of the artery to the internal auditory canal as well as cranial nerves VII and VIII (18, 19). In our study, 3 patients presented with an aneurysm of the anterior inferior cerebellar artery, and 1 of these patients had a cranial nerve deficit.
A range of visual deficits were reported in our patient cohort (14% [64/454]). Aneurysm-related visual changes commonly reported in the literature may include diplopia, ptosis, pupillary dilation, and lateral deviation of the affected eye, likely secondary to oculomotor palsy. Decreased visual acuity as a result of optic pathway compression has also been reported. In fact, 2 of the most common causes of non–pupil-sparing oculomotor nerve palsy are posterior communicating artery aneurysms and distal basilar artery aneurysms (20). Less commonly, aneurysms of the cavernous portion of the ICA have been reported to cause third cranial nerve palsy (21). Although the pathophysiology behind oculomotor nerve palsy is thought to be due to pulsatility and compressive mass effect of large aneurysms, there are reports of small aneurysms causing third cranial nerve palsy, refuting the idea that these deficits are strictly related to aneurysm size (21–23). Any aneurysm of the circle of Willis can cause anterior optic pathway compression and result in visual deficits; however, the most common locations include the ICA, specifically the paraclinoid region. In a study conducted by Park et al. (8), the most common aneurysms causing visual deficits arose from the ophthalmic segment of the ICA (31 of 33 cases). The close approximation of the ICA to the optic nerve and optic chiasm may yield compression from UIAs and is likely the cause of visual disturbances. Direct compression is thought to be the most common etiology; however, other causes include diminished blood supply due to compromise of the ophthalmic artery or small arterial branches in the parasellar and suprasellar regions (24–26).
In the ISUIA trial, the second most common symptom reported among patients presenting with UIAs was an ischemic cerebrovascular event (2). In our cohort of 454 patients, 17 (4%) presented with signs and symptoms of either a transient ischemic attack or cerebrovascular infarction, and an aneurysm was discovered upon further investigation. Both hemodynamic and biological factors of aneurysms contribute to the prothrombotic environment within the aneurysmal sac, which may result in subsequent thrombosis of the parent vessel or dislodged emboli from the thrombus (27). Compression of nearby vasculature can also result in ischemic events. In a retrospective study of 3,202 patients, reported by Calviere et al. (28), 15 patients (0.47%) were found to have stroke or transient ischemic attack solely caused by UIAs, among whom 10 had evidence of aneurysmal thrombosis. Aneurysmal thrombosis was significantly associated with ischemic stroke (p = 0.02) in that study. Additionally, cases of large vessel occlusion caused by a thrombus formed in the aneurysmal sac have been documented in the literature (29–31). Although aneurysms can be an exclusive cause of ischemia, a UIA discovered in the presence of an ischemic event is usually thought to be an incidental finding due to the overlapping risk factors of both strokes and aneurysms, which include hypertension, diabetes, hyperlipidemia, and smoking (28, 32). As such, UIAs are more commonly found in patients who have experienced an ischemic stroke than in the general population. In our cohort, among patients with hypertension and patients with diabetes, the proportion who were symptomatic at presentation was greater than the proportion who were asymptomatic at presentation.
Vertigo was the second most common presenting symptom (21% [94/454]) in our UIA cohort. In 1 case reported by Oh et al. (33), a patient who was later found to have a large left vertebral artery aneurysm had a clinical presentation consisting of positional vertigo and vomiting, which resolved after aneurysm resection. The likely cause of this patient's clinical presentation was presumed to be secondary to mass effect on the inferior cerebellum around the fourth ventricle and compression of the area postrema. In another case, a patient presented with headache, nausea, vomiting, and vertigo; an unruptured aneurysm, partially eroding the floor of the sella and causing hydrocephalus, was identified (34). Cerebral aneurysms of the ICA and anterior communicating artery can mimic sellar lesions; in the reported case, cerebral angiography identified an aneurysm of the right carotid artery at the intracavernous tract.
The functional disability of people with seizures often prompts further workup, yet it is unclear whether seizures are a direct result of aneurysms or incidental findings. In our analysis, only 2% (10/454) of the patients had an aneurysm that was discovered upon workup for seizures. However, a series of articles have reported seizure as a primary presenting symptom of UIA (12, 35–38). Of 662 surgically managed unruptured intracranial aneurysms, Patil et al. (38) reported a total of 3 patients with unruptured anterior communicating artery aneurysms who presented with seizures as the only symptom. In a separate study, Hanggi et al. (39) assessed 347 UIA patients, and 9 presented with seizures, all of which resolved after aneurysm treatment. In the same study, a comprehensive review of seizures secondary to aneurysms suggested direct or intermittent cortical compression can cause cortical gliosis and resultant epileptogenesis. The authors concluded that surgical resection of the surrounding gliosis leads to a seizure-free postoperative course (39).
Additional and extremely vague symptoms displayed in our analysis of 454 patients included syncope and near syncope (24 [5%]), limb weakness (29 [6%]), paresthesia (35 [8%]), gait imbalance (58 [13%]), and cognitive-related impairments (68 [15%]). Many of these symptoms are nonspecific, and reports of them in the literature are limited to case studies. Syncope was identified in 1 other case of a woman with transient syncopal episodes prompting presentation to the emergency department, at which time an unruptured fusiform mid-basilar artery aneurysm was incidentally found (40). A case report published in 2021 details a patient with a giant thrombosed middle cerebral artery aneurysm, with gait disturbance as the sole presenting symptom (41). A case series published in 1980 described 3 patients with episodic weakness and numbness of the arms and legs and 1 patient with confusion spells leading to incidental findings of UIAs; 3 of the 4 patients reported symptom resolution after treatment (42). It is unclear whether these extremely vague symptoms are a direct result of the aneurysms, and a direct correlation remains difficult to assess.
Limitations of this study include its retrospective design and an inability to account for all confounding variables. Additionally, the external validity of this study was limited because it was conducted at a single institution that receives high volumes of patient referrals with atypical and complex clinical presentations. Furthermore, it is difficult to ascertain whether clinical symptoms upon presentation are related to the aneurysm or whether the aneurysm is an incidental finding.
The clinical presentation of patients with UIAs can consist of vague and nonspecific symptoms. In the setting of nonspecific neurologic symptoms, such as headache, cranial nerve deficits, ischemic events, and even seizures, UIAs should be considered as a potential etiology. In many cases, it is unclear whether aneurysms cause these symptoms or are simply an incidental finding. However, a substantial proportion of patients with UIAs experience resolution of nonspecific symptoms after aneurysm treatment. Aneurysms may therefore be the origin of these symptoms through varied pathogenesis. Because early detection is crucial to prevent aSAH, it is imperative that physicians not rule out aneurysms in the setting of nonspecific neurologic symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The ethics committee waived the requirement of written informed consent for participation.
Conception and design: AH, SK, and JC. Acquisition of data: ER and LS. Analysis and interpretation of data: SK and AN. Drafting the article: AH, JR, EN, and AE. Critically revising the article: JC, JH, BF, EW, and ML. Statistical analysis: SK and AN. Study supervision: JC and ML. All authors contributed to the article and approved the submitted version.
We thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
aSAH, aneurysmal subarachnoid hemorrhage; ICA, internal carotid artery; ISUIA, International Study of Unruptured Intracranial Aneurysms; SAH, subarachnoid hemorrhage; SD, standard deviation; UIA, unruptured intracranial aneurysm.
1. Tawk RG, Hasan TF, D’Souza CE, Peel JB, Freeman WD. Diagnosis and treatment of unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage. Mayo Clin Proc. (2021) 96(7):1970–2000. doi: 10.1016/j.mayocp.2021.01.005
2. Malhotra A, Wu X, Gandhi D. Management of unruptured intracranial aneurysms. Neuroimaging Clin N Am. (2021) 31(2):139–46. doi: 10.1016/j.nic.2021.02.001
3. Cianfoni A, Pravata E, De Blasi R, Tschuor CS, Bonaldi G. Clinical presentation of cerebral aneurysms. Eur J Radiol. (2013) 82(10):1618–22. doi: 10.1016/j.ejrad.2012.11.019
4. Jersey AM, Foster DM. Cerebral aneurysm. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
5. Mayer PL, Awad IA, Todor R, Harbaugh K, Varnavas G, Lansen TA, et al. Misdiagnosis of symptomatic cerebral aneurysm. Prevalence and correlation with outcome at four institutions. Stroke. (1996) 27(9):1558–63. doi: 10.1161/01.str.27.9.1558
6. Vega C, Kwoon JV, Lavine SD. Intracranial aneurysms: current evidence and clinical practice. Am Fam Physician. (2002) 66(4):601–8. 12201551
7. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362(9378):103–10. doi: 10.1016/s0140-6736(03)13860-3
8. Park W, Park JC, Han K, Ahn JS, Kwun BD. Anterior optic pathway compression due to internal carotid artery aneurysms: neurosurgical management and outcomes. J Stroke. (2015) 17(3):344–53. doi: 10.5853/jos.2015.17.3.344
9. King JT Jr, Kassam AB, Yonas H, Horowitz MB, Roberts MS. Mental health, anxiety, and depression in patients with cerebral aneurysms. J Neurosurg. (2005) 103(4):636–41. doi: 10.3171/jns.2005.103.4.0636
10. Connolly ES Jr, Solomon RA. Management of symptomatic and asymptomatic unruptured aneurysms. Neurosurg Clin N Am. (1998) 9(3):509–24. doi: 10.1016/S1042-3680(18)30247-X
11. Date I. Symptomatic unruptured cerebral aneurysms: features and surgical outcome. Neurol Med Chir. (2010) 50(9):788–99. doi: 10.2176/nmc.50.788
12. Raps EC, Rogers JD, Galetta SL, Solomon RA, Lennihan L, Klebanoff LM, et al. The clinical spectrum of unruptured intracranial aneurysms. Arch Neurol. (1993) 50(3):265–8. doi: 10.1001/archneur.1993.00540030031010
13. Dandurand C, Parhar HS, Naji F, Prakash S, Redekop G, Haw CS, et al. Headache outcomes after treatment of unruptured intracranial aneurysm: a systematic review and meta-analysis. Stroke. (2019) 50(12):3628–31. doi: 10.1161/STROKEAHA.119.026864
14. Lebedeva ER, Gurary NM, Sakovich VP, Olesen J. Migraine before rupture of intracranial aneurysms. J Headache Pain. (2013) 14(1):15. doi: 10.1186/1129-2377-14-15
15. Witvoet EH, Pelzer N, Terwindt GM, Rinkel GJE, Vlak MHM, Algra A, et al. Migraine prevalence in patients with unruptured intracranial aneurysms: a case-control study. Brain Behav. (2017) 7(5):e00662. doi: 10.1002/brb3.662
16. Kong DS, Hong SC, Jung YJ, Kim JS. Improvement of chronic headache after treatment of unruptured intracranial aneurysms. Headache. (2007) 47(5):693–7. doi: 10.1111/j.1526-4610.2006.00630.x
17. Tateshima S, Murayama Y, Gobin YP, Duckwiler GR, Guglielmi G, Vinuela F. Endovascular treatment of basilar tip aneurysms using guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery. (2000) 47(6):1332–9; discussion 9–42. doi: 10.1097/00006123-200012000-00012
18. Liang B, Brammeier T, Huang J, Benardete EA. “Plugged” anterior inferior cerebellar artery aneurysm causing facial palsy, hearing loss, and subarachnoid hemorrhage treated by a translabyrinthine approach. Cureus. (2020) 12(12):e12282. doi: 10.7759/cureus.12282
19. Mizushima H, Kobayashi N, Yoshiharu S, Kazuo H, Dohi K, Sasaki K, et al. Aneurysm of the distal anterior inferior cerebellar artery at the medial branch: a case report and review of the literature. Surg Neurol. (1999) 52(2):137–42. doi: 10.1016/s0090-3019(99)00042-7
20. Scumpia AJ, Serak J, Ritchie KL, Kohl S. Posterior communicating artery aneurysm in 20-year-old female with Noonan’s syndrome. West J Emerg Med. (2013) 14(2):175–6. doi: 10.5811/westjem.2012.11.14368
21. Almaghrabi N, Fatani Y, Saab A. Cavernous internal carotid artery aneurysm presenting with ipsilateral oculomotor nerve palsy: a case report. Radiol Case Rep. (2021) 16(6):1339–42. doi: 10.1016/j.radcr.2021.03.008
22. Arle JE, Abrahams JM, Zager EL, Taylor C, Galetta SL. Pupil-sparing third nerve palsy with preoperative improvement from a posterior communicating artery aneurysm. Surg Neurol. (2002) 57(6):423–6; discussion 6–7. doi: 10.1016/S0090-3019(02)00717-6
23. Nam KH, Choi CH, Lee JI, Ko JG, Lee TH, Lee SW. Unruptured intracranial aneurysms with oculomotor nerve palsy: clinical outcome between surgical clipping and coil embolization. J Korean Neurosurg Soc. (2010) 48(2):109–14. doi: 10.3340/jkns.2010.48.2.109
24. de Oliveira JG, Borba LA, Rassi-Neto A, de Moura SM, Sanchez-Junior SL, Rassi MS, et al. Intracranial aneurysms presenting with mass effect over the anterior optic pathways: neurosurgical management and outcomes. Neurosurg Focus. (2009) 26(5):E3. doi: 10.3171/2009.3.FOCUS0924
25. Heran NS, Song JK, Kupersmith MJ, Niimi Y, Namba K, Langer DJ, et al. Large ophthalmic segment aneurysms with anterior optic pathway compression: assessment of anatomical and visual outcomes after endosaccular coil therapy. J Neurosurg. (2007) 106(6):968–75. doi: 10.3171/jns.2007.106.6.968
26. Mendez Roberts A, Grimes AL. Enlargement of internal carotid artery aneurysm presenting with severe visual sequela: a case report and anatomy review. Optometry. (2009) 80(2):76–82. doi: 10.1016/j.optm.2008.05.009
27. Ribeiro de Sousa D, Vallecilla C, Chodzynski K, Corredor Jerez R, Malaspinas O, Eker OF, et al. Determination of a shear rate threshold for thrombus formation in intracranial aneurysms. J Neurointerv Surg. (2016) 8(8):853–8. doi: 10.1136/neurintsurg-2015-011737
28. Calviere L, Viguier A, Da Silva NA Jr, Cognard C, Larrue V. Unruptured intracranial aneurysm as a cause of cerebral ischemia. Clin Neurol Neurosurg. (2011) 113(1):28–33. doi: 10.1016/j.clineuro.2010.08.016
29. Fomenko A, Kaufmann AM. Spontaneous thrombosis of an unruptured saccular aneurysm causing mca infarction. Can J Neurol Sci. (2016) 43(6):856–8. doi: 10.1017/cjn.2016.282
30. Kamogawa M, Koide T, Kikuchi R, Nakamura A, Tagawa A, Miyazaki H. A case of Wallenberg’s syndrome presenting with spontaneous thrombosis of a vertebral artery aneurysm. J Stroke Cerebrovasc Dis. (2020) 29(1):104492. doi: 10.1016/j.jstrokecerebrovasdis.2019.104492
31. Yoshihara R, Shindo K, Ogino T, Nakamura H. Acute middle cerebral artery occlusion caused by spontaneous thrombosis of a small internal carotid artery aneurysm: illustrative case. J Neurosurg Case Lessons. (2022) 4(18):1–4. doi: 10.3171/CASE22335
32. Jiranukool J, Thiarawat P, Galassi W. Prevalence of intracranial aneurysms among acute ischemic stroke patients. Surg Neurol Int. (2020) 11:341. doi: 10.1097/MAO.0000000000002072
33. Oh D, Lee ES, Shin DS, Lee TK. A rare, but dangerous cause of vertigo: central positional vertigo due to a large vertebral artery aneurysm. Otol Neurotol. (2019) 40(2):e170–e2. doi: 10.1097/MAO.0000000000002072
34. Locatelli M, Spagnoli D, Caroli M, Isalberti M, Branca V, Gaini SM, et al. A potential catastrophic trap: an unusually presenting sellar lesion. Eur J Neurol. (2008) 15(1):98–101. doi: 10.1111/j.1468-1331.2007.02004.x
35. Currie S, Heathfield KW, Henson RA, Scott DF. Clinical course and prognosis of temporal lobe epilepsy. A survey of 666 patients. Brain. (1971) 94(1):173–90. doi: 10.1093/brain/94.1.173
36. Jomin M, Lesoin F, Lozes G, Fawaz A, Villette L. Surgical prognosis of unruptured intracranial arterial aneurysms. Report of 50 cases. Acta Neurochir. (1987) 84(3-4):85–8. doi: 10.1007/BF01418829
37. Morley TP, Barr HW. Giant intracranial aneurysms: diagnosis, course, and management. Clin Neurosurg. (1969) 16:73–94. doi: 10.1093/neurosurgery/16.cn_suppl_1.73
38. Patil A, Menon GR, Nair S. Unruptured anterior communicating artery aneurysms presenting with seizure: report of three cases and review of literature. Asian J Neurosurg. (2013) 8(3):164. doi: 10.4103/1793-5482.121693
39. Hanggi D, Winkler PA, Steiger HJ. Primary epileptogenic unruptured intracranial aneurysms: incidence and effect of treatment on epilepsy. Neurosurgery. (2010) 66(6):1161–5. doi: 10.1227/01.NEU.0000369515.95351.2A
40. Ansari SA, Lassig JP, Nicol E, Thompson BG, Gemmete JJ, Gandhi D. Thrombosis of a fusiform intracranial aneurysm induced by overlapping neuroform stents: case report. Neurosurgery. (2007) 60(5):E950–1; discussion E-1. doi: 10.1227/01.NEU.0000255427.08926.DC
41. Mura J, Alvarez VH, Oberman DZ, Cardenas AR, Rabelo NN, Figueiredo EG. Management of giant thrombosed MCA aneurysm: double sSTA-MCA revascularization. World Neurosurg. (2021) 149:1. doi: 10.1016/j.wneu.2021.01.125
Keywords: cerebrovascular, microsurgical treatment, retrospective analysis, symptoms, unruptured intracranial aneurysms
Citation: Hackett AM, Koester SW, Rhodenhiser EG, Scherschinski L, Rulney JD, Naik A, Nico E, Eberle AT, Hartke JN, Fox BM, Winkler EA, Catapano JS and Lawton MT (2023) A comprehensive assessment of self-reported symptoms among patients harboring an unruptured intracranial aneurysm. Front. Surg. 10:1148274. doi: 10.3389/fsurg.2023.1148274
Received: 19 January 2023; Accepted: 29 March 2023;
Published: 21 April 2023.
Edited by:
Chloe Dumot, Hospices Civils de Lyon, FranceReviewed by:
Steve M. Cordina, University of South Alabama, United States© 2023 Hackett, Koester, Rhodenhiser, Scherschinski, Rulney, Naik, Nico, Eberle, Hartke, Fox, Winkler, Catapano and Lawton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael T. Lawton bmV1cm9wdWJAYmFycm93bmV1cm8ub3Jn
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.