
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 03 March 2023
Sec. Thoracic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1136166
Background: Thymomas and thymic carcinoma are thymic epithelial tumors (TETs) of the anterior mediastinum. On the basis of The AJCC 8th Edition of TNM classification, no prognostic prediction model has been established for TETs patients undergoing surgical resection. In this study, based on data from Qilu Hospital of Shandong University, we identified prognostic factors and developed a nomogram to predict the prognosis for TETs patients undergoing extended thymectomy.
Methods: Patients with TETs who underwent thymectomy between 2010 and 2020 were consecutively enrolled. An analysis of multivariate Cox regression and stepwise regression using the Akaike information criterion (AIC) was conducted to identify prognostic factors, and a nomogram for TETs was derived from the results of these analyses. The model was validated internally with the Kaplan-Meier curves, ROC curves and calibration curves.
Results: There were 350 patients with TETs enrolled in the study, and they were divided into a training group (245,0.7) and a validation group (105,0.3). Age, histological type, tumor size, myasthenia gravis, and TNM stage were independent prognostic factors for CSS. The Kaplan-Meier curves showed a significant difference between high nomorisk group and low nomorisk group. A nomogram for CSS was formulated based on the independent prognostic factors and exhibited good discriminative ability as a means of predicting cause-specific mortality, as evidenced by the area under the ROC curves (AUCs) of 3-year, 5-year, and 10-year being 0.946, 0.949, and 0.937, respectively. The calibration curves further revealed excellent consistency between the predicted and actual mortality when using this nomogram.
Conclusion: There are several prognostic factors for TETs. Based on TNM stage and other prognostic factors, the nomogram accurately predicted the 3-, 5-, and 10-year mortality rates of patients with TETs in this study. The nomogram could be used to stratify risk and optimize therapy for individual patients.
Thymomas and thymic carcinoma are both thymic epithelial tumors (TETs), which are relatively rare anterior mediastinal tumors. The WHO classifies TETs into five types: A, AB, B1, B2, B3, and TC. Type A/AB/B1 is a low-risk group with excellent overall survival (OS), and the 10-year overall survival rate is over 90%-95%. B2/B3/TC is a high-risk group, with 5-year survival rates of 75%, 70%, and 48%, respectively (1).
At present, the Masaoka-Koga staging system and American Joint Commission on Cancer (AJCC) 8th Edition of TNM classification are the two most commonly used staging systems for TETs. The Masaoka-Koga staging system relies primarily on primary tumor extension and the degree of involvement beyond the thymus (2). The AJCC 8th edition of the TNM classification, based on the combination of primary tumor local invasion, nodal involvement and metastatic spread, has been confirmed to play an important role in the diagnosis and treatment of TETs (3).
Currently, surgical resection remains the optimal treatment for TETs. Complete resection is of prognostic importance for patients with thymoma at any stage (4, 5).The standard surgical approach for stage I or II thymic tumors is thymectomy, in which the entire thymus is removed along with the tumor. Currently, extended thymectomy has been used because thymic tissue is often present in the mediastinal fat and may contribute to the non-remission of postoperative myasthenia gravis or the development of postoperative myasthenia gravis (1). Most patients can achieve satisfactory outcomes after extended thymectomy. In clinical treatment, patients with advanced stages are often treated with radiotherapy and chemotherapy after surgery. Studies have shown that postoperative radiotherapy for Masaoka-Koga stage III/IV could improve OS (6).
The well-recognized prognostic factors for TETs include tumor stage and resection status (5, 7, 8), and studies have reported that age, completeness of resection, and histological type are also important prognostic factors except staging (7–9). At present, a few nomograms have been established to predict the prognosis of TETs. Zhang et al. (10) established a prediction model based on the SEER database, but there are shortcomings such as excessive missing data, which affects the integrity and accuracy of the predictive model. In this study, we aimed to establish an effective prognostic prediction model based on TNM stage and other important clinicopathological parameters for TETs patients following extended thymectomy and provide a reference for patient postoperative therapy.
The study was approved by the Qilu Hospital of Shandong University institutional review board (KYLL-202008-023-1). Written informed consent was signed by all patients to obtain their clinical information.
From January 2010 to December 2020, a total of 378 patients were diagnosed with TETs. In this study, 350 patients were treated with extended thymectomy, surgical approaches include median sternotomy and Video-Assisted Thoracic Surgery, recovered and were discharged (Figure 1).
The inclusion criteria are shown in Table 1.
The following data of eligible patients were collected from the database of Qilu Hospital: age at diagnosis, sex, histological type, tumor size, pleural effusion, lymph node dissection, positive lymph nodes, myasthenia gravis, surgical margin, TNM stage, postoperative radiotherapy, and postoperative chemotherapy. Some of the variables were regrouped, such as the age of diagnosis, which was divided into “<50” and “≥50”, since 50 years old was considered an important point(10, 11). Histological type was determined by the patient's pathology report and regrouped into “A/AB/B1”, “B2”, and “B3/CA”, because they were considered as low risk, intermediate risk and high risk groups for aggressiveness, recurrence and survival, respectively (1, 11–14). The cutoff point of tumor size (6.0 cm) was determined using X-tile (version 3.6.1) by Kaplan-Meier curve, in other studys, the cutoff point is selected as 5.5 or 6.6 cm (15, 16), which are close to our cutoff point, and tumor size was then divided into “<6 cm” and “≥6 cm”. For surgical resection margin, emphasis on completeness of excision (1), was divided into “R0” and “R1/R2”. The TNM stage was determined by the intraoperative findings and pathology reports each patient and was divided into “I”, “II”, and “III/IV”, as the invasion of adjacent organs in T satge(resectable or unresectable)(17, 18), is similar to the grouping of other studies with masaoka staging(15). The primary endpoint of the study was cancer-specific survival (CSS), which was measured from the time of diagnosis to (1) death from TETs and (2) the last follow-up.
The multivariate Cox regression model and stepwise regression based on the Akaike information criterion (AIC) were used to explore the prognostic factors of TETs and select important variables for the model, and then shown as a forest map. Once the model was established, we used it to predict risk, and the effect of the prediction was assessed using the Kaplan-Meier curves, time-dependent receiver operating characteristic (tdROC) curves and calibration curves. The above analyses were performed using R, version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) by package survminer, survival, rms, foreign, ggDCA, car, timeROC, ggforest and ggplot2. Variables were described using the medians [IQR] and numbers (%). Differences in these variables were assessed by the chi-squared or Fisher exact test. The analyses were performed using IBM SPSS Statistics 20. The hypothesis tests were two-sided, and p < 0.05 was considered to be statistically significant.
According to the inclusion criteria, 350 patients were enrolled in this study. The demographic, tumor and treatment characteristics of this cohort are shown in Table 2. There was no statistical difference between the inclusion and exclusion groups(Supplementary Table S1). Overall, the majority of the patients were ≥50 years old (210, 60%). In terms of treatment, most patients had surgical margins of R0 (329, 94%), no lymph node dissection (292, 83.4%), radiotherapy (117, 33.4%) or chemotherapy (55, 15.7%). In Table 2, from the perspective of survival status, among the surviving patients, most patients had the histological type A/AB/B1 (138, 42.9%) and a tumor size <6 cm (207, 64.3%). The surgical margin of most patients was R0 (312, 96.9%), Masaoka-Koga stage I/II (200, 62.1%), and TNM stage I (266, 82.6%). Among the deceased patients, most were histological type B3/CA (21, 75%), Masaoka-Koga stage III/IV (25, 89.3%), and TNM stage III/IV (15, 50%). In the majority of the patients who died, they died because of postoperative recurrence (24, 85.7%). The patients included in the study had a median follow-up of 45 months (interquartile range, 5–133 months). As of the last follow-up, 28 patients (8.0%) had died during the follow-up period, all from Ts and TC.
There were 350 patients with Ts or TC enrolled in the study, and the patients were divided into a training group (245,0.7) and a validation group (105,0.3). The multivariate Cox regression model was used to explore the prognostic risk factors for TET, and stepwise regression based on AIC was used to select important variables for the model. After screening, age, histological type, tumor size, myasthenia gravis, surgical margin, TNM stage, radiotherapy, and chemotherapy were important variables, except for surgical margin, radiotherapy, and chemotherapy, which were all statistically significant (Table 3). Age ≥ 50(HR = 7.47, P = 0.002), histological type B3/Ca(HR = 14.4, P = 0.036), tumor size ≥6 cm(HR = 6.36, P = 0.008), myasthenia gravis(HR = 3.77,P = 0.038), TNM stage II (HR = 14.1, P = 0.001) or III/IV (HR = 43.7, P < 0.001) were risk factors (Figure 2).
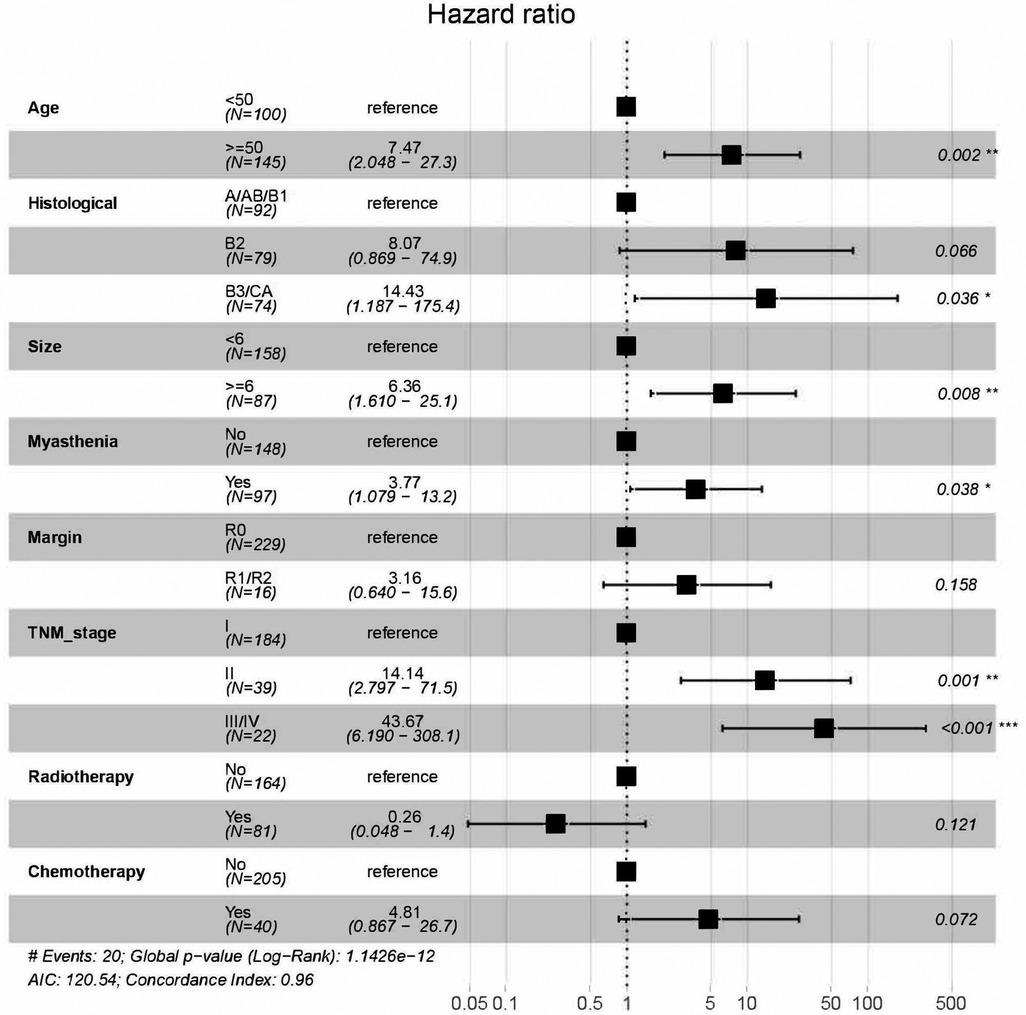
Figure 2. Hazard ration of variables based on multivariate cox regression model and stepwise regression based on the Akaike information criterion.
The significant variables in the multivariate Cox regression analysis and stepwise regression based on AIC were included in the nomogram, and each variable was given a score according to the HR (Figure 3). Then, by summing the total scores for each variable and locating them on a total subscale, the probability of CSS at 3 and 5 years and 10 years for the patients was derived. For example, if a 65-year man with type B2, TNM stage III, tumor size 4.5 cm, and myasthenia gravis underwent extended thymectomy, he would score 17 points, which means that this patient has an approximately 80% possibility of survival in the fifth year and an approximately 15% possibility of survival in the tenth year.
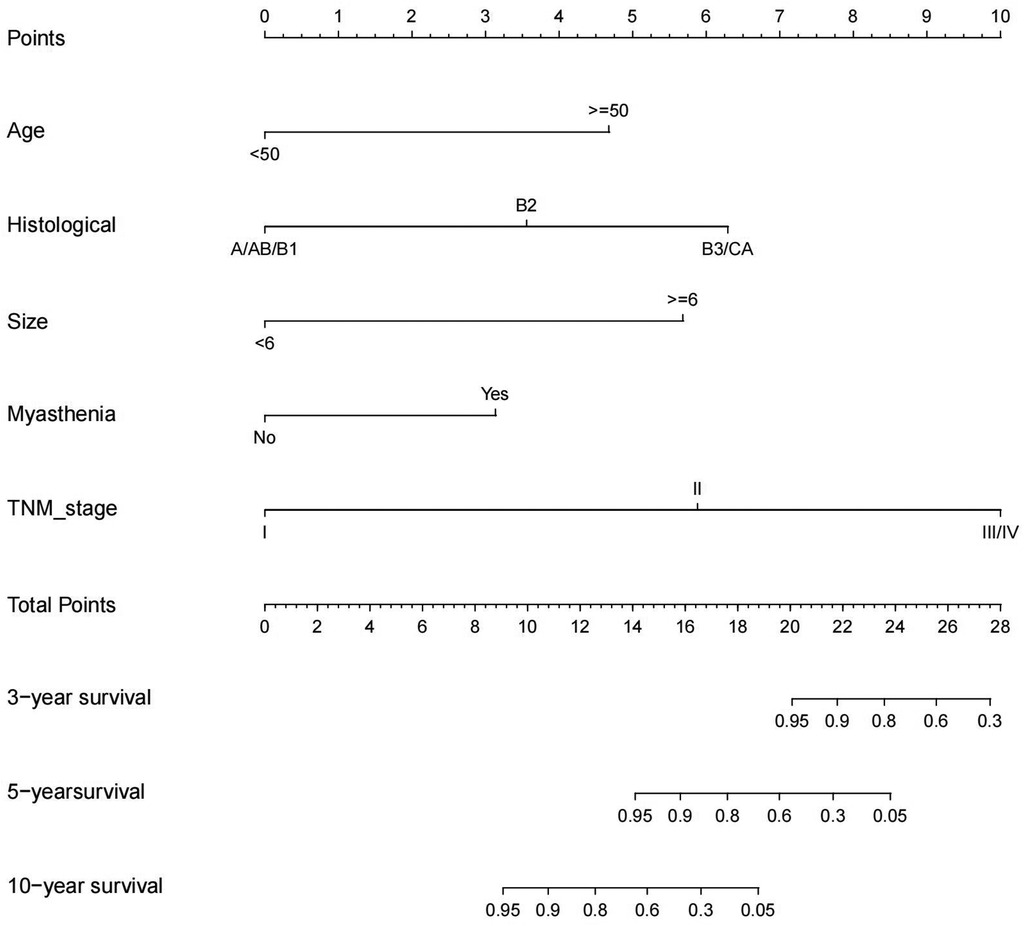
Figure 3. Competing risk nomogram for the prediction of 3-, 5-, 10-year cause-specific survival associated with TETs.
Both the training and validation sets were validated for the model. Divided into two groups according to nomorisk: high risk and low risk, then the Kaplan-Meier curves were established and showed a significant difference(Figure 4). In the time-dependent receiver operating characteristic (ROC) curve of the validation group (Figure 5), the areas under the ROC curves (AUCs) at 3-year, 5-year, and 10-year were 0.946, 0.949, and 0.937, respectively, indicating that the prediction accuracy of this nomogram was high at these three time points. The calibration curve (Figure 6) also showed a good predictive ability of the model.
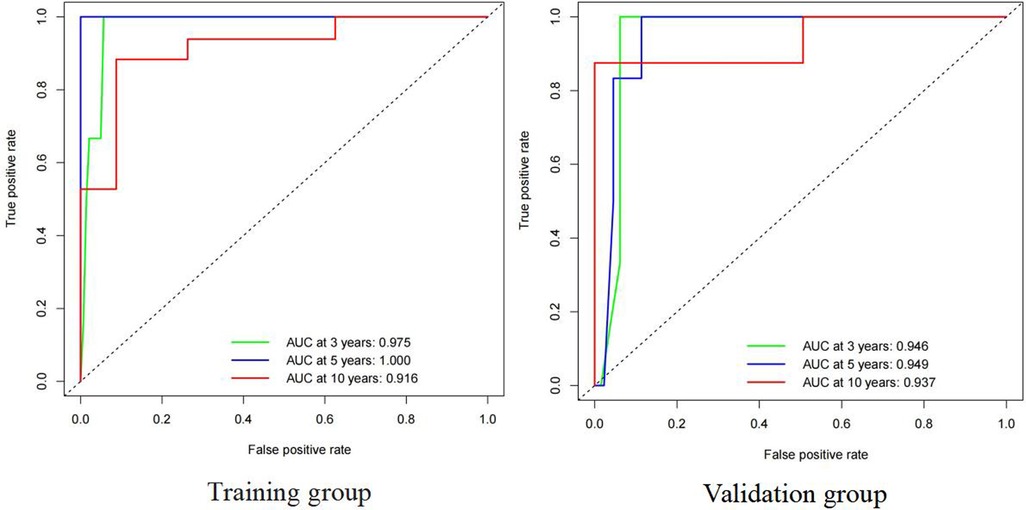
Figure 5. Time-dependent receiver operating characteristic (ROC) curve for cause-specific survival nomogram in TETs of training group ang validation group.
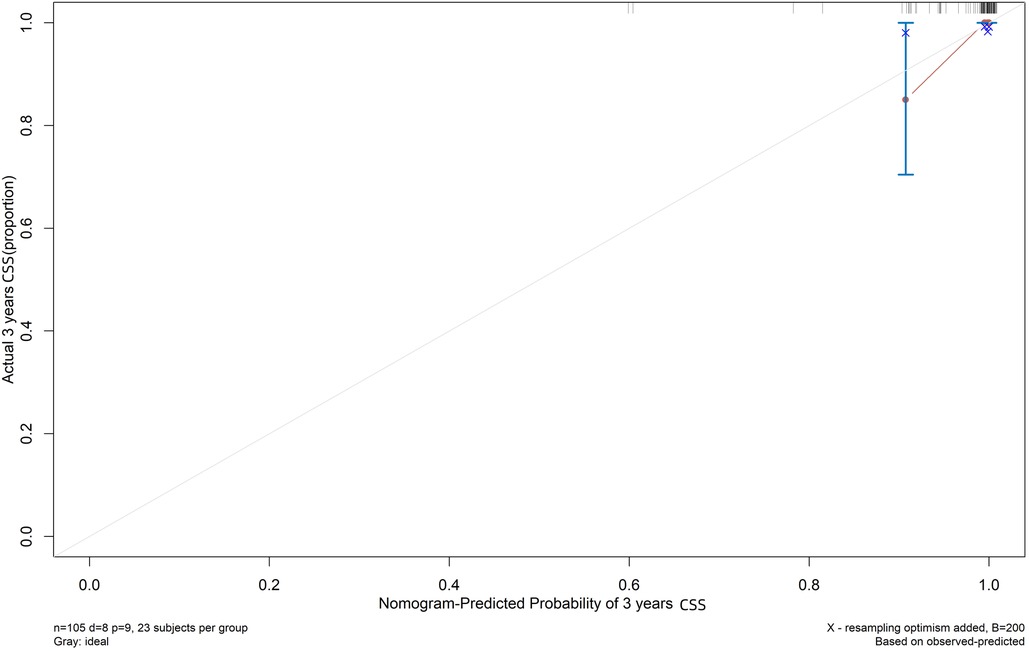
Figure 6. Calibration plot for cause-specific mortality nomogram in TETs. The x-axis and y-axis respectively correspond to the predicted odds of cause-specific survival and the actual observed incidence of cause-specific survival (3-year) of validation group.
As a relatively rare anterior mediastinal tumor, the incidence of TETs is higher in China (4.09/1 million) than in other areas(1.3∼3.2/1 million) (19). Currently, surgical resection is the best treatment for TETs, and most cases can be cured by surgery (20). At the same time, postoperative radiotherapy and chemotherapy are also widely used. To date, no randomized controlled trials have been conducted to evaluate the effect of postoperative chemotherapy on TETs (15), and the effect of postoperative chemotherapy on patient survival is still controversial. However, for R2 resection and metastatic TETs, postoperative chemotherapy is recommended (21–23). Some studies have proposed prognostic models using the SEER database, but the data in the SEER database are different and missing the surgical methods and postoperative treatment data. Masaoka-Koga staging is the most commonly used method for TETs, and TNM staging was introduced later. As demonstrated by Meurgey A et al. (24), when switching from the Masaoka-Koga stage to TNM stage (AJCC 8th Edition), histological types were associated with tumor stage (3, 25, 26), and the good and significant correlation between them contributes to the prognostic value of WHO classification. Therefore, it is necessary to establish a prognostic model of TETs on TNM stage.
In the establishment of this prediction model, five factors were included: age, histological type, tumor size, myasthenia gravis, and TNM stage. Among them, myasthenia gravis and TNM stage were the variables included for the first time. Age has been reported to be a prognostic factor for TETs (9, 27); however, Yanagiya et al. demonstrated that age and histological type were not meaningful prognostic factors for thymoma compared to stage (28). In this study, the risk of patients ≥50 years of age was significantly higher than that of patients <50 years of age, which suggested that age is a meaningful prognostic factor for TETs, and older patients may have higher possibilities of experiencing worse CSS outcomes. Among the histological types, the risk of B3/Ca was the highest (HR = 14.4, P < 0.05), followed by B2 (HR = 8.07, P = 0.066), and the results were consistent with the clinical consensus on the prognosis of the pathological subtype. In comparison, the patients with type A/AB/B1 have fewer malignant tumors and longer survival. Tumor size has been shown to be an independent risk factor for prognosis, with larger tumors having higher recurrence and mortality (29), and the patients with tumor size ≥6 cm in this study had a higher risk for mortality (p < 0.01). A larger tumor size usually means more difficulties for resection and higher recurrence rates. However, in A/AB/B1 TETs, tumors tend to grow within the membrane, and large tumor diameters may have early TNM stages. In this study, myasthenia gravis was also listed as a risk factor after screening (p < 0.05). TETs are often associated with myasthenia gravis (30–32); Tian W et al. (33) believed that patients with myasthenia gravis had smaller tumors and a higher proportion of advanced tumors; and myasthenia gravis was significantly associated with poorer OS and recurrence free survival in TETs. Of course, some studies have concluded that myasthenia gravis affecting neurologic related survival (34).This study indicated that myasthenia gravis is associated with poor prognosis for TET patients, and although its risk in the model is lower than that of other factors, we believe that patients with myasthenia gravis need more attention in postoperative therapy. The surgical margin is an important factor for the prognosis of TET patients and is a measure of the effectiveness of surgical excision. R1/R2 patients tend to be more prone to recurrence and higher mortality.Stages I and II have very high rates of R0 resection, but stages III and IV have much lower rates (50%) and 25%, respectively (5). The stage III prognosis significantly improves after a radical resection, almost reaching a stage I prognosis (35, 36). In the Cox multivariate regression, surgical margin status was not significant (P > 0.05). We recognize that it is related to a small amount of R1/R2 data (21,0.06), because of the goal of expanded thymectomy is R0 resection, so we cannot assert that margin status is not a prognostic factor in our study. In Surgical treatment, R0 resection still improves prognosis significantly, especially in advanced patients. TNM stage showed an important prognostic role in the Cox multivariate regression. The risk of stage II was significantly higher than that of stage I, which was not significant in the Masaoka-Koga stage in previous studies. Chiappetta et al. (37) believed that there was no difference in survival between patients with Masaoka-Koga staging in stage I and stage II, while there was a difference in survival between patients with stage I and II after TNM staging. TNM stage and Masaoka-Koga stage have their own advantages and disadvantages in diagnosis and treatment. Masaoka staging concentrates more on the concept of continuous invasion (stage III) and discontinuous progression (stage IV). In contrast, the TNM system respects the localization of the involved area and prioritizes the surgical outcome (38). By the classification of TNM stage, more early stage patients with better prognosis were enrolled in stage I, and the risk of stage II (HR = 14.1, P = 0.001) or III/IV (HR = 43.7, P < 0.001) was significantly higher. We believed that after surgery, patients with TNM stage could have better performance in the prediction of prognosis, and the nomogram was established based on TNM stage. Our study also analyzed postoperative radiotherapy and postoperative chemotherapy in the multivariate Cox regression model. As a result, postoperative radiotherapy was observed to be a protective factor (P = 0.121 > 0.05) but was not statistically significant. We considered postoperative chemotherapy to have marginal statistical significance (P = 0.072 > 0.05) because the sample size of the patients (55,0.157) who received postoperative chemotherapy in this study was small. At present, there is still some controversy about the effect of postoperative chemotherapy on the treatment of TETs. In Zhao M's study (15), postoperative chemotherapy was a risk factor in the prediction model. Our analysis yielded the same result, HR = 4.81. Advanced stage, the small sample size, and some patients receiving no standardized chemotherapy cycles may be the reason for this conclusion. Furthermore, lymph node dissection and positive lymph nodes were not considered to be significant prognostic factors in the analysis. Due to the low number of patients with lymph node metastasis within the TET patients, lymph node dissection is still the current surgical controversy (39). Wang et al. (40) reported that the prognosis of patients who did not receive lymph node dissection was significantly worse than that of patients who received lymph node dissection and were positive for lymph node metastasis; however, there was no significant difference in the patients with negative lymph node metastasis. There were few lymph node dissection patients in this study, and the results need to be further confirmed.
Our nomogram is innovative and rational in the following aspects. First, our nomogram is the first method to predict the prognosis of TETs based on TNM stage, which makes the individualized prediction of CSS and individualized treatment guidance possible. Second, many characteristics are involved in our analysis, not only the TNM stage but also other variables such as age, histological type, tumor size, and myasthenia gravis, in patients with TETs. In particular, myasthenia gravis was associated with poor prognosis in the nomogram, which has important clinical significance. Third, as a result of the data from Qilu Hospital and because of the rigorous algorithm, the performance of the nomograms was reliable. In conclusion, our prognostic model is innovative and rational enough to be effective in clinical practice.
However, there are still some limitations of this study. First, compared to the SEER database-based analysis, our analysis has a relatively small sample size, which needs to be extended in the follow-up. Second, as a retrospective study, the nomogram needs to be validated in the next prospective cohort before it can be formally applied in clinical practice. In addition, some factors, such as margin status and postoperative chemotherapy, were not included in the nomogram because of the small sample size, and these factors may also be associated with the prognosis of TETs. Therefore, a more complete model that includes margin status and postoperative treatment is needed in the future. Besides,the surgical approach may also be an important prognostic factor that needs to be explored in subsequent studies. Finally, although the AUCs of the 3-year, 5-year, and 10-year tdROC curves are all greater than 0.9, indicating that the model for CSS has high precision, it is not perfect because approximately 20% of predictions are still wrong. In fact, it is impossible for any predictive model to achieve 100% accuracy, but we will do our best to improve the quality and quantity of data and the reliability of our algorithms to achieve this goal.
In this study, our prognostic model demonstrated that demographic characteristics, clinical characteristics, and TNM stage were all significantly associated with survival outcomes in TET patients following extended thymectomy. More importantly, we built an accurate and visible nomogram to predict individual CSS in postoperative patients with TETs. The nomogram will help clinicians assess the risk of patients with TETs and guide more individualized treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Qilu Hospital of Shandong. University (KYLL-202008-023-1). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Conception and design: YL and HT. Administrative support: HT. Provision of study materials or patients: YL, and ZT. Collection and assembly of data: YL and XZ. Data analysis and interpretation: YL. All authors contributed to the article and approved the submitted version.
This work was funded by National Key Research and Development Program (2021YFC2500900), Key Research and Development Program of Shandong Province (2020CXGC011303) and Natural Science Foundation of Shandong Province (ZR2021LSW006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1136166/full#supplementary-material.
1. Scorsetti M, Leo F, Trama A, D'Angelillo R, Serpico D, Macerelli M, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. (2016) 99:332–50. doi: 10.1016/j.critrevonc.2016.01.012
2. Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. (1994) 44:359–67. doi: 10.1111/j.1440-1827.1994.tb02936.x
3. Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG thymic epithelial tumors staging project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. (2014) 9:S65–72. doi: 10.1097/JTO.0000000000000290
4. Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J. (2013) 126:2186–91. doi: 10.3760/cma.j.issn.0366-6999.20130177
5. Detterbeck F, Youssef S, Ruffini E, Okumura M. A review of prognostic factors in thymic malignancies. J Thorac Oncol. (2011) 6:S1698–1704. doi: 10.1097/JTO.0b013e31821e7b12
6. Tateishi Y, Horita N, Namkoong H, Enomoto T, Takeda A, Kaneko T. Postoperative radiotherapy for completely resected masaoka/masaoka-koga stage II/III thymoma improves overall survival: an updated meta-analysis of 4746 patients. J Thorac Oncol. (2021) 16:677–85. doi: 10.1016/j.jtho.2020.12.023
7. Ruffini E, Detterbeck F, Van Raemdonck D, Rocco G, Thomas P, Weder W, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg. (2014) 46:361–8. doi: 10.1093/ejcts/ezt649
8. Weis CA, Yao X, Deng Y, Detterbeck FC, Marino M, Nicholson AG, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. (2015) 10:367–72. doi: 10.1097/JTO.0000000000000393
9. Li JF, Hui BG, Li X, Xiao RX, Jiang GC, Liu J, et al. Video-assisted thoracic surgery for thymoma: long-term follow-up results and prognostic factors-single-center experience of 150 cases. J Thorac Dis. (2018) 10:291–7. doi: 10.21037/jtd.2017.12.34
10. Zhang T, Liu L, Qiu B. Development of a competing risk nomogram for the prediction of cause-specific mortality in patients with thymoma: a population-based analysis. J Thorac Dis. (2021) 13:6838–47. doi: 10.21037/jtd-21-931
11. Lamarca A, Moreno V, Feliu J. Thymoma and thymic carcinoma in the target therapies era. Cancer Treat Rev. (2013) 39:413–20. doi: 10.1016/j.ctrv.2012.11.005
12. Moser B, Scharitzer M, Hacker S, Ankersmit J, Matilla JR, Lang G, et al. Thymomas and thymic carcinomas: prognostic factors and multimodal management. Thorac Cardiovasc Surg. (2014) 62:153–60. doi: 10.1055/s-0032-1322611
13. Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, et al. The 2015 world health organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. (2015) 10:1383–95. doi: 10.1097/JTO.0000000000000654
14. Cheng B, Xue Y, Gu S, Yang H, Liu P, Qi G. Developing and validating a nomogram to predict myasthenia gravis exacerbation in patients with postoperative thymoma recurrence. Gland Surg. (2022) 11:1712–21. doi: 10.21037/gs-22-549
15. Zhao M, Yin J, Yang X, Jiang T, Lu T, Huang Y, et al. Nomogram to predict thymoma prognosis: a population-based study of 1312 cases. Thorac Cancer. (2019) 10:1167–75. doi: 10.1111/1759-7714.13059
16. Li Y, Jiang A, Zhao Y, Shi C, Ma Y, Fu X, et al. A novel risk classifier for predicting the overall survival of patients with thymic epithelial tumors based on the eighth edition of the TNM staging system: a population-based study. Front Endocrinol (Lausanne). (2022) 13:1050364. doi: 10.3389/fendo.2022.1050364
17. Carter BW, Benveniste MF, Madan R, Godoy MC, Groot PM, Truong MT, et al. IASLC/ITMIG staging system and lymph node map for thymic epithelial neoplasms. Radiographics. (2017) 37:758–76. doi: 10.1148/rg.2017160096
18. Markowiak T, Hofmann HS, Ried M. Classification and staging of thymoma. J Thorac Dis. (2020) 12:7607–12. doi: 10.21037/jtd-2019-thym-01)
19. Chinese Guideline for clinical diagnosis and treatment of thymic epithelial tumors (2021). Zhonghua Zhong liu za zhi [Chin J Oncol. (2021) 43:395–404. doi: 10.3760/cma.j.cn112152-20210313-00226
20. Berghmans T, Durieux V, Holbrechts S, Jungels C, Lafitte JJ, Meert AP, et al. Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer. (2018) 126:25–31. doi: 10.1016/j.lungcan.2018.10.018
21. Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26(Suppl 5):v40–55. doi: 10.1093/annonc/mdv277
22. Girard N, Lal R, Wakelee H, Riely GJ, Loehrer PJ. Chemotherapy definitions and policies for thymic malignancies. J Thorac Oncol. (2011) 6:S1749–1755. doi: 10.1097/JTO.0b013e31821ea5f7
23. Rajan A, Giaccone G. Chemotherapy for thymic tumors: induction, consolidation, palliation. Thorac Surg Clin. (2011) 21:107–14, viii. doi: 10.1016/j.thorsurg.2010.08.003
24. Meurgey A, Girard N, Merveilleux du Vignaux C, Maury JM, Tronc F, Thivolet-Bejui F, et al. Assessment of the ITMIG statement on the WHO histological classification and of the eighth TNM staging of thymic epithelial tumors of a series of 188 thymic epithelial tumors. J Thorac Oncol. (2017) 12:1571–81. doi: 10.1016/j.jtho.2017.06.072
25. Chalabreysse L, Roy P, Cordier JF, Loire R, Gamondes JP, Thivolet-Bejui F. Correlation of the WHO schema for the classification of thymic epithelial neoplasms with prognosis: a retrospective study of 90 tumors. Am J Surg Pathol. (2002) 26:1605–11. doi: 10.1097/00000478-200212000-00008
26. Okumura M, Ohta M, Tateyama H, Nakagawa K, Matsumura A, Maeda H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. (2002) 94:624–32. doi: 10.1002/cncr.10226
27. Mou H, Liao Q, Hou X, Chen T, Zhu Y. Clinical characteristics, risk factors, and outcomes after adjuvant radiotherapy for patients with thymoma in the United States: analysis of the surveillance, epidemiology, and End results (SEER) registry (1988-2013). Int J Radiat Biol. (2018) 94:495–502. doi: 10.1080/09553002.2018.1454618
28. Yanagiya M, Nitadori JI, Nagayama K, Anraku M, Sato M, Nakajima J. Prognostic significance of the preoperative neutrophil-to-lymphocyte ratio for complete resection of thymoma. Surg Today. (2018) 48:422–30. doi: 10.1007/s00595-017-1602-y
29. Okumura M, Yoshino I, Yano M, Watanabe SI, Tsuboi M, Yoshida K, et al. Tumour size determines both recurrence-free survival and disease-specific survival after surgical treatment for thymoma. Eur J Cardiothorac Surg. (2019) 56:174–81. doi: 10.1093/ejcts/ezz001
30. Filosso PL, Evangelista A, Ruffini E, Rendina EA, Margaritora S, Novellis P, et al. Does myasthenia gravis influence overall survival and cumulative incidence of recurrence in thymoma patients? A retrospective clinicopathological multicentre analysis on 797 patients. Lung Cancer. (2015) 88:338–43. doi: 10.1016/j.lungcan.2015.03.007 ).25819383
31. Wang F, Pang L, Fu J, Shen Y, Wei Y, Tan L, et al. Postoperative survival for patients with thymoma complicating myasthenia gravis-preliminary retrospective results of the ChART database. J Thorac Dis. (2016) 8:711–7. doi: 10.21037/jtd.2016.02.07
32. Tassi V, Vannucci J, Ceccarelli S, Gili A, Matricardi A, Avenia N, et al. Stage-related outcome for thymic epithelial tumours. BMC Surg. (2019) 18:114. doi: 10.1186/s12893-018-0434-z
33. Tian W, Li X, Sun Y, Wang J, Jiang G, Tong H. Myasthenia gravis affects overall survival in patients with thymoma: an analysis of multicentre database using propensity score matching. Interact Cardiovasc Thorac Surg. (2021) 33:250–7. doi: 10.1093/icvts/ivab074
34. Lococo F, Nachira D, Chiappetta M, Evangelista J, Falcoz PE, Ruffini E, et al. Does myasthenia Gravis affect long-term survival in thymic carcinomas? An ESTS database analysis. Diagnostics (Basel, Switzerland). (2022) 12(7):1764. doi: 10.3390/diagnostics12071764
35. Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. (1996) 112:376–84. doi: 10.1016/S0022-5223(96)70265-9
36. Yagi K, Hirata T, Fukuse T, Yokomise H, Inui K, Ike O, et al. Surgical treatment for invasive thymoma, especially when the superior vena cava is invaded. Ann Thorac Surg. (1996) 61:521–4. doi: 10.1016/0003-4975(95)00983-3
37. Chiappetta M, Lococo F, Pogliani L, Sperduti I, Tabacco D, Bria E, et al. Masaoka-Koga and TNM staging system in thymic epithelial tumors: prognostic comparison and the role of the number of involved structures. Cancers (Basel). (2021) 13(21):5254. doi: 10.3390/cancers13215254
38. Moran CA. Thymoma staging: an analysis of the different schemas. Adv Anat Pathol. (2021) 28:298–306. doi: 10.1097/PAP.0000000000000315
39. Clermidy H, Maury JM, Collaud S, Drevet G, Ginoux M, Chalabreysse L, et al. Lymph node dissection in thymoma: is it worth it. Lung Cancer. (2021) 157:156–62. doi: 10.1016/j.lungcan.2021.05.022
Keywords: thymic epithelial tumors (TETs), nomogram, prognostic factor, TNM stage, cancerspecific survival (CSS)
Citation: Li Y, Tang Z, Zhu X and Tian H (2023) Nomogram based on TNM stage to predict the prognosis of thymic epithelial tumors (TETs) patients undergoing extended thymectomy. Front. Surg. 10:1136166. doi: 10.3389/fsurg.2023.1136166
Received: 2 January 2023; Accepted: 15 February 2023;
Published: 3 March 2023.
Edited by:
Marco Scarci, Hammersmith Hospital, United KingdomReviewed by:
Yener Aydin, Atatürk University, Türkiye© 2023 Li, Tang, Zhu and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Tian dGlhbmh1aXFsQGVtYWlsLnNkdS5lZHUuY24=
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.