
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 31 May 2023
Sec. Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1126596
This article is part of the Research TopicAdvanced Technological Applications in Neurosurgery.View all 5 articles
Background: Primary palmar hyperhidrosis (PPH) is a condition marked by an overactive secretion of the hand's exocrine glands and is frequently hereditary. The profuse sweating associated with this condition can significantly impair the patient's daily activities and quality of life.
Objective: The objective of this study was to compared the benefits and drawbacks of thoracic sympathetic block and thoracic sympathetic radiofrequency in the treatment of PPH.
Methods: A retrospective analysis was conducted on 69 patients. They were divided into groups A and B according to their treatment. Group A (34 cases) received CT-guided percutaneous thoracic sympathetic nerve chain anhydrous alcohol chemical damage block, and group B (35 cases) received CT-guided percutaneous thoracic sympathetic nerve chain radiofrequency thermocoagulation.
Results: Palmar sweating disappeared immediately after the operation. The recurrence rates at 1, 3, 6, 12, 24, and 36 months were 5.88% vs. 2.86% (P > 0.05), 20.59% vs. 5.71% (P > 0.05), 32.35% vs. 11.43% (P < 0.05),32.35% vs. 11.43% (P < 0.05), 25% vs. 14.71% (P < 0.05), and 68.75% vs. 20.59% (P < 0.05), respectively. The incidence of intercostal neuralgia and compensatory hyperhidrosis was higher in group A compared with of group B (52.94% vs. 22.86%, P < 0.05; 55.88% vs. 22.86%, P < 0.05).
Conclusion: Both methods were found to be effective in treating PPH, but thoracic sympathetic radiofrequency had a longer-term effect, a lower recurrence rate, and a lower incidence of intercostal neuralgia and compensatory hyperhidrosis than a thoracic sympathetic block.
Primary palmar hyperhidrosis (PPH) is a condition characterized by excessive secretion of the exocrine glands in the hand, which is often accompanied by hyperhidrosis in other areas of the body, including the head, face, armpit, or foot (1). In China, the disease rate in China is estimated to be 2.08%, with 25.40% of patients having a family history of PPH (2). Despite extensive research, the pathogenesis of PPH remains unknown (3). Thoracoscopic sympathectomy (ETS), is considered the most effective treatment for PPH (4), but its use is limited due to a high incidence of compensatory hyperhidrosis (5). We have previously employed CT-guided thoracic sympathetic chain anhydrous alcohol chemical damage block to treat palmar hyperhidrosis (6–8). However, this treatment has a limited duration, and anhydrous alcohol can spread to the intercostal nerve, leading to intercostal neuralgia and a high incidence of compensatory hyperhidrosis. Therefore, we introduced CT-guided radiofrequency thermocoagulation (9) in the treatment of PPH. The aim of this study was to assess the pros and cons of two novel technologies for managing PPH.
This study was approved by the ethics committee of the First Hospital of Jiaxing, the Affiliated Hospital of Jiaxing University (Approval number: ls2018-141). From January 2017 to December 2018, we collected data from patients who underwent surgery for PPH at our hospital. The patients were divided into two groups, A and B based on the surgical procedures they received. Group A (n = 34) received a CT-guided percutaneous thoracic sympathetic nerve block with anhydrous alcohol, while group B (n = 35) received CT-guided percutaneous radiofrequency thermocoagulation to physically damage the thoracic sympathetic nerve chain.
Inclusion criteria:
(1) Patients who met the diagnostic criteria for primary hyperhidrosis established by the multidisciplinary working group of the American Academy of Dermatology (10, 11);
(2) Patients between the ages of 18 and 45;
(3) Patients who had not undergone previous surgical treatment;
(4) Patients with a Hyperhidrosis Disease Severity Scale (HDSS) (3, 12) scored 3–4.
Exclusion criteria:
(1) Patients who were allergic to alcohol or local anesthetics;
(2) Pregnancy or preparing for pregnancy, patients with severe thoracic deformity, lung or puncture site infection, or other serious systemic diseases that would preclude the operation;
(3) Patients with secondary hyperhidrosis.
Prior to the operation, patients were informed about the operation process and risks and were required to sign an informed consent form. Patients were advised to refrain from consuming any food or drink for at least 8 h before the operation. A trocar was inserted in the upper limb vein for later use. The patient was then sent to the CT operating room, and asked to lie prone on the CT console and position their hands close to the outside of their body with palms up. Vital signs, palm temperature (T), and pulse index (PI) were monitored throughout the operation.
All patients underwent treatment at the sympathetic nerve chain at the level of the 4th thoracic vertebra level (T4). The CT scan frame was set on the chest CT localization image, covering the 1st to 4th ribs. Subsequently, an axial scan with a layer thickness of 3 mm was conducted to determine the layer encompassing the upper border of the small head of the fourth rib, which was identified as the optimal puncture site. A puncture path was then charted based on this layer. The process for each group is described below:
Group A (6–8): Puncture target points on both sides were pulled straight to the back skin through the lamina-transverse process gap, and the intersection point with the skin was identified as the puncture entry point. The puncture depth and angle were measured using the CT software ruler. Following the administration of local anesthesia, a No. 7 puncture needle with a length of 10 cm was used to puncture the target point according to the designed puncture path (Figure 1). The needle core was then removed, and 3 ml of 1% lidocaine (0.1 ml of 30% iohexol contrast agent for development) was injected on each side. CT scanning was then repeated. If the injected drug solution wrapped around the small head of the fourth rib and the lateral edge of the fourth vertebral body without entering the spinal canal along the intervertebral foramen (Figure 2), and T and PI were observed to increase significantly, 2.5 ml of absolute alcohol (with iohexol) was slowly injected into each side after 20 min. CT scanning was repeated, and a three-dimensional reconstruction of the image was performed (Figure 3). The distribution of the injected liquid and complications such as pneumothorax and bleeding were then observed. Finally, the needle was pulled out, and the puncture point was covered with a sterile dressing.
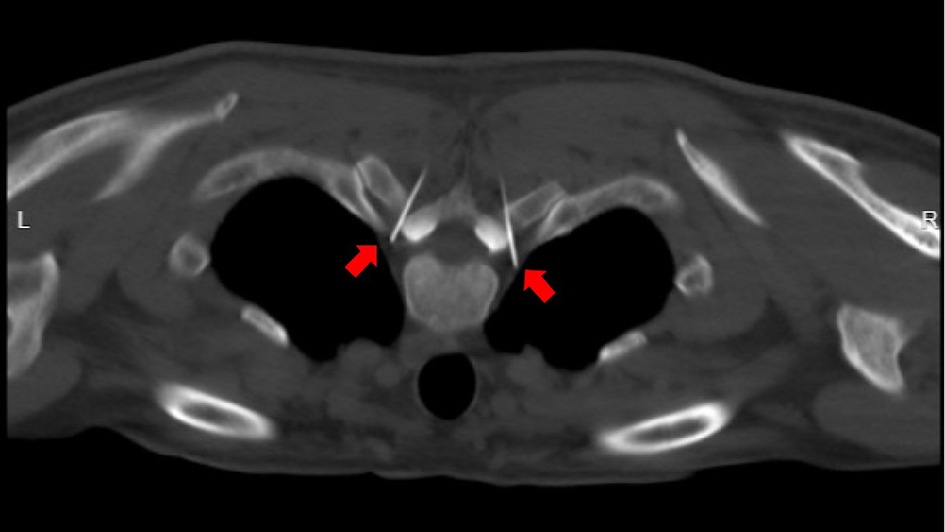
Figure 1. Under CT guidance, the needle tip reaches the predetermined target point (indicated by the red arrow) along the designed puncture path.
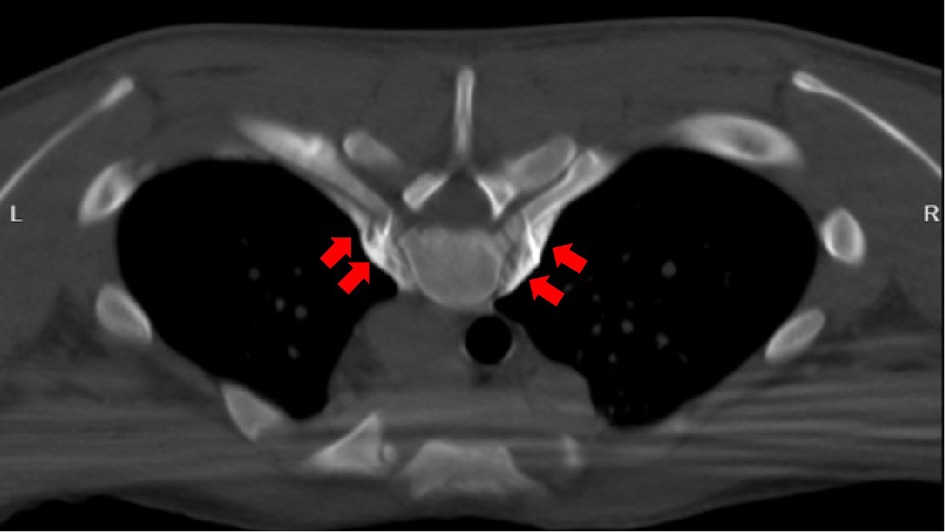
Figure 2. Successful puncture with lidocaine test. 2.5 ml of absolute alcohol (each 1 ml contains 0.9 ml of absolute alcohol and 0.1 ml of 30% iohexol) is injected on both sides, as shown by the red arrow in the figure.
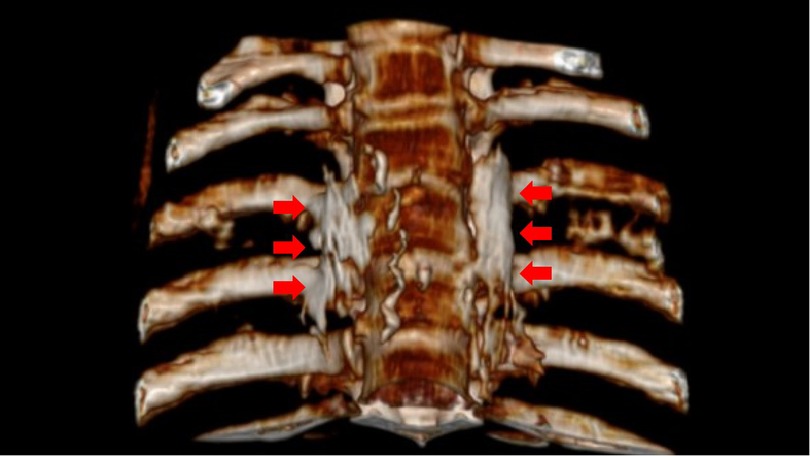
Figure 3. The distribution of absolute alcohol on both sides after three-dimensional CT reconstruction, which wraps around the small head of the 4th rib and the lateral edge of the 4th vertebral body, as indicated by the red arrow in the figure.
Group B (9): Puncture paths were designed as in Group A. Following local anesthesia, a 10 cm No.7 blunt thoracic sympathetic RF puncture needle (with a bare end of 10 mm) was used to puncture the target point until the needle tip was close to the front upper edge of the 4th rib small head (Figure 4). A three-dimensional reconstruction of the CT image was carried out to confirm the position of the puncture tip (Figure 5). The RF needle core was removed, and the RF electrode was inserted to perform electrophysiological tests on motor and sensory nerves, respectively. RF thermocoagulation was conducted at 95°C for 300 s if a current below 1.5 mA elicited no numbness or muscle twitching within the spinal nerve innervation area. T and PI were monitored before and after the procedure, after which the treatment was terminated.
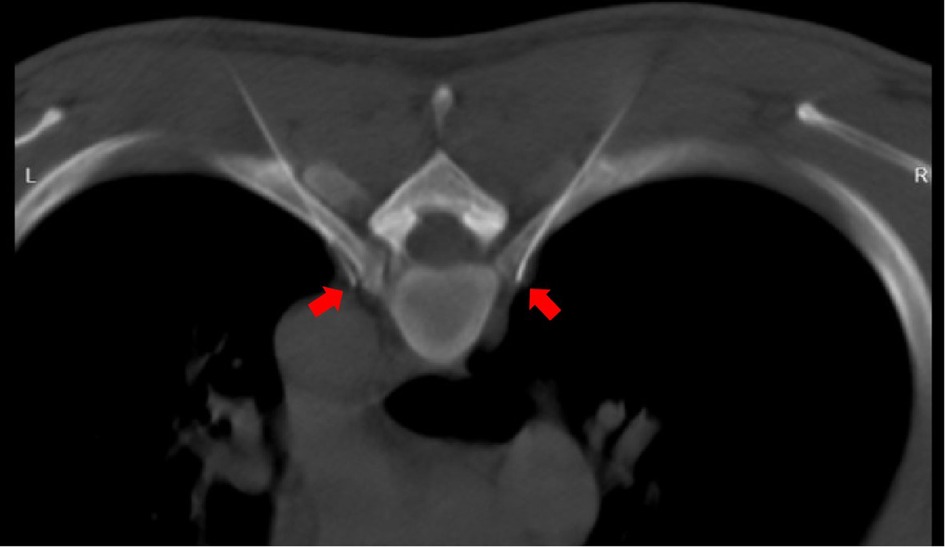
Figure 4. CT-guided puncture reaching the predetermined target point along the designed path (indicated by the red arrow).
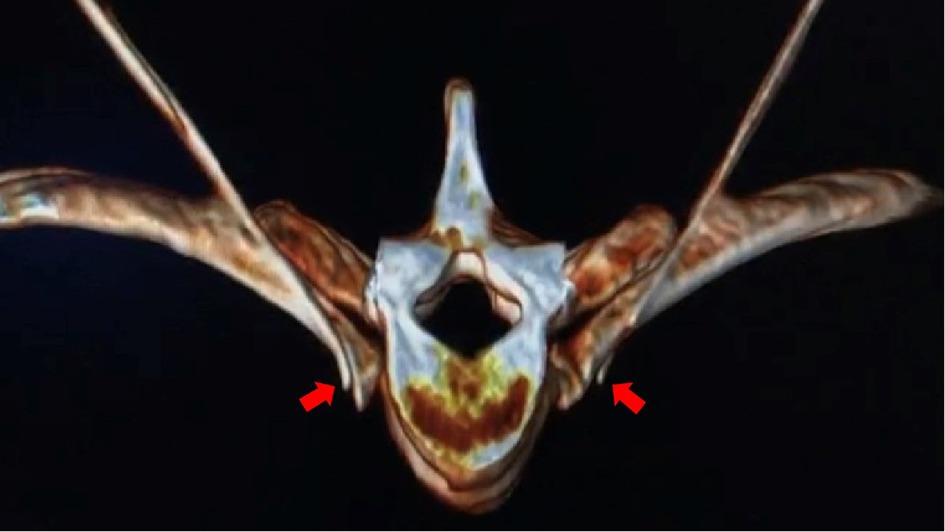
Figure 5. After the three-dimensional reconstruction of CT, the needle tips on both sides reach the anterior superior edge of the 4th rib head and the lateral wall of the T4 vertebral body. (indicated by the red arrow).
To evaluate the relief of hyperhidrosis in the hands of patients after the operation, observed the changes in palm temperature (T), perfusion index (PI), finger pulse oxygen saturation (SPO2), and heart rate (HR) before and after treatment, as well as any occurrences of local hematoma, spinal cord injury, vascular embolism, pneumothorax, Horner's syndrome, and other complications. We conducted telephone follow-ups on the 1st, 3rd, 6th, 12th, 24th, and 36th months after the operation. We evaluated the incidence of unrelated nerve injury (intercostal neuralgia), compensatory sweating (13), and the recurrence rate of hand sweating.
The degree of postoperative hyperhidrosis was scored 1–2 points based on the severity of the symptoms and the HDSS score scale, indicating a satisfactory curative effect. A postoperative recurrence was considered if one or both hands perspired again after the operation, and the HDSS score reached 3–4 points. We subsequently recorded the recurrence time.
Data were analyzed using SPSS 22.0 software. Measurement data were expressed as mean standard deviation (x ± s). The t-test was used to make a comparison between the two groups, and the rank sum test to compare the skewness distribution measurement data between the two groups. The rank data of different groups was expressed as a percentage and compared the average rank values between groups using the Mann-Whitney U test. Data were compared between the two groups using the χ2 test (Chi-squared test). PI, T, and SpO2 were corrected by repeated measurement ANOVA and the Greenhouse-Geisser method. The inspection level was set at P < 0.05.
Analysis of the revealed that age, sex, and BMI were not significantly different between the two groups (P > 0.05) (Table 1).
The study involved 69 patients (138 sides), all of whom underwent either chemical damage to the sympathetic nerve chain or radiofrequency thermocoagulation with successful outcomes. Immediately following the procedure, all patients' hands exhibited dryness, and no significant complications such as hematoma, vascular embolism, or paraplegia were observed. During the operation, three patients experienced a decrease in blood pressure and HR, but they immediately recovered after using an atropine needle.
In group A, CT scans during the operation revealed unilateral drug infiltration into the cervical sympathetic ganglia at the head in four cases, and a slight Horner's syndrome was observed. However, the syndrome disappeared within 30 min after injecting 5 ml of normal saline into the stellate ganglion (14). In group B, CT scans after a radiofrequency operation revealed a small amount of pneumothorax in one patient who had no complaints. The pneumothorax did not worsen after the patient stayed in bed for 24 h and took oxygen.
There was no significant difference in HR and SpO2 between the two groups before and after the operation (P > 0.05). After the operation, both groups showed a significantly higher palm T in both hands significantly increased compared to before the operation, with a statistically significant difference (P < 0.05). Additionally, both groups' peripheral PI of both hands significantly increased after surgery compared to before surgery (P < 0.05) (Tables 2–4).
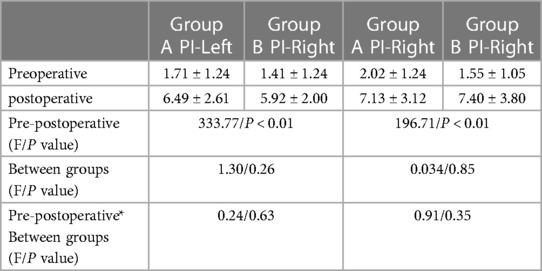
Table 3. Comparison of peripheral perfusion index (PI) before and after operation between the two groups.
During follow-up, one patient in group A was lost at 12 months, and one patient in group B was lost at 24 months after the operation. All other patients completed the follow-up for 36 months.
The recurrence rates of the two groups were compared at 1 and 3 months after the operation (5.88% vs. 2.86%, 20.59% vs. 5.71%), with no significant difference (P > 0.05). However, at 6, 12, 24, and 36 months (32.35% vs. 11.43%, 32.35% vs. 11.43%, 56.25% vs. 14.71%, 68.75% vs. 20.59%), there was a statistically significant difference (P < 0.05) (Table 5). The difference in intercostal neuralgia between the two groups (52.94% vs. 22.86%) was also statistically significant (P < 0.05). All patients with intercostal neuralgia had a pain score of NRS < 4, which had no significant impact on their lives, and they all recovered within 3 months of the operation. The incidence of postoperative compensatory sweating was 19 cases (55.88%) in group A and 8 cases (22.86%) in group B, which was statistically significant (P > 0.05). However, all cases of compensatory hyperhidrosis in the two groups were mild to moderate, and no severe compensatory sweating occurred (Table 6).
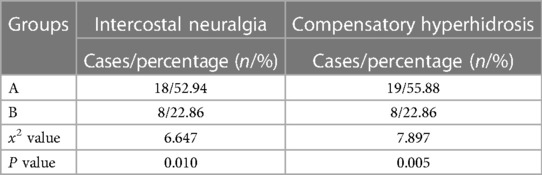
Table 6. Comparison of the incidence of intercostal neuralgia and compensatory hyperhidrosis between the two groups.
Currently, there are few effective treatments for PPH. The commonly used drugs include oral oxybutynin (15), local botulinum toxin injection (16, 17), local iontophoresis (18), local microneedle radiofrequency (19, 20), or other conservative treatments, but they have some limitations. Although ETS has high efficacy and long-term curative effects in the treatment of PPH and was once considered the “gold standard” treatment (4, 5, 21), its use is limited due to the high incidence of compensatory hyperhidrosis, which can be as high as 78% (5, 22). Compensatory hyperhidrosis is a significant problem for both doctors and patients following ETS since there is currently no effective treatment. Although some scholars claim that comprehensive bilateral thoracic sympathetic nerve chain resection performed in one sitting may be efficacious, the long-term impact of such extensive resection on human physiology remains unknown. Moreover, ETS requires general anesthesia and alternate single lung collapse, which can result in complications such as atelectasis, pneumothorax, and other postoperative complications. Under the funding of the Zhejiang Medical and Health Platform's key project, we conducted research on the treatment technology of CT-guided absolute alcohol chemical destructive block of the thoracic sympathetic nerve chain to explore a new treatment technology for palmar hyperhidrosis (6–8). This technique has been effectively utilized for managing palmar hyperhidrosis, cephalic hyperhidrosis, axillary hyperhidrosis, perineal hyperhidrosis of the lower limbs, and Raynaud's syndrome (6–9, 23, 24), as well as a combined thoracic and lumbar sympathetic nerve block for palmar and foot hyperhidrosis (25, 26). Resen et al. conducted a study on the effectiveness of CT-guided chemical destruction of the thoracic sympathetic nerve chain with absolute alcohol in treating palmar hyperhidrosis. The study included 86 cases and the results showed that the short-term curative effect was observed to be 100% (27), and the curative effect is comparable to ETS within one year but with fewer complications (28). However, although absolute alcohol chemical damage block has high immediate efficiency, the time effect is far less lasting than ETS, with a 3 year recurrence rate as high as 68.75% and a 55.88% incidence of postoperative compensatory hyperhidrosis. The liquid medication exhibits limited controllability and can easily spread along the pleura, leading to alcoholic aseptic inflammation of the affected intercostal nerve. This may result in intercostal nerve pain that can last for a duration of 1–3 months. Horner's syndrome (14) can also occur if it flows upward to the level of the pleura crest. If the injected absolute alcohol accidentally enters the subarachnoid space or causes a spinal root artery embolism, it can cause serious complications such as paraplegia (29). With the support of Jiaxing's special funds for important scientific research, our focus was redirected toward radiofrequency thermocoagulation and its corresponding physical damage to the sympathetic nerve chain (9). In a meta-analysis conducted by Hasimoto et al. (30), nine studies on the radiofrequency treatment of palmar hyperhidrosis with the thoracic sympathetic nerve chain were analyzed. The results revealed that radiofrequency thermocoagulation therapy was found to have a short-term curative effect that was comparable to ETS. Although the long-term recurrence rate is slightly higher than that of ETS, the incidence of compensatory hyperhidrosis of radiofrequency therapy is significantly lower than that of ETS. According to Andrade et al., percutaneous radiofrequency ablation of the thoracic sympathetic nerve chain is a potentially feasible and safe method to treat palmar hyperhidrosis (31). Using relatively rough C-arm x-ray guidance to perform radiofrequency thermocoagulation of the thoracic sympathetic nerve chain to treat palmar hyperhidrosis has a better curative effect than local carnitine injection, as stated by Mostafa et al. (31). In this study, our results showed that both the immediate effectiveness rate of absolute alcohol damage block of the thoracic sympathetic chain and radiofrequency can achieve a 100% success rate. There was no significant difference in the recurrence rate between the two groups at 1 month and 3 months after operation (P > 0.05). In contrast, the long-term recurrence rate of the latter is markedly lower compared with that of the former. The incidence of intercostal neuralgia and hypobaric hyperhidrosis after surgery was significantly lower than that of the former. The reason for the regenerative and resurrective properties of the sympathetic nerve chain may be attributed to the relatively low cytotoxicity of absolute alcohol towards the nerve tissue. Palmar hyperhidrosis was difficult to recur after radiofrequency because the nerve chain was completely physically damaged and lost its ability to regenerate and revive. According to Garca-Barqun et al.,139 cases of palmar hyperhidrosis were treated with bilateral T2, T3, and T4 thoracic sympathetic nerve chains at 90°C for 8 min (32). The remission rate after one month was only 77.38%, significantly lower than the remission rate after radiofrequency treatment of only one segment (T4) in this group. Following meticulous examination of the described method, it was ascertained that the radiofrequency needle failed to accurately target the anterior superior edge of the small head of the ribs. Instead, it inadvertently penetrated the paravertebral space situated external to the sympathetic nerve chain within the body (Figure 6). Therefore, even more, RF time and RF segments are insufficient to achieve the desired effect. The therapeutic target is located at the anterior superior edge of the capitulum in the fourth rib, and precise adherence of the radiofrequency puncture needle to the parietal pleura is necessary to avoid the potential risk of pneumothorax resulting from accidental pleural puncture. To address this concern, we developed and manufactured a unique puncture needle for thoracic sympathetic nerve radiofrequency. The needle has a depth scale on the needle body and a blunt tip, reducing the occurrence of pneumothorax complications (33).
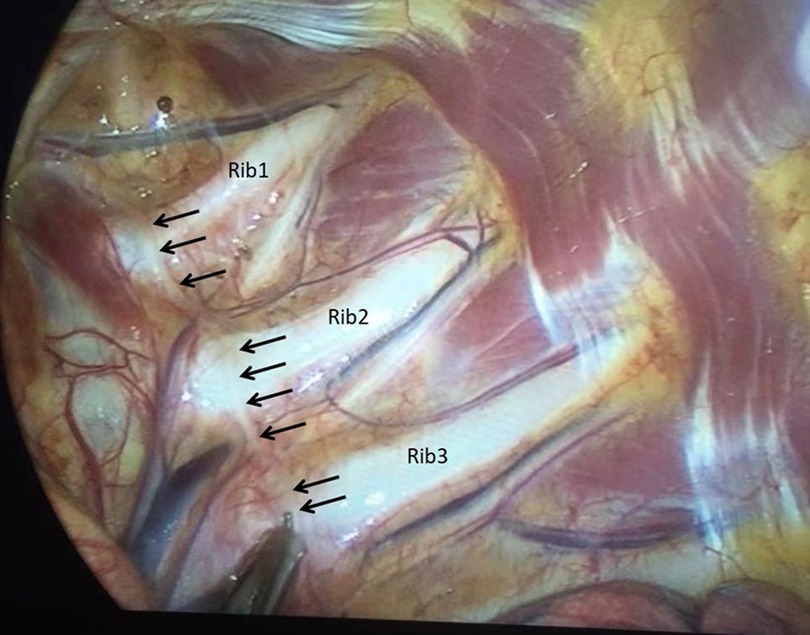
Figure 6. The thoracic sympathetic nerve chain is observed to cling to the pleura in front of the small head of ribs (indicated by the black arrow), rather than in the paraspinal space.
In conclusion, our study suggests that CT-guided percutaneous thoracic sympathetic nerve chain nerve block with absolute alcohol and radiofrequency thermocoagulation are both effective treatments for PPH. However, radiofrequency thermocoagulation appears to be more durable, with a lower recurrence rate and fewer complications such as intercostal neuralgia and compensatory sweating. These findings highlight the potential of radiofrequency thermocoagulation as a safe and effective treatment option for PPH.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the First Hospital of Jiaxing(the Affiliated Hospital of Jiaxing University) (Approval number: ls2018-141). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors met the ICMJE guidelines for contribution, and read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by funding from the key project of the Zhejiang Medical and Health Platform (2012ZDA043); Pain medicine (2019-ss-ttyx), a key discipline jointly established by Zhejiang Province; and Jiaxing Key Science and Technology Project (2020AY30011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nawrocki S, Cha J. The etiology, diagnosis and management of hyperhidrosis: a comprehensive review. Part II. Therapeutic options. J Am Acad Dermatol. (2019) 81(3):669–80. doi: 10.1016/j.jaad.2018.11.066
2. Lai F, Tu Y, Li Y, Li X, Lin M, Chen J, et al. Nationwide epidemiological surver of primary palmar hyperhidrosis in the people’s republic of China. Clin Auton Res. (2015) 25:105–8. doi: 10.1007/s10286-014-0259-5
3. Vorkamp T, Foo FJ, Khan S, Schmitto J, Wilson P. Hyperhidrosis: evolving concepts and a comprehensive review. Surgeon. (2010) 8(5):287–92. doi: 10.1016/j.surge.2010.06.002
4. Xie H, Lu T, Zhu L, Zhu D, Wei T, Yuan G, et al. A retrospective cohort study of T3 versus T4 thoracoscopic sympathectomy for primary palmar hyperhidrosis and primary palmar hyperhidrosis with axillary and plantar sweating. Videosurgery Miniinv Techniques. (2020) 15(3):488–95. doi: 10.5114/wiitm.2019.89656
5. Chen J, Liu Y, Yang J, Hu J, Peng J, Gu L, et al. Endoscopic thoracic sympathicotomy for primary palmar hyperhidrosis: a retrospective multicenter study in China. Surgery. (2019) 166(6):1092–8. doi: 10.1016/j.surg.2019.05.039
6. Huang B, Yao M, Zhou X, Cao H, Zhu Z, Hou J, et al. Clinical effect of CT-guided percutaneous thoracic sympathectomy for palmar hyperhidrosis. Chin J Med. (2011) 91:2710–3. doi: 10.3760/cma.j.issn.0376-2491.2011.38.015
7. Huang B, Sun K, Zhu Z, Zhou C, Wu Y, Zhang F, et al. Oximetry-derived perfusion index as an early indicator of CT-guided thoracic sympathetic blockade in palmar hyperhidrosis. Clin Radiol. (2013) 68(12):1227–32. doi: 10.1016/j.crad.2013.07.003
8. Guo JG, Fei Y, Huang B, Yao M. CT-guided thoracic sympathetic blockade for palmar hyperhidrosis: immediate results and postoperative quality of life. J Clin Neurosci. (2016) 34:89–93. doi: 10.1016/j.jocn.2016.05.031
9. Huang H, Qiu W, Chen Q, Sun K, Huang B. Computed tomography (CT)-guided percutaneous thoracic sympathetic chain radiofrequency thermocoagulation for raynaud disease. Med Sci Monit. (2019) 25:7391–5. doi: 10.12659/MSM.917392
10. Hornberger J, Grimes K, Naumann M, Glaser DA, Lowe NJ, Naver H, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. (2004) 51(2):274–86. doi: 10.1016/j.jaad.2003.12.029
11. Henning MAS, Thorlacius L, Ibler KS, Jemec GBE. How to diagnose and measure primary hyperhidrosis: a systematic review of the literature. Clin Auton Res. (2021) 31(4):673–9. doi: 10.1007/s10286-021-00794-6
12. Kirsch BM, Burke L, Hobart J, Angulo D, Walker PS. The hyperhidrosis disease severity measure-axillary: conceptualization and development of item content. J Drugs Dermatol. (2018) 17(7):707–14.30005091
13. Cai SW, Huang SH, An J, Li Y, Weng Y, Liao H, et al. Effect of lowering or restricting sympathectomy levels on compensatory sweating. Clin Auton Res. (2014) 24(3):143–9. doi: 10.1007/s10286-014-0242-1
14. Liu Q, Huang B, Chen Y, Yao M, Zhang L, Fei Y, et al. Effect of thoracic sympathetic modulation on Horner’s syndrome in the treatment of cephalic sweating. Analysis. Chin Med J. (2017) 97:3624–7. doi: 10.3760/cma.j.issn.0376-2491,2017.46.005
15. Millán-Cayetano JF, Del Boz J, Rivas-Ruiz F, Blázquez-Sánchez N, Hernández Ibáñez C, de Troya-Martín M. Oral oxybutynin for the treatment of hyperhidrosis: outcomes after one-year follow-up. Australas J Dermatol. (2017) 58(2):31–5. doi: 10.1111/ajd.12473
16. Nawrocki S, Cha J. Botulinum toxin: pharmacology and injectable administration for the treatment of primary hyperhidrosis. J Am Acad Dermatol. (2020) 82(4):969–79. doi: 10.1016/j.jaad.2019.11.042
17. Farrell J, Stewart T, Singh B, Singh G, Rosen R. Retrospective analysis of the efficacy and duration of botulinumtoxin A injections in 30 patients with palmar hyperhidrosis. Intern Med J. (2021) 51:1517–21. doi: 10.1111/imj.15489
18. Kim DH, Kim TH, Lee SH, Lee AY. Treatment of palmar hyperhidrosis with tap water iontophoresis: a randomized, sham-controlled, single-blind, and parallel-designed clinical trial. Ann Dermatol. (2017) 29(6):728–34. doi: 10.5021/ad.2017.29.6.728
19. Abtahi-Naeini B, Naeini FF, Saffaei A, Behfar S, Pourazizi M, Mirmohammadkhani M, et al. Treatment of primary axillary hyperhidrosis by fractional microneedle radiofrequency: is it still effective after long-term follow-up? Indian J Dermatol. (2016) 61(2):234–9. doi: 10.4103/0019-5154.177789
20. Cohen SR, Goodacre AK, Leong TS, Southwell L, Nomachi T. Short-term clinical outcomes and safety associated with percutaneous radiofrequency treatment for excessive sweating. Aesthet Surg J. (2019) 39(12):1390–9. doi: 10.1093/asj/sjy277
21. Toolabi K, Parsaei R, Farid R, Zamanian A. Endoscopic thoracic sympathotomy for primary hyperhidrosis: predictors of outcome over a 10- year period. Surg Endosc. (2021) 36(5):3585–91. doi: 10.1007/s00464-021-08684-8
22. WoloskerI N, Kauffman P, Silva M, da Silva MFA, Faustino CB, Tedde ML, et al. Cohort study on 20 years’ experience of bilateral videoassisted thoracic sympathectomy (VATS) for treatment of hyperhidrosisin 2431 patients. Sao Paulo Med J. (2022) 140:284–9. doi: 10.1590/1516-3180.2021.0078.r1.23072021
23. Lu Y, Huang B, Sun J, Yao M, Hu Y, Zhou X, et al. CT-guided percutaneous puncture of thoracic sympathetic nerve for the treatment of head and face hyperhidrosis. Chin J Neurosurg. (2013) 29:520–3. doi: 10.3760/cma.j.issn.1001-2346,2013.05.027
24. Hou J, Huang B, Yao M, Wang H, Deng F, Zhou X, et al. CT-guided percutaneous thoracic sympathectomy for Raynaud’s syndrome. Chin J Neurosurg. (2012) 28:197–9. doi: 10.3760/cma.j.issn.1001-2346,2012.02.028
25. Huang B, Guo J, Ren X, Yao M, Zhang L, Fei Y, et al. CT-guided combined thoracic and lumbar sympathectomy for hyperhidrosis of hands and feet. Chin J Neuromed. (2015) 32:1055–8. doi: 10.3760/cma.j.issn.1671-8925.2015.10.017
26. Liu M, Ni H, Tao J, Xie K. Lumbar sympathetic nerve modulation using absolute ethanol for the treatment of primary lower-extremity hyperhidrosis: a dose-effect pilot study. Med Sci Monit. (2021) 27:e928209. doi: 10.12659/MSM.928209
27. Andresen JR, Scheer F, Schlöricke E, Andresen R. CT-assisted thoracic sympathicolysis for therapy of primary hyperhidrosis palmaris-retrospective analysis of the influence of the amount and position of the sympathetic agent on the therapeutic outcome and side effects. Fortschr Röntgenstr. (2021) 193(5):574–81. doi: 10.1055/a-1299-2098
28. Andresen JR, Scheer F, Schlöricke E, Sallakhi A, Liedke MO, Andresen R. CT-guided thoracic sympathicolysis versus VATS sympathectomy in the therapeutic concept for severe primary palmar hyperhidrosis. Thorac Cardiovasc Surg. (2022) 70(2):152–8. doi: 10.1055/s-0041-1725205
29. Elizondo ME, Linazasoro CG. Reversible partial paraplegia after sympathetic lumbar block. Neurologia. (1995) 10(2):101–3.7695936
30. Hasimoto FN, Cataneo DC, Hasimoto EN, Ximenes AMG, Cataneo AJM. Radiofrequency in the treatment of primary hyperhidrosis: systematic review and meta-analysis. Clin Auton Res. (2020) 30(2):111–20. doi: 10.1007/s10286-019-00640-w
31. Mostafa TAH, Hamed AA, Mohammed BM, El Sheikh NA, Shama AAA. C-Arm guided percutaneous radiofrequency thoracic sympathectomy for treatment of primary palmar hyperhidrosis in comparison with local botulinum toxin type A injection, randomized trial. Pain Physician. (2019) 22(6):591–9. doi: 10.36076/ppj/2019.22.591
32. García-Barquín P, Aquerreta Beola JD, Bondía Gracía JM, España Alonso A, Pérez Cajaraville J, Bartolomé Leal P, et al. Percutaneous CT guided sympathicolysis with radiofrequency for the treatment of palmar hyperhidrosis. J Vasc Interv Radiol. (2017) 28(6):877–85. doi: 10.1016/j.jvir.2017.02.025
Keywords: hyperhidrosis, palmar, sympathetic, nerve block, autonomic nerve block, radiofrequency therapy
Citation: Zhang L, Xu S-s, Liu X-l, Zhao W, Ma Y and Huang B (2023) Comparison of CT-guided thoracic sympathetic nerve block and radiofrequency in the treatment of primary palmar hyperhidrosis. Front. Surg. 10:1126596. doi: 10.3389/fsurg.2023.1126596
Received: 18 December 2022; Accepted: 16 May 2023;
Published: 31 May 2023.
Edited by:
Tsung-Hsi Tu, Taipei Veterans General Hospital, TaiwanReviewed by:
Ilker Sengul, Giresun University, Türkiye© 2023 Zhang, Xu, Liu, Zhao, Ma and Haung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Huang MDAxODE3MjdAemp4dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.