
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 27 February 2023
Sec. Reconstructive and Plastic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1131293
 Samuel Knoedler1,2*
Samuel Knoedler1,2* Dany Y. Matar2
Dany Y. Matar2 Leonard Knoedler1,3
Leonard Knoedler1,3 Doha Obed2,4
Doha Obed2,4 Valentin Haug2,5
Valentin Haug2,5 Sabina M. Gorski5
Sabina M. Gorski5 Bong-Sung Kim6
Bong-Sung Kim6 Martin Kauke-Navarro7
Martin Kauke-Navarro7 Ulrich Kneser5
Ulrich Kneser5 Adriana C. Panayi2,5
Adriana C. Panayi2,5 Dennis P. Orgill2
Dennis P. Orgill2 Gabriel Hundeshagen5*
Gabriel Hundeshagen5*
Introduction: Burn injuries are associated with significant morbidity, often necessitating surgical management. Older patients are more prone to burns and more vulnerable to complications following major burns. While the relationship between senescence and major burns has already been thoroughly investigated, the role of age in minor burns remains unclear. To better understand differences between elderly and younger patients with predominantly minor burns, we analyzed a multi-institutional database.
Methods: We reviewed the 2008-2020 ACS-NSQIP database to identify patients who had suffered burns according to ICD coding and underwent initial burn surgery.
Results: We found 460 patients, of which 283 (62%) were male and 177 (38%) were female. The mean age of the study cohort was 46 ± 17 years, with nearly one-fourth (n = 108; 23%) of all patients being aged ≥60 years. While the majority (n = 293; 64%) suffered from third-degree burns, 22% (n = 99) and 15% (n = 68) were diagnosed with second-degree burns and unspecified burns, respectively. An average operation time of 46 min, a low mortality rate of 0.2% (n = 1), a short mean length of hospital stay (1 day), and an equal distribution of in- and outpatient care (51%, n = 234 and 49%, n = 226, respectively) indicated that the vast majority of patients suffered from minor burns. Patients aged ≥60 years showed a significantly prolonged length of hospital stay (p<0.0001) and were significantly more prone to non-home discharge (p<0.0001). In univariate analysis, advanced age was found to be a predictor of surgical complications (p = 0.001) and medical complications (p = 0.0007). Elevated levels of blood urea nitrogen (p>0.0001), creatinine (p>0.0001), white blood cell count (p=0.02), partial thromboplastin time (p = 0.004), and lower levels of albumin (p = 0.0009) and hematocrit (p>0.0001) were identified as risk factors for the occurrence of any complication. Further, complications were more frequent among patients with lower body burns.
Discussion: In conclusion, patients ≥60 years undergoing surgery for predominantly minor burns experienced significantly more complications. Minor lower body burns correlated with worse outcomes and a higher incidence of adverse events. Decreased levels of serum albumin and hematocrit and elevated values of blood urea nitrogen, creatinine, white blood count, and partial thromboplastin time were identified as predictive risk factors for complications.
Burns injuries can be devastating and are associated with high morbidity and mortality. In the United States, more than 1 million burn injuries and up to 3,500 burn-related deaths are recorded annually (1, 2). Burns are typically classified according to the percentage of total body surface area involvement (%TBSA) and the burn depth (3). In the traditional classification, first-degree burns are limited to the epidermis, second-degree burns extend to the dermal layer (superficial or partial thickness) and third-degree burns are marked by full-thickness skin burns and subcutaneous fat layer involvement. While first-degree and superficial second-degree burns are commonly treated non-surgically, deep second and third-degree burns often require surgical excision and skin grafting, e.g., autologous split-thickness skin grafts or synthetic skin replacement, in the immediate phase after the injury (4, 5).
It has been thoroughly demonstrated that the area and degree of burn directly correlate with patient morbidity and mortality (6–10). In this context, advanced age has been identified as a crucial risk factor, with geriatric burn patients showing worse survival rates and postoperative sequelae (11–15). It is, therefore, not surprising that common burn prognosis indexes, such as the abbreviated burn severity index and the Baux-score integrate patient's age as a determining factor for survival prediction (16, 17). Jeschke et al. found, that in elderly patients even relatively small burn injuries become increasingly life-threatening and, therefore, require adequate (surgical) care (18). Accordingly, in a 2020 study, Goei et al. reported significantly higher burn surgery rates among elderly burn patients while reiterating their particular vulnerability (15).
To date, however, knowledge of burn surgery outcomes has been widely derived from retrospective analyses of single-institution experience, intervention-specific medical records, and/or cases of severe burn injuries. As a result, research significance and transferability are limited, with scarce evidence on burn surgery in elderly patients suffering from less drastic burn lesions. In 2020, the Committee on Elderly Burn Care acknowledged this paucity and called for an improvement in the identification and prediction of outcomes among elderly burn patients (11). By pooling patient data with geographical and institutional variance, the analysis of a multicenter database can help to fill this literature gap and provide an age-stratified overview of burn surgery outcomes also in patients with less-extensive injuries.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) includes a diverse surgical patient population, capturing validated data from more than 700 hospitals. As a risk-adjusted and multi-institutional clinical registry, the ACS-NSQIP provides information on over 150 pre-, peri-, and post-operative variables for patients undergoing surgery. Selective audits and peer reviews ensure the quality, reliability, and accuracy of the information entered. Therefore, we queried this multi-institutional database to investigate perioperative outcomes and predictive risk factors of burn surgery, with a special focus on elderly patients.
Data were collected between 2008 and 2020 from the ACS-NSQIP database. Originally, these data stem from 607 hospitals within the US and 100 hospitals in 11 other countries. Hospitals have a randomized process for patient selection and most of these hospitals are not designated burn centers. Currently, 35 of the 123 American burn centers (Supplementary Table S1) report patient data to the ACS-NSQIP. The records analyzed contain strictly de-identified information. Ethical approval to complete this retrospective analysis was obtained from the Brigham and Women's Hospital (Protocol #: 2013P001244).
The ACS-NSQIP database was queried to identify all burn patients who were diagnosed with burn injuries (via ICD coding) and underwent initial surgical management. The ACS-NSQIP is a database capturing surgical cases of patients aged 18 years and older. Therefore, a priori, non-surgical cases and pediatric or adolescent patients were not included in this study. 13 annual datasets between 2008 and 2020 were screened for the ICD-9-CM codes 940–949 (“Burns”) and ICD-10-CM codes T20-T32 (“Burns and corrosions”). Patients with other and/or more extensive diagnoses, such as polytrauma with concurrent burn injuries were excluded. This initial ICD-based search yielded 565 patient cases, which were then manually reviewed against the inclusion criteria by two investigators (SK and DYM). A third investigator (LK) was consulted to resolve any discrepant assessments. A total of 105 cases were excluded due to the following reasons: surgical treatment of burn sequela, internal organ burns, non-burn care, and corrosions. Thus, all cases with treatments beyond the scope of initial skin burn surgery and/or concurrent non-burn surgery interventions were excluded. In addition, one patient case with a body mass index below 7 kg/m2 was excluded due to miscoding. As a result, we compiled a homogeneous cohort of patients who had suffered isolated skin burns and underwent initial surgical management thereof. The flowchart illustrating the screening process is shown in Figure 1. The NSQIP utilizes the traditional description of burns (first, second, and third degree). To investigate the relevance and impact of age on morbidity and mortality in surgical burn care, we dichotomized the patient pool into patients aged ≥60 years and patients <60 years. In this regard, we followed previous burn-related studies and adopted the United Nations' threshold defining older people as those aged ≥60 years (19–21).
We extracted pre-, peri-, and postoperative variables for analysis. In terms of pre- and perioperative data, we evaluated all variables that have been consistently recorded by the ACS-NSQIP throughout the 13-year study period: (i) patient demographics such as sex, age, race, height (inches), weight (pounds), body mass index, (ii) comorbidities (history of chronic obstructive pulmonary disease [COPD], congestive heart failure [CHF], diabetes mellitus, and disseminated cancer, hypertension requiring treatment, active dialysis therapy, dyspnea, nicotine abuse within 12 months prior to surgery, current use of corticosteroids, ventilator dependency, weight loss exceeding 10% of body weight, preoperative wound infections, and functional health status), (iii) preoperative scores (American Society of Anesthesiology (ASA) physical status classification [score 1–4]) and wound classification [score 1–4], and (iv) preoperative laboratory parameters including serum albumin, serum creatinine, serum sodium, serum glutamic-oxaloacetic transaminase (SGOT), alkaline phosphatase (AP), blood urea nitrogen (BUN), total bilirubin, white blood count (WBC), platelet count, hematocrit, international normalized ratio (INR), partial thromboplastin time (PTT), and prothrombin time (PT). Additionally, we evaluated the operative setting (in- or outpatient). Table 1 provides a detailed list of all retrieved pre- and perioperative variables.
As 30-day postoperative outcomes, we analyzed the discharge destination and the length of hospital stay (LOS). LOS is calculated as the difference in days between the date of admission and the date of discharge. Any complication was delineated as the occurrence of one of the following: Mortality, reoperation, readmission or unplanned readmission, surgical or medical complications. All surgical complications captured in the ACS-NSQIP database (i.e., superficial and deep incision site infections, organ space infections, wound dehiscences, and blood transfusions) were analyzed. Similarly, while considering all medical complications recorded in the ACS-NSQIP database, we concentrated on those that had occurred at least once. Details on postoperative outcomes and complications following burn surgery are shown in Tables 2, 3.
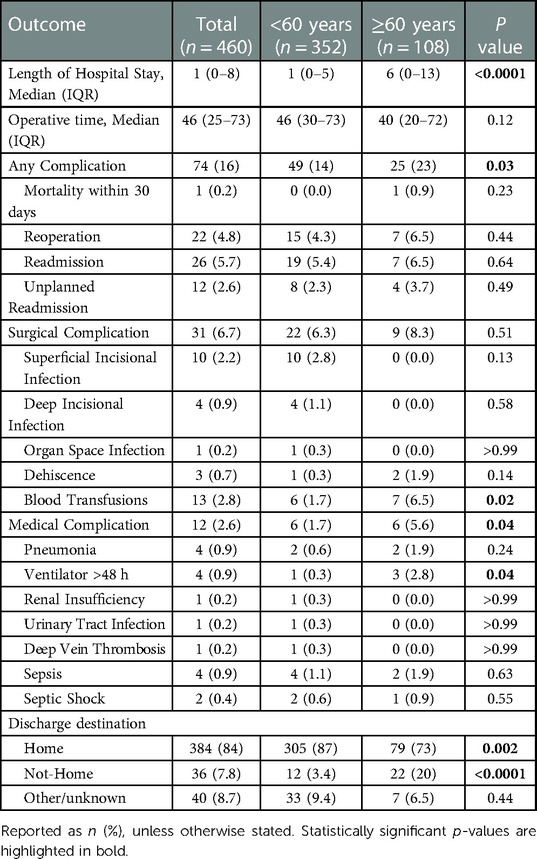
Table 3. Comparison of outcomes following burn surgery between patients younger and older than 60 years of age.
We attempted to extract burn-specific variables. Since the %TBSA was not specified in the ACS-NSQIP database, the main classification was based on the burn degree. We refined this classification pattern by specifying which body area was affected by the burn injury (head and neck, upper body, lower body, unspecified area). When sorting and classifying each case, we closely adhered to the nomenclature and diagnostic details provided in the NSQIP database. Owing to the limited information recorded in the database, in some cases, a more accurate specification of the burn severity and the affected body part(s) was not possible. The classification scheme and the prevalence of each burn (sub)type are shown in Table 2.
The raw data of the ACS-NSQIP annual datasets were converted into analyzable Microsoft Excel (Version 16, Microsoft Corporation, Redmond, WA, USA) files via IBM SPSS Statistics for Windows, version 29 (IBM Corporation, Armonk, NY, USA). Subsequently, all ACS-NSQIP datasets between 2008 and 2020 were standardized into a consistent format. These data were collected and saved in an electronic laboratory notebook (LabArchives, LLC, San Marcos, CA, USA), and analyzed using GraphPad Prism (V9.00 for MacOS, GraphPad Software, La Jolla California, United States). We used independent t-tests to analyze continuous variables. The results were reported as means with standard deviations. Differences in categorical variables were calculated with Pearson's χ2 test. In cases with less than 10 events, Fisher's exact test was applied. To identify differences between groups with non-normally distributed data, we employed the Mann–Whitney U-test. The threshold for statistical significance was set at p < 0.05. After partitioning the cohort into three subgroups according to the occurrence of any, surgical, and medical complications, a univariate subgroup analysis was performed to identify risk factors for complications. All variables identified as significant predictors of the occurrence of any complication were included in a multivariate regression to compensate for confounding. For these confounder-adjusted results, we reported the odds ratio with 95% confidence interval to quantify the correlation between risk factors and outcomes.
The study population included 460 burn patients who underwent initial surgery over a 13-year review period between 2008 and 2020. The mean patient age and BMI were 46 ± 17 years and 29 ± 7.5 kg/m2, respectively. About one-fourth (n = 108; 23%) of all patients were aged ≥60. While male (n = 283; 62%) and white patients (n = 329; 72%) represented the majority of our patient cohort, preoperative wound infections (n = 202; 44%) and obesity (BMI > 30; n = 170; 37%) accounted for the most common comorbidities. Table 1 presents the demographic data and comorbidities of the total study population, all patients aged ≥60 years and all patients <60 years of age.
The majority of patients suffered third-degree burns (n = 293; 64%), with the upper body affected in 143 (49%) and the lower body in 124 (42%) of cases (Table 2). In our patient cohort, second-degree burns accounted for 22% (n = 99) of cases. While our patient pool included no case of first-degree burn, the degree of burn remained unspecified in 68 (15%) cases. A nearly equal number of cases was performed in the outpatient setting (n = 226; 49%) and in the inpatient (n = 234; 51%) setting.
The median operative time was 46 (IQR: 25–73) minutes. Following a LOS of one day (IQR: 0–8), 384 (84%) patients were discharged home. The LOS was significantly different (p < 0.0001) between patients aged <60 years (1 day, IQR: 0–5) and those ≥60 years (6 days, IQR: 0–13). Significant differences were also found between the two age groups with regard to the discharge destination: While 305 (87%) patients aged <60 years and 79 (73%) patients ≥60 years were discharged home, 12 (3.4%) non-seniors and 22 (20%) seniors did not return to a home-based facility. Table 3 lists the perioperative outcomes in detail.
One (0.2%) death was reported within the 30-day follow-up period and 22 (4.8%) patients returned to the operating room. Readmission and unplanned readmissions were indicated in 26 (5.7%) and 12 (2.6%) cases, respectively. Any adverse events, including mortality, reoperation, (unplanned) readmission, surgical and medical complications, were reported in 74 (16%) cases. Surgical complications occurred in 31 (6.7%) cases, with superficial incisional infections (n = 10; 2.2%) and blood transfusions (n = 13; 2.8%) being the most common surgical complications. While seven (6.5%) of the 108 patients aged ≥60 years received blood transfusions, 352 patients <60 years of age received only six (1.7%) blood transfusions, marking a significant difference (p = 0.02) in the age-stratified incidence of transfusions. The rate of medical complications differed significantly (p = 0.04) between patients aged <60 years and those ≥60 years: in both groups, six cases of medical complications (1.7% and 5.6%, respectively) were reported. Table 3 provides detailed information on postoperative outcomes and complications. In both patients < and ≥60 years of age, any complications, surgical and medical complications were relatively and absolutely most frequent among patients with third-degree burns of the upper and lower body (Supplementary Table S2 and Figure 2).
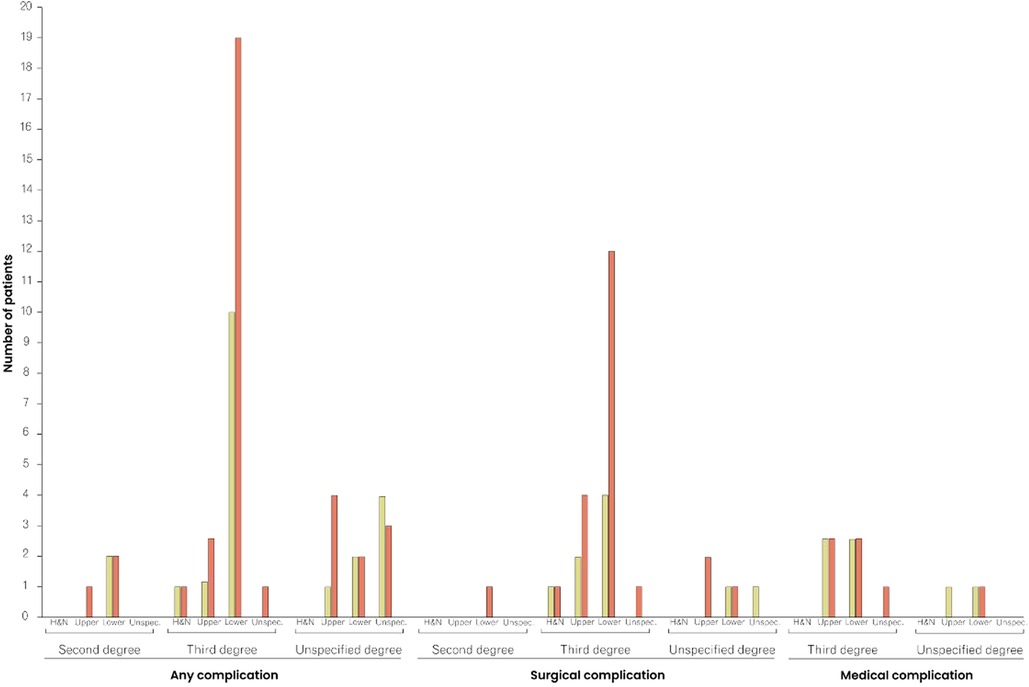
Figure 2. Comparison of complication rates between patients <60 and ≥60 years of age, stratified by type of complication, burn degree, and affected area. The red columns represent the frequency of complications in patients ≥60 years of age, and the yellow columns in patients <60 years of age. H&N, Head and Neck.
Advanced age (p < 0.0001), diabetes mellitus (p = 0.003), hypertension (p = 0.01), wound infection (p = 0.0003), higher ASA scores (p < 0.0001), and higher wound classes (p = 0.001) were identified as risk factors for the occurrence of any complication. Similarly, advanced age (p = 0.01), hypertension (p = 0.04), higher ASA scores (p < 0.0001), and higher wound classes (p = 0.0008) were predictors of surgical complications. For medical complications, again, advanced age (p = 0.0007), higher ASA scores (p < 0.0001), higher wound classes (p = 0.01), and wound infection (p = 0.0007) were found to be risk factors. Significant correlations between the occurrence of any, surgical and medical complications and inpatient procedures were noted (p < 0.0001, p = 0.0006, and p = 0.0004, respectively). Vice versa, the multivariate analysis confirmed the association (OR = −0.11, 95% CI: −0.18 to −0.03; p = 0.004) between an outpatient setting and a lower occurrence of any complications. Multivariate analysis revealed dialysis (OR = 0.44, 95% CI: 9.15–0.74; p = 0.003) and sepsis (OR=0.22, 95% CI: 0.06–0.37; p = 0.006) as independent risk factors for the occurrence of any complications, with dialysis (OR=0.29, 95% CI: 0.08–0.50; p = 0.006) and sepsis (OR=0.09, 95% CI: 0.02–0.15; p = 0.02) being significantly associated with surgical and medical complications, respectively. Tables 4, 5 provide a detailed breakdown of the risk factors for adverse events and the multivariate assessment of any, surgical, and medical complications. Analyzing the preoperative laboratory values, we identified elevations of BUN (p > 0.0001), creatinine (p > 0.0001), WBC (p = 0.02), and PTT (p = 0.004), as well as decreases of albumin (p = 0.0009) and hematocrit (p > 0.0001) as risk factors for the occurrence of any complications. Further details regarding the predictive value of preoperative lab parameters are described in Table 6.
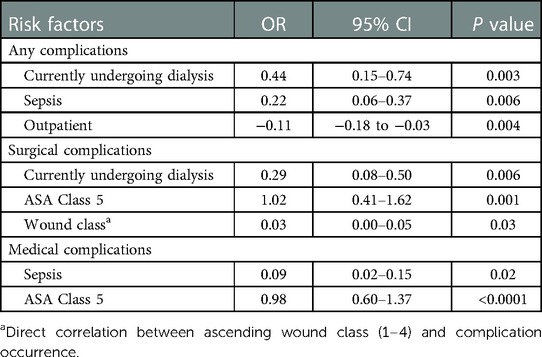
Table 5. Multivariate assessment of any, surgical or medical complication occurrence for all patients undergoing burn surgery.
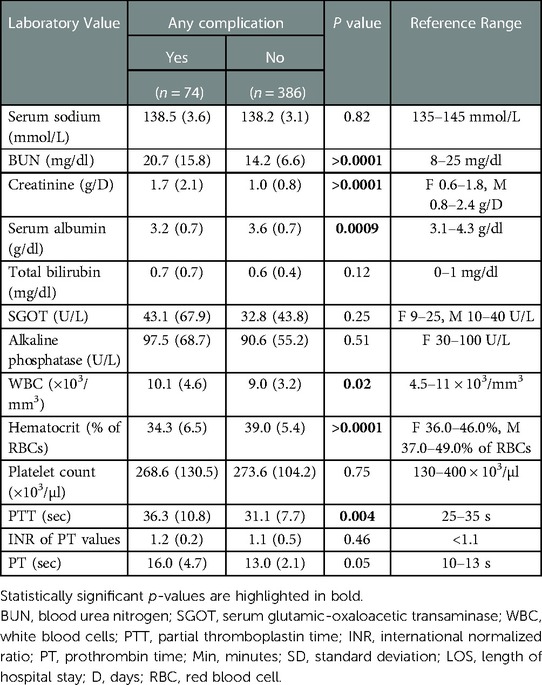
Table 6. Preoperative laboratory values (reported as mean values with standard deviation) and their association with the occurrence of any complication.
Most studies examining burn patients focus exclusively on outcomes in critically ill patients, with extensive and major burn injuries. Thus, there is a paucity of studies that investigate minor burn surgery holistically—across varying institutions, settings, and age groups. To address this knowledge gap, we queried the ACS-NSQIP multi-institutional database, analyzing early outcomes, medical and surgical complications, and predictive risk factors in 460 burn surgery cases. We included burn injuries of different degrees and stratified based on age, that were treated in both inpatient and outpatient settings. Albeit the %TBSA was not specified in the ACS-NSQIP database, a large body of evidence indicates that the vast majority of our patient cohort suffered minor burns with <10% TBSA: Namely, (i) the average operation time of less than one hour, (ii) the comparatively low mortality and morbidity, (iii) the relatively short LOS, and (iv) the nearly equal number of in- and outpatient care support the hypothesis of predominantly minor burn injuries (8, 15, 22–26).
With this assumption in mind, it was intriguing to explore the role of patient age in such non-extensive burns. Furthermore, as mentioned at the beginning, minor burns may lead to major harm. It remains, therefore, essential to identify—also among patients with minor burn injuries—specific perioperative risk factors.
In this study, 23% of all included patients were ≥60 years old. This proportion generally corresponds with burn injury demographics in the United States, where geriatric burns account for 20% of all burn patients (27). While elderly patients accounted for only about one-fourth of the patient cohort in our study, more than half of all observed complications occurred in patients aged ≥60 years (Table 3). Accordingly, our analysis revealed that patients ≥60 years of age were at significantly higher risk of any complications, with particular proneness to bleeding/blood transfusions and medical adverse events. Further, elderly patients were discharged more frequently to non-home skilled facilities.
A 2020 nationwide study from the Netherlands study found a considerably higher risk of morbidity, mortality, and decreased functional status, requiring facility discharge in patients over 65 years old after surgical burn management (15). Cobert et al. analyzed non-fatal burn hospitalizations in older adults concluding that higher age was predictive of discharge to non-independent living such as nursing homes or rehabilitation centers (28). While our findings generally align with previous reports, our work also revealed elevated complication incidences and non-home discharges in elderly burn surgery patients with minor, less-drastic injuries.
In addition, in our analysis, burn patients aged ≥60 years showed a significantly prolonged LOS, with a surplus of five LOS days on average. An Iranian study including 899 hospitalized burn patients reported a mean LOS of 3.2 days, while Lundy et al. identified advanced age as a risk factor for prolonged LOS (29, 30). While we found a slightly decreased LOS in our study cohort with predominantly minor burn injuries, we could confirm the predisposing role of age for prolonged LOS, with patients ≥60 years reporting a median LOS of 6 days. This finding carries high clinical relevance as prolonged LOS is correlated with increased costs and risk of nosocomial infections (31, 32).
Ultimately, the American Burn Association advises that elderly and other high-risk patients be treated for burns in burn centers, when possible, to receive specialized care and reduce complications. Although 35 of the 123 American burn centers (Supplementary Table S1) currently report patient data to the NSQIP (which includes 707 institutions), only a handful of analyses published by American burn centers investigate post-burn complications in the elderly (33, 34). Analyzing a cohort of 644 patients, Iles et al. found that elderly patients were most likely to have higher frailty, higher LOS, morbidity, and non-home discharge. They also reported an increased mortality rate in elderly patients, the majority of which suffered from minor burns (35). Similarly, in their prospective study of burn patients aged ≥65 years, Maxwell et al. noted that frail and elderly patients suffered from significantly more complications, such as sepsis, and higher mortality (36). Our findings of higher morbidity and complication rates, LOS, and non-home discharge agree with these reports. The increased incidence of such adverse events may be due to a higher prevalence of comorbidities such as diabetes and hypertension and an overall worse preoperative health status among patients aged 60 years and older (Table 1). For example, the calculated increased risk of bleeding/blood transfusion in the elderly patient cohort is likely a consequence of the lower hematological and coagulation values. Yet, underlying causalities need to be investigated more accurately in further large-scale studies.
Strikingly, while our univariate analysis highlighted age as a significant risk factor for any complication, a confounder-eliminating multivariate analysis demonstrated that age was no longer a risk factor (Tables 4, 5). Instead, the multivariate analysis revealed significant correlations between the occurrence of any complications and current dialysis treatment (OR = 0.44, 95% CI: 9.15–0.74; p = 0.003) and history of sepsis (OR = 0.22, 95% CI: 0.06–0.37; p = 0.006). Taken together, our findings point to a predisposing role of age in minor burn injuries and identify patients aged ≥60 years as particularly susceptible to adverse events. However, surgeons should be wary of focusing on the patient's age as an isolated clinical variable and rather also account for the surrounding comorbidities and concomitant treatment modalities. These findings also call for a re-thinking of the prevailing understanding that mere biological age is intrinsically associated with increased risk in burn surgery. The deliberation of patient eligibility, preoperative planning, and perioperative monitoring should be decoupled from the mono-perspective age consideration and replaced with a holistic view of the patient's characteristics.
When analyzing the affected burn area, and its association with postoperative complications, the lower body appeared to be a risk factor for both patients aged <60 years and ≥60 years (Figure 2 and Supplementary Table S2). Indeed, we demonstrated that minor lower body burns accounted for the largest proportion of all cases with any complication. Yeong et al. found an increased rate of tissue-expansion failure in burn patients with an affection of the lower limbs (37). Remarkably, Momeni et al. analyzed 995 burn cases and calculated that with every 1% increase in lower extremity burn surface area, the mortality increased by 9%. This statistical link was even more evident in elderly patients (38). Thus, while the lower body has previously been identified as a risk site, our study confirmed this observation for non-extensive burn lesions (39).
We found markedly decreased serum albumin and hematocrit levels as well as elevated BUN, creatinine, and WBC levels in burn patients experiencing any postoperative complication. Further, the PTT was prolonged in patients suffering any complication. Previously, lower preoperative levels of hematocrit have been shown to correlate with an increased risk of intraoperative bleeding and the need for blood transfusion (40, 41). Hypoalbuminemia was found to worsen postoperative wound healing and negatively affect surgical site infections, while elevated BUN, creatinine, and WBC levels, and prolonged PTT have been linked with an increased risk of postoperative complications in general surgery and intensive care (42–50). Of note, albumin and hematocrit levels are generally lower in geriatric patients, whereas the same patient population has shown elevated BUN, and creatinine levels as well as a higher PTT (51–58). Accordingly, these risk-associated laboratory values may be considered proxy indicators of senescence, thereby underscoring the clinical relevance of advanced age in surgical burn care. With our results in mind, we propose particular attention to be paid to (elderly) patients with out-of-range preoperative levels of the above biomarkers in anticipation of postoperative adverse events. In addition, we suggest incorporating these laboratory values into frailty scores for burn surgery to further improve their sensitivity and specificity.
Ultimately, surgical interventions utilized for the treatment of burns in older patients remain a field of controversy and ongoing debate. Clinicians should take into consideration a variety of factors when deciding what surgical intervention to choose, especially when treating geriatric burns. Generally speaking, first-degree burns do not require surgical management, while deep second and third-degree burns often necessitate excision and grafting. However, the dermis in elderly patients is comparably thinner, which delays wound healing and may complicate harvesting and re-harvesting skin donor sites (59). Additionally, a well-vascularized wound bed is necessary for successful grafting, and older patients, especially those suffering from peripheral vascular disease, diabetes mellitus, or limb ischemia, are at higher risk for graft failure (60). Many surgeons contend that aggressive, early excision of deeply burned tissues and early grafting using an autologous split-thickness skin graft or synthetic skin replacement is essential in older patients to decrease infections, hospital stay, and accelerate recovery (15, 61, 62). While other clinicians advocate for a more conservative and delayed treatment approach to avoid surgical complications, previous reports have shown that early surgery in the elderly is better tolerated and leads to fewer complications (63, 64). Clinicians should consider the physiological changes that accompany older age, as well as the predictive risk factors and preoperative lab values mentioned in this study when evaluating a patient's eligibility for surgery and optimizing surgical outcomes and postoperative care. Furthermore, burn surgeons should also be aware that minor burn lesions may cause major damage and, therefore, require adequate care—particularly in the advanced patient age.
Due to the multi-institutional nature of the ACS-NSQIP database, the analyzed patient pool can be considered relatively diverse and large. However, the inherent limitations should be considered. General limitations include the retrospective structure of the NSQIP database, which is innately subject to confounding factors and bias. Herein, we report only the presence of statistical correlations, while underlying causal-effect relationships need to be investigated in future studies. The accuracy and reliability of the records depend on the party responsible for data capture. Therefore, varying personal expertise and subjective rating may explain differing assessments, for example regarding the wound classification. In addition, the scope of the database has also been suggested as a potential source of bias, as there may be variability in quality both between and within the participating institution. Nonetheless, when assessing the data quality and interrater-reliability of the ACS-NSQIP database, Shiloach et al. found little variance in the heterogeneity of the catalog (65). Further, the standardized data collection results in a lack of potentially relevant information. The ACS-NSQIP database misses details on the patients' preoperative health (such as history of history of ischemic heart disease, coronary artery disease, or peripheral vascular disease) as well as on short-term (<30 days) procedure-specific complications including hematoma and edema. The postoperative follow-up is limited to 30 days, leaving long-term (>30 days) complications uncovered. The long-term success of burn surgery also depends on functional factors, such as pain and sensation, and aesthetic aspects, such as the presence and appearance of scars. These parameters are not included in the database. Similarly, the discharge destination may be biased by pre-admission domicile (at home or in a care facility), which is not reported and, therefore, not taken into account. The ACS-NSQIP database does not record information on the severity and chronicity of the comorbidities. Moreover, the burned body area (%TBSA), a crucial parameter in burn care, is not reported. Therefore, no relationship between the extent of burn and postoperative complications can be established in this study. Yet, we could estimate the extent of burn wounds in our patient cohort by considering the operative time, mortality and morbidity, LOS, and in- and outpatient distribution. In addition, in some cases, due to the limited information provided in the ACS-NSQIP database, the affected body region could not be clearly defined and was, therefore, left unspecified. One may speculate that patients who had suffered burns to multiple body regions may be in this category of “unspecified area”.
In this study, we utilized the NSQIP-ACS database to characterize patients receiving initial surgical care for predominately minor burn injuries. We also identified predictive risk factors for perioperative and postoperative complications, such as age and involvement of lower body parts. Although our multivariate analysis delineated age as a non-risk factor for complications, age can be a predictive risk factor when considering other coinciding factors such as dialysis treatment and sepsis. We demonstrated the relevance of preoperative laboratory values such as serum albumin and hematocrit in predicting postoperative adverse events. By including these novel variables and insights in the perioperative algorithm, surgeons may improve overall patient care and surgical outcomes in burn surgery.
The datasets presented in this article are not readily available because formal restrictions apply to the availability of these data. Requests to access the datasets should be directed to American College of Surgeons—National Surgical Quality Improvement Program, https://accreditation.facs.org/programs/nsqip.
The studies involving human participants were reviewed and approved by Brigham and Women's Hospital (Protocol #: 2013P001244). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conceptualization: SK, DPO, and GH; Methodology: SK, DYM, ACP, DPO, and GH; Data Curation: SK, DYM, and ACP; Visualization: SK and LK; Writing – original draft: SK and GH; Writing – review & editing: DYM, LK, DO, VH, SMG, BSK, MKN, UK, ACP, and DPO; Supervision: UK, DPO, and GH; Project administration: ACP and DPO. All authors contributed to the article and approved the submitted version.
We would like to thank our Quality Program Manager Jill Steinberg MPH, RN, for her help with the ACS NSQIP data acquisition. American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1131293/full#supplementary-material.
1. Kaddoura I, Abu-Sittah G, Ibrahim A, Karamanoukian R, Papazian N. Burn injury: review of pathophysiology and therapeutic modalities in major burns. Ann Burns Fire Disasters. (2017) 30(2):95–102. PMID: 29021720; PMCID: PMC5627559
2. Garcia-Espinoza JA, Navarro-Delgadillo CI, Costa-Dulché A, Flores-Soto D, Barrera-García G, Márquez-Espriela C. Epidemiology of burn injuries: 2 years’ experience in a specialized hospital in Mexico City. Ann Burns Fire Disasters. (2019) 32(4):261–6. PMID: 32431574; PMCID: PMC7197908
3. Browning JA, Cindass R. Burn debridement, grafting, and reconstruction. Treasure Island, FL: StatPearls Publishing LLC (2022).
4. Niţescu C, Calotă DR, Florescu IP, Lascăr I. Surgical options in extensive burns management. J Med Life. (2012) 5(Spec Issue):129–36. PMID: 31803300; PMCID: PMC6880225
5. Gacto-Sanchez P. Surgical treatment and management of the severely burn patient: review and update. Med Intensiva. (2017) 41(6):356–64. doi: 10.1016/j.medin.2017.02.008
6. Swanson JW, Otto AM, Gibran NS, Klein MB, Kramer CB, Heimbach DM, et al. Trajectories to death in patients with burn injury. J Trauma Acute Care Surg. (2013) 74(1):282–8. doi: 10.1097/TA.0b013e3182788a1c
7. Güldoğan CE, Kendirci M, Gündoğdu E, Yastı A. Analysis of factors associated with mortality in major burn patients. Turk J Surg. (2019) 35(3):155–64. doi: 10.5578/turkjsurg.4065
8. Jeschke MG, Pinto R, Kraft R, Nathens AB, Finnerty CC, Gamelli RL, et al. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. (2015) 43(4):808–15. doi: 10.1097/CCM.0000000000000790
9. Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: extending and updating the baux score. J Trauma. (2010) 68(3):690–7. doi: 10.1097/TA.0b013e3181c453b3
10. Obed D, Salim M, Dastagir N, Knoedler S, Dastagir K, Panayi AC, et al. Comparative analysis of composite mortality prediction scores in intensive care burn patients. Int J Environ Res Public Health. (2022) 19(19):12321. doi: 10.3390/ijerph191912321
11. Jeschke MG, Phelan HA, Wolf S, Romanowski K, Rehou S, Saetamal A, et al. State of the science burn research: burns in the elderly. J Burn Care Res. (2020) 41(1):65–83. doi: 10.1093/jbcr/irz163
12. Abu-Sittah GS, Chahine FM, Janom H. Management of burns in the elderly. Ann Burns Fire Disasters. (2016) 29(4):245–9. PMID: 28289356; PMCID: PMC5347309
13. Romanowski KS, Sen S. Wound healing in older adults with severe burns: clinical treatment considerations and challenges. Burns Open. (2022) 6(2):57–64. doi: 10.1016/j.burnso.2022.01.002
14. Caetano P, BrandÃo C, Campos I, TÃo J, Laíns J, Cabral L. Aging and burn: a five-year retrospective study in a major burn centre in Portugal. Ann Burns Fire Disasters. (2018) 31(3):163–7. PMID: 30863245; PMCID: PMC6367867
15. Goei H, van Baar ME, Dokter J, Vloemans J, Beerthuizen G, Middelkoop E, et al. Burns in the elderly: a nationwide study on management and clinical outcomes. Burns Trauma. (2020) 8:tkaa027. doi: 10.1093/burnst/tkaa027
16. Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann Emerg Med. (1982) 11(5):260–2. doi: 10.1016/S0196-0644(82)80096-6
17. Heng JS, Clancy O, Atkins J, Leon-Villapalos J, Williams AJ, Keays R, et al. Revised baux score and updated Charlson comorbidity index are independently associated with mortality in burns intensive care patients. Burns. (2015) 41(7):1420–7. doi: 10.1016/j.burns.2015.06.009
18. Jeschke MG, Pinto R, Costford SR, Amini-Nik S. Threshold age and burn size associated with poor outcomes in the elderly after burn injury. Burns. (2016) 42(2):276–81. doi: 10.1016/j.burns.2015.12.008
19. European Commission, Eurostat. Ageing Europe: Looking at the lives of older people in the EU: 2019 edition. Publications Office (2019). doi: 10.2785/811048
20. United Nations. Department of Economic and Social Affairs, Population Division, World Population Ageing 2013. (2013) New York. ST/ESA/SER.A/348
21. Bayuo J, Botchway AE. Burns among older persons: a narrative review. Burns Open. (2017) 1(1):2–8. doi: 10.1016/j.burnso.2017.05.006
22. Johnson LS, Shupp JW, Pavlovich AR, Pezzullo JC, Jeng JC, Jordan MH. Hospital length of stay–does 1% TBSA really equal 1 day? J Burn Care Res. (2011) 32(1):13–9. doi: 10.1097/BCR.0b013e318204b3ab
23. Abali AE. Cost-effective outpatient burn-care for minor burns. Burns. (2015) 41(3):639–40. doi: 10.1016/j.burns.2014.11.015
24. Moss LS. Outpatient management of the burn patient. Crit Care Nurs Clin North Am. (2004) 16(1):109–17. doi: 10.1016/j.ccell.2003.09.002
25. Mertens DM, Jenkins ME, Warden GD. Outpatient burn management. Nurs Clin North Am. (1997) 32(2):343–64. doi: 10.1016/S0029-6465(22)02191-0
26. Ziolkowski N, Rogers AD, Xiong W, Hong B, Patel S, Trull B, et al. The impact of operative time and hypothermia in acute burn surgery. Burns. (2017) 43(8):1673–81. doi: 10.1016/j.burns.2017.10.001
27. Dissanaike S, Rahimi M. Epidemiology of burn injuries: highlighting cultural and socio-demographic aspects. Int Rev Psychiatry. (2009) 21(6):505–11. doi: 10.3109/09540260903340865
28. Cobert J, Sheckter C, Pham TN. A national analysis of discharge disposition in older adults with burns-estimating the likelihood of independence at discharge. J Burn Care Res. (2022) 43(6):1221–6. doi: 10.1093/jbcr/irac104
29. Chukamei G, et al Z. The length of stay and cost of burn patients and the affecting factors. Int J Burns Trauma. (2021) 11(5):397–405. PMID: 34858720; PMCID: PMC8610821
30. Lundy JB, Chung KK, Pamplin JC, Ainsworth CR, Jeng JC, Friedman BC. Update on severe burn management for the intensivist. J Intensive Care Med. (2016) 31(8):499–510. doi: 10.1177/0885066615592346
31. Liu JY, Dickter JK. Nosocomial infections: a history of hospital-acquired infections. Gastrointest Endosc Clin N Am. (2020) 30(4):637–52. doi: 10.1016/j.giec.2020.06.001
32. Koljonen V, Laitila M, Rissanen AM, Sintonen H, Roine RP. Treatment of patients with severe burns-costs and health-related quality of life outcome. J Burn Care Res. (2013) 34(6):e318–25. doi: 10.1097/BCR.0b013e3182779c90
33. American College of Surgeons, Hospital and Facilities. (2022). Available at: https://www.facs.org/hospital-and-facilities/?searchTerm=&institution=NsqipHospital&address&page=1 (Cited November 30, 2022).
34. American Burn Association, Burn Center Regional Map. (2022). Available at: https://ameriburn.org/resources/burn-center-regional-map/ (Cited November 30, 2022).
35. Gregg D, Patil S, Singh K, Marano MA, Lee R, Petrone SJ, et al. Clinical outcomes after burns in elderly patients over 70 years: a 17-year retrospective analysis. Burns. (2018) 44(1):65–9. doi: 10.1016/j.burns.2017.09.018
36. Maxwell D, Rhee P, Drake M, Hodge J, Ingram W, Williams R. Development of the burn frailty Index: a prognostication index for elderly patients sustaining burn injuries. Am J Surg. (2019) 218(1):87–94. doi: 10.1016/j.amjsurg.2018.11.012
37. Yeong EK, Chen KW, Chan ZH. Risk factors of tissue-expansion failure in burn-scar reconstruction. J Plast Reconstr Aesthet Surg. (2011) 64(12):1635–40. doi: 10.1016/j.bjps.2011.07.006
38. Momeni M, Sediegh-Marufi S, Safari-Faramani R, Akhoondinasab MR, Karimi H, Karimi AM. Lower extremity burns, complications, and outcome. J Burn Care Res. (2020) 41(2):409–15. doi: 10.1093/jbcr/irz182
39. Mohammadi AA, Pakyari MR, Seyed Jafari SM, Tavakkolian AR, Tolide-Ie HR, Moradi Z, et al. Effect of burn sites (upper and lower body parts) and gender on extensive burns’ mortality. Iran J Med Sci. (2015) 40(2):166–9. PMID: 25821297; PMCID: PMC4359937
40. Muñoz M, Gómez-Ramírez S, Campos A, Ruiz J, Liumbruno GM. Pre-operative anaemia: prevalence, consequences and approaches to management. Blood Transfus. (2015) 13(3):370–9. doi: 10.2450/2015.0014-15
41. Cahill CM, Alhasson B, Blumberg N, Melvin A, Knight P, Gloff M, et al. Preoperative anemia management program reduces blood transfusion in elective cardiac surgical patients, improving outcomes and decreasing hospital length of stay. Transfusion. (2021) 61(9):2629–36. doi: 10.1111/trf.16564
42. Issangya CE, Msuya D, Chilonga K, Herman A, Shao E, Shirima F, et al. Perioperative serum albumin as a predictor of adverse outcomes in abdominal surgery: prospective cohort hospital based study in Northern Tanzania. BMC Surg. (2020) 20(1):155. doi: 10.1186/s12893-020-00820-w
43. He Z, Zhou K, Tang K, Quan Z, Liu S, Su B. Perioperative hypoalbuminemia is a risk factor for wound complications following posterior lumbar interbody fusion. J Orthop Surg Res. (2020) 15(1):538. doi: 10.1186/s13018-020-02051-4
44. Liu J, Sun LL, Wang J, Ji G. Blood urea nitrogen in the prediction of in-hospital mortality of patients with acute aortic dissection. Cardiol J. (2018) 25(3):371–6. doi: 10.5603/CJ.a2017.0075
45. Knos GB, Berry AJ, Isaacson IJ, Weitz FI. Intraoperative urinary output and postoperative blood urea nitrogen and creatinine levels in patients undergoing aortic reconstructive surgery. J Clin Anesth. (1989) 1(3):181–5. doi: 10.1016/0952-8180(89)90039-1
46. Faisst M, Wellner UF, Utzolino S, Hopt UT, Keck T. Elevated blood urea nitrogen is an independent risk factor of prolonged intensive care unit stay due to acute necrotizing pancreatitis. J Crit Care. (2010) 25(1):105–11. doi: 10.1016/j.jcrc.2009.02.002
47. Amaranto DJ, Wang EC, Eskandari MK, Morasch MD, Rodriguez HE, Pearce WH, et al. Normal preoperative white blood cell count is predictive of outcomes for endovascular procedures. J Vasc Surg. (2011) 54(5):1395–403.e2. doi: 10.1016/j.jvs.2011.04.063
48. O'Brien MM, Gonzales R, Shroyer AL, Grunwald GK, Daley J, Henderson WG, et al. Modest serum creatinine elevation affects adverse outcome after general surgery. Kidney Int. (2002) 62(2):585–92. doi: 10.1046/j.1523-1755.2002.00486.x
49. Castle J, Mazmudar A, Bentrem D. Preoperative coagulation abnormalities as a risk factor for adverse events after pancreas surgery. J Surg Oncol. (2018) 117(6):1305–11. doi: 10.1002/jso.24972
50. Hu B, Xu G, Jin X, Chen D, Qian X, Li W, et al. Novel prognostic predictor for primary pulmonary hypertension: focus on blood urea nitrogen. Front Cardiovasc Med. (2021) 8:724179. doi: 10.3389/fcvm.2021.724179
51. Weaving G, Batstone GF, Jones RG. Age and sex variation in serum albumin concentration: an observational study. Ann Clin Biochem. (2016) 53(Pt 1):106–11. doi: 10.1177/0004563215593561
52. Chen S, Liu Y, Cai L, Ren C, Xiong T, Jin L, et al. Erythropoiesis changes with increasing age in the elderly Chinese. Int J Lab Hematol. (2021) 43(5):1168–73. doi: 10.1111/ijlh.13615
53. Lanier JB, Park JJ, Callahan RC. Anemia in older adults. Am Fam Physician. (2018) 98(7):437–42. PMID: 30252420
54. Snoeck HW. Aging of the hematopoietic system. Curr Opin Hematol. (2013) 20(4):355–61. doi: 10.1097/MOH.0b013e3283623c77
55. Miao G. Reference range of hematocrit in the elderly with respect to altitude. Clin Hemorheol Microcirc. (2003) 29(1):25–31. PMID: 14561901
56. Liu Q, Wang Y, Chen Z, Guo X, Lv Y. Age- and sex-specific reference intervals for blood urea nitrogen in Chinese general population. Sci Rep. (2021) 11(1):10058. doi: 10.1038/s41598-021-89565-x
57. Nguyen T, Foster Y, Cekaj S. Older adult kidney function assessment and rounding creatinine led to medication dosing error. Am J Ther. (2018) 25(4):e439–46. doi: 10.1097/MJT.0000000000000568
58. Zhang GM, Zhang W, Zhang GM. Age-specific reference intervals for PT, aPTT, fibrinogen and thrombin time for parturient women. Thromb Haemost. (2019) 119(6):894–8. doi: 10.1055/s-0039-1683932
59. Still JM, Law EJ, Belcher K, Thiruvaiyaru D. A regional medical center’s experience with burns of the elderly. J Burn Care Rehabil. (1999) 20(3):218–23. doi: 10.1097/00004630-199905000-00011
60. Pomahac B, Matros E, Semel M, Chan RK, Rogers SO, Demling R, et al. Predictors of survival and length of stay in burn patients older than 80 years of age: does age really matter? J Burn Care Res. (2006) 27(3):265–9. doi: 10.1097/01.BCR.0000216795.90646.4E
61. Papini R. Management of burn injuries of various depths. Br Med J. (2004) 329(7458):158–60. doi: 10.1136/bmj.329.7458.158
62. Kara M, Peters WJ, Douglas LG, Morris SF. An early surgical approach to burns in the elderly. J Trauma. (1990) 30(4):430–2. doi: 10.1097/00005373-199004000-00011
63. Abu-Sittah GS, Sarhane KA, Dibo SA, Ibrahim A. Cardiovascular dysfunction in burns: review of the literature. Ann Burns Fire Disasters. (2012) 25(1):26–37. PMID: 23012613; PMCID: PMC3431724
64. Burdge JJ, Katz B, Edwards R, Ruberg R. Surgical treatment of burns in elderly patients. J Trauma. (1988) 28(2):214–7. doi: 10.1097/00005373-198802000-00016
65. Shiloach M, Frencher SK Jr., Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. (2010) 210(1):6–16. doi: 10.1016/j.jamcollsurg.2009.09.031
Keywords: burn surgery, burns, burn injury, minor burns, age, elderly, geriatric
Citation: Knoedler S, Matar DY, Knoedler L, Obed D, Haug V, Gorski SM, Kim B-S, Kauke-Navarro M, Kneser U, Panayi AC, Orgill DP and Hundeshagen G (2023) Association of age with perioperative morbidity among patients undergoing surgical management of minor burns. Front. Surg. 10:1131293. doi: 10.3389/fsurg.2023.1131293
Received: 28 December 2022; Accepted: 13 February 2023;
Published: 27 February 2023.
Edited by:
Jan A. Plock, Aarau Cantonal Hospital, SwitzerlandReviewed by:
Guangtao Xu, Jiaxing University Medical College, China© 2023 Knoedler, Matar, Knoedler, Obed, Haug, Gorski, Kim, Kauke-Navarro, Kneser, Panayi, Orgill and Hundeshagen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Knoedler c2FtdWVsLmtub2VkbGVyQHN0dWQudW5pLXJlZ2Vuc2J1cmcuZGU= Gabriel Hundeshagen Z2FicmllbC5odW5kZXNoYWdlbkBiZ3UtbHVkd2lnc2hhZmVuLmRl
Specialty Section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.