- 1School of Medicine, Nankai University, Tianjin, China
- 2Graduate School of Tianjin Medical University, Tianjin, China
- 3Department of Spinal Surgery, Tian-jin Union Medical Centre, Nankai University People's Hospital, Tianjin, China
- 4Department of Respiratory Medicine, Tian-jin Union Medical Centre, Nankai University People's Hospital, Tianjin, China
Objective: Patients with osteoporotic vertebral fractures (OVFs) often suffer from residual low back pain (LBP) after percutaneous kyphoplasty (PKP). The purpose of this study was to identify risk factors for postoperative residual LBP and to develop a nomogram to predict the occurrence of residual LBP.
Methods: We retrospectively reviewed 236 patients who underwent PKP for OVFs and had a minimum follow-up of 12 months. The mean age was 72.1 ± 6.3, 74.3% were female and 25.7% were male. Patients with LBP VAS scores ≥ 3.5 at the 12th month postoperatively were considered to have residual LBP. Risk factors for residual LBP were identified by univariate and multifactorial logistic regression analysis. Then, a predictive nomogram was constructed and validated using the bootstrap method. The discrimination, calibration, and clinical utility of the nomogram were assessed using a receiver operating characteristic curve (ROC), a calibration curve, and a decision curve analysis (DCA).
Results: univariate and multifactorial logistic regression analysis identified depression (P = 0.02), intravertebral vacuum cleft (P = 0.01), no anti-osteoporosis treatment (P < 0.001), cement volume <3 ml (P = 0.02), and cement distrubution (P = 0.01) as independent risk factors for residual LBP. The area under the ROC was 0.83 (0.74–0.93) and further validated by bootstrap method was 0.83 (0.73–0.92). The calibration curve illustrated the consistency between the predicted probability and the observed results. DCA showed that nomogram exhibits clinical utility and net benefit when the threshold probability is between 6% and 73%.
Conclusions: Our study found that depression, intravertebral vacuum cleft, no anti-osteoporosis treatment, cement volume <3 ml and cement distribution represent independent risk factors for residual LBP. The nomogram containing the above five predictors can accurately predict the risk of residual LBP after surgery.
Introduction
Osteoporotic vertebral fractures (OVFs) result from decreased bone density and bone strength (1). OVFs mainly cause chronic and persistent low back pain, secondary kyphosis, and even cardiopulmonary complications, which seriously affect the quality of life of middle-aged and older adults (2, 3). Percutaneous balloon kyphoplasty (PKP) is performed by expanding the compressed vertebral body with a balloon and injecting polymethyl methacrylate (PMMA) bone cement into the expanded cavity. This method can provide pain alleviation and kyphosis correction and is considered to be the current preferred minimally invasive surgical treatment (4). However, some patients still have persistent moderate to severe low back pain after PKP with an incidence ranging from 1.8% to 15.6% (5, 6). The presence of postoperative residual low back pain may weaken the outcomes of the PKP procedure, decrease the patients' quality of life and increase their financial burden. Therefore, it is crucial to explore the baseline risk factors for residual LBP in patients with OVFs following PKP.
Studies have examined various potential risk factors for residual LBP, such as bone mineral density, intravertebral vacuum cleft, posterior fascia edema, fracture of adjacent vertebral bodies, and vertebral compression ratio (5, 7, 8). However, preoperative mental status and intraoperative factors, such as cement volume and distribution, were not analyzed as risk factors (9, 10). More importantly, shorter follow-up periods (1 month) and higher pain thresholds (VAS ≥ 4) may not accurately identify patients with residual LBP (10) because we cannot be certain whether early postoperative residual pain will be resolved spontaneously during long-term follow-up. Therefore, a predictive model based on long-term follow-up and including mental status and intraoperative factors should be developed to guide therapeutic interventions for residual LBP.
Our study analyzed multiple baseline factors associated with residual LBP after PKP in OVFs. Furthermore, we developed and validated a nomogram to provide individualized guidance for the treatment of residual LBP.
Methods
Patients
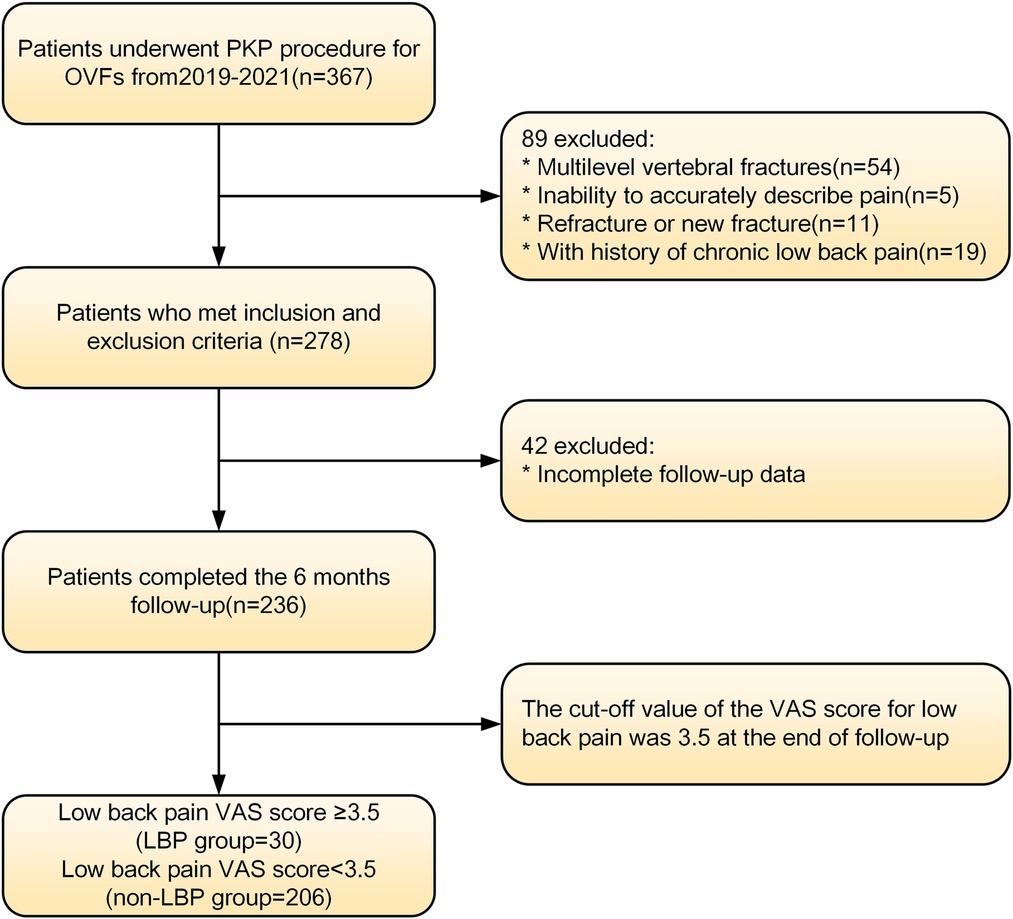
We prospectively collected data from patients treated with PKP for osteoporotic vertebral fractures between May 2019 and June 2021 at a hospital in Tianjin, China. These patients were analyzed retrospectively. The details of the inclusion and exclusion criteria were as follows. Inclusion criteria: (1) The patient had a clear history of osteoporosis or was diagnosed with osteoporosis by DXA. (2) Vertebral fractures caused by low-energy injuries and further confirmed by spinal MRI (11). (3) Only patients with single-segment vertebral fractures were included in this study. (4) The patients had obvious low back pain symptoms because of the vertebral fractures (VAS > 3.5). (5) Patients were treated with PKP rather than conservative or other treatments. (6) Information on the clinical and demographic characteristics required for the study was complete and accessible. The exclusion criteria were as follows: (1) Pathological fractures caused by tumors, infections, tuberculosis or other diseases. (2) Spinal cord compression and obvious neural symptoms, such as numbness and/or muscle weakness. (3) Patients had chronic low back pain(VAS > 3.5) prior to the fractures, either due to osteoporosis, degenerative kyphosis or scoliosis, idiopathic pain, previous back surgery, or other diseases. (4) Patients with cognitive disorders who could not communicate independently. Figure 1 shows the screening process for enrolling patients. This study was approved by the Medical Ethics Committee of the Tianjin Union Medical Centre and conducted in accordance with the Declaration of Helsinki. Informed consent was waived because this is a retrospective study that does not involve personal privacy or commercial interests.
Surgical technique
The patient was placed in a prone position, raised to make the abdomen hang, and routinely sterilized. The C-arm machine was used to locate the fractured vertebrae following local anesthesia or general anesthesia. Using pedicle approach, the puncture probe was inserted into the vertebrae. The tip in the lateral fluoroscopy was located in the front 1/3 of the vertebral body and close to or exceeded the midline of the spinous process in the front fluoroscopy. Then, the tube core of the guide needle was removed, and the working channel was established. The inflatable balloon was placed into the working channel, and the contrast medium was slowly injected into the balloon. The balloon was expanded to reposition the vertebral body. The vertebral body was filled with bone cement under continuous fluoroscopic guidance to eliminate the expansion gap. The procedure should be stopped immediately in cases with bone cement leakage. After curing the bone cement, the working channel was withdrawn. Then, the skin incision was sutured, and the cells were observed for 10–20 min. Radiographs and computed tomography (CT) scans of the spine were taken for all patients on the first postsurgical day. All patients were discharged 2 to 3 days after surgery.
Identification of residual low back pain
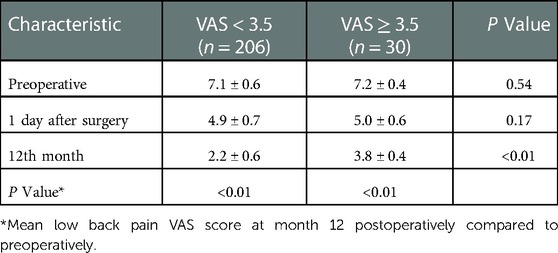
A visual analog scale was used to evaluate patients' low back pain intensity at the 12th month. Patients' low back pain intensity is the average intensity for the month. A VAS score <3.5 corresponds to mild pain, whereas a VAS score ≥3.5 corresponds to moderate to severe pain and is considered to indicate residual low back pain (12). Spine MRI should be performed at any time to exclude the suspicion of a refracture or a new fracture at another level.
Data collection
Baseline risk factors were extracted from the medical records, operative records, radiological image management system, and questionnaire surveys. Two independent spinal surgeons were involved in the radiographic evaluation. When a disagreement occurred between the surgeons, a consensus meeting was held.
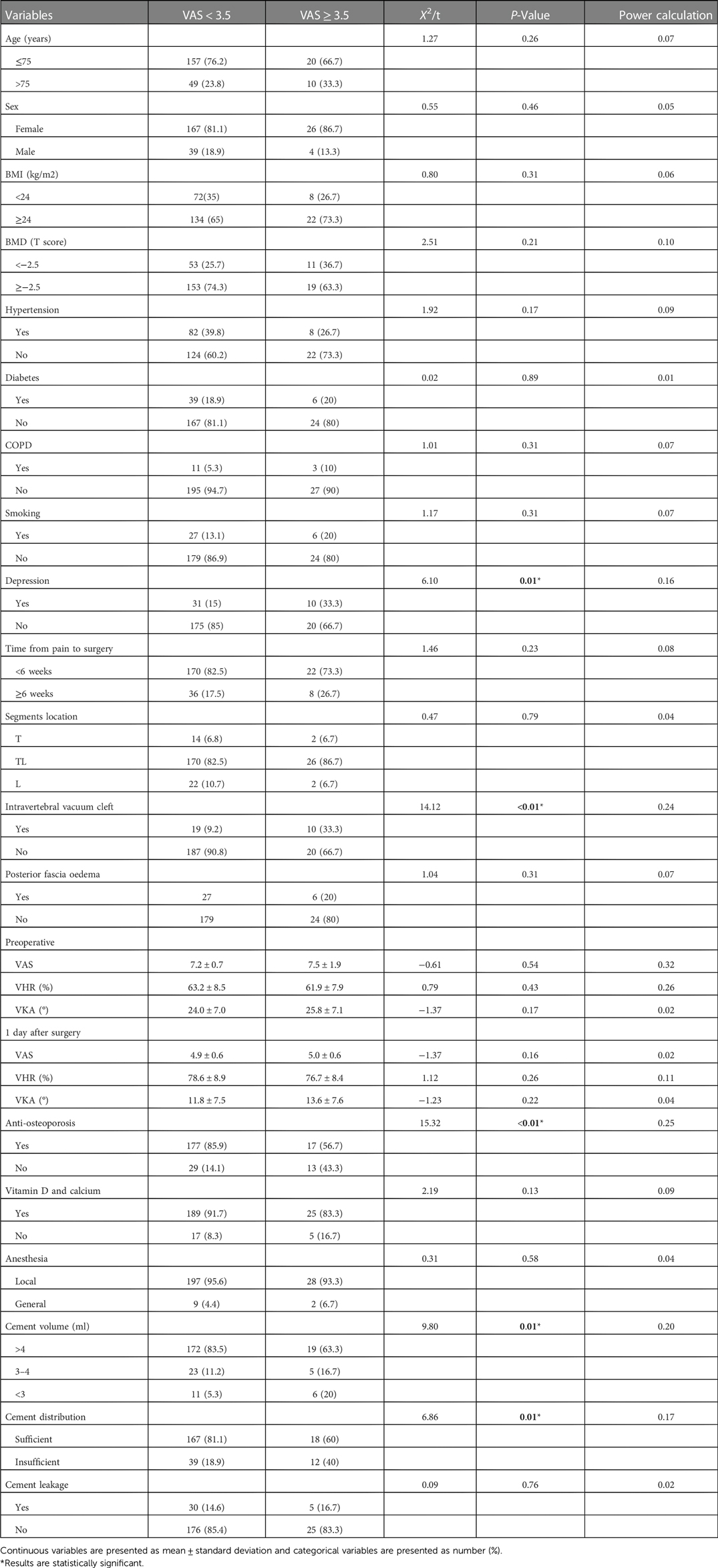
Baseline demographic characteristics included age, sex, body mass index (BMI), bone mineral density (BMD), comorbidities [hypertension, diabetes, and chronic obstructive pulmonary disease (COPD)], smoking, and depression. The depression status of patients was determined based on the mental component summary of the Short Form-36 (SF-36 MCS) (13). SF-36 MCS > 50 indicates depression; otherwise, depression is not noted.
The following baseline clinical and radiological factors were assessed: (1) Time from pain to surgery; (2) Segment location—thoracic spine (T4–T9), thoracolumbar spine (T10-L2), and lumbar spine (L3–L5); (3) VAS score for low back pain; (4) Vertebral height ratio (VHR) and vertebral kyphosis angle (VKA)—The vertebral height ratio is the ratio of the anterior height of the fractured vertebral body to the average anterior height of the adjacent upper and lower segments (Figure 2), and the vertebral kyphosis angle is the angle between the upper and lower endplates of a fractured vertebral body (Figure 2); (5) Anti-osteoporosis therapy—there are two anti-osteoporosis protocols, intravenous zoledronic acid (5 mg/year) or oral alendronate sodium (70 mg/week). Given compliance issues with alendronate administration, we considered patients who had been taking alendronate for less than 6 months as not receiving regular anti-osteoporosis treatment; (6) Vitamin D (800–1,200 IU/d) and calcium supplements (1000 mg/d)—Given issues with medication adherence, we considered patients who did not receive or those who received vitamin D and calcium for less than 6 months as not receiving a standardized anti-osteoporosis basal supplement. (7) Intravertebral vacuum cleft (IVC; Figure 3); (8) Posterior fascia edema (Figure 4).
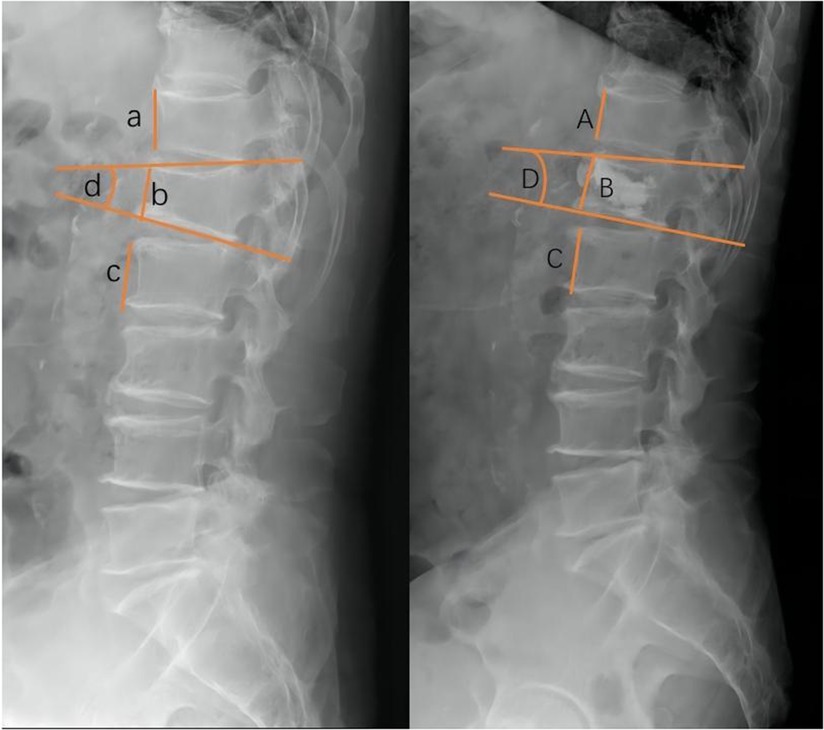
Figure 2. Radiographic evaluation of compressed vertebrae. Preoperative VHR (left,%):2b/a + c, VKA (left,°):∠d; Postoperative VHR (right,%):2B/A + C, VKA (right,°):∠D.
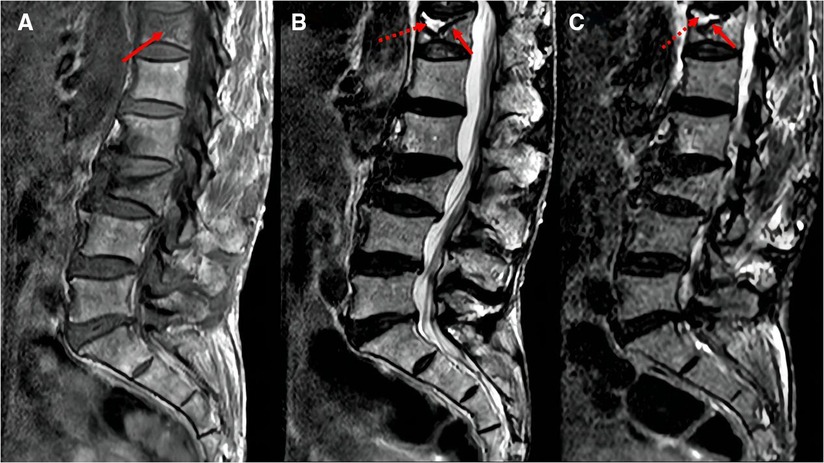
Figure 3. MRI images of intravertebral vacuum cleft. Hypointense on T1-weighted images (A, arrows); hyperintensity or hypointensity on T2-weighted images (B) or STIR images (C), depending on fluid (dotted arrows) or gas (solid arrows) filling.
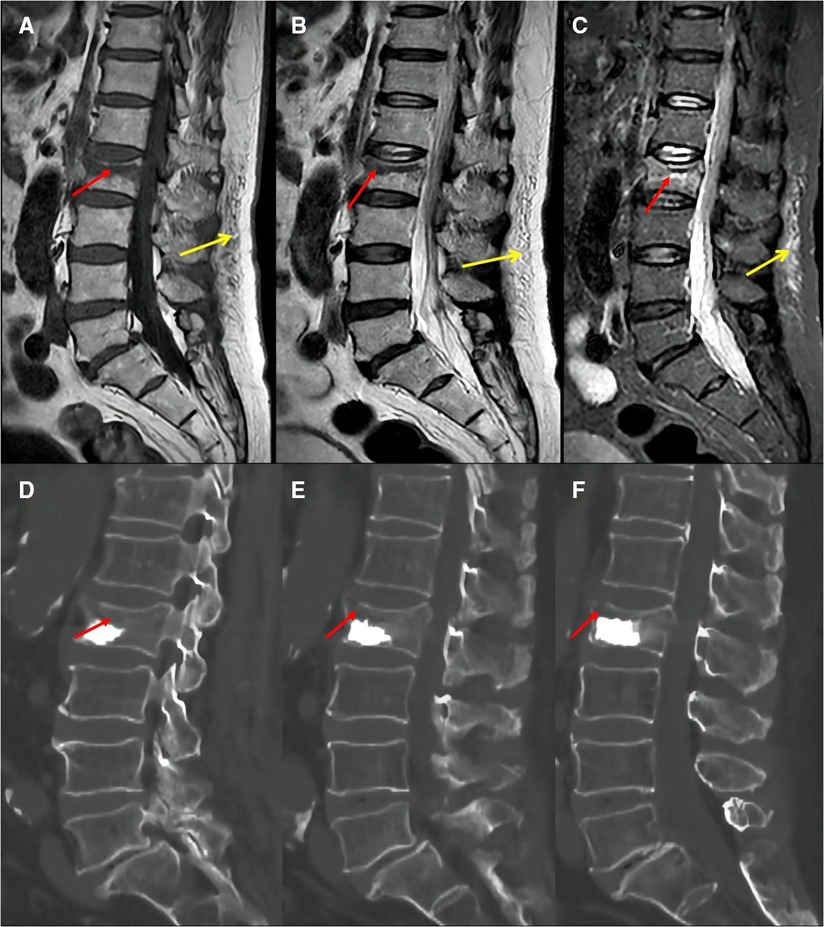
Figure 4. Preoperative MRI images showed that the fracture area was located in the upper third of the vertebral body (A: T1, B: T2, C: STIR, red arrow); postoperative CT images showed insufficient cement filling in the vertebral fractured area (D: left in median sagittal section; E: median sagittal section; F: right in median sagittal section, red arrow). Posterior fascia edema:hypointense on T1-weighted images (A, yellow arrow); hyperintensity on T2-weighted images (B, yellow arrow) or STIR images (C, yellow arrow).
Intraoperative factors: (1) Anesthesia—local or general anesthesia; (2) Cement volume; (3) Cement distribution—Cement distribution was classified into two states of sufficient and insufficient based on whether the cement filled the majority of the fractured area of the vertebrae (Figure 4); (4) Cement leakage.
Statistical analysis
Univariate analysis was conducted to determine whether these factors were associated with residual LBP. The normal distribution of continuous data was assessed using the Shapiro‒Wilk test, and the between-group differences were evaluated using the Student's t test or the Mann‒Whitney U test according to the data distribution characteristics. Chi-squared or Fisher's exact test was used to investigate differences in categorical data between the two groups. Variables with P value less than 0.05 in the univariate analysis were included in further multifactorial analyses to identify independent risk factors. The nomogram was then developed using independent risk factors. SPSS 26 software (IBM, USA) was used for data analysis, and R software (version 4.1.1, Foundation for Statistical Computing, Vienna, Austria) was utilized for nomogram construction. The predictive ability and performance of the model were assessed based on the receiver operating characteristic (ROC) curve, the area under the ROC curve (AUC), the calibration curve, and decision curve analysis (DCA). In general, the AUC values between 0.5 and 1.0 indicate that the model can accurately predict and discriminate (14). The calibration curve was used to assess the consistency between the actual diagnosis of LBP and the projected likelihood of LBP. Furthermore, by estimating the net benefit and threshold probabilities of the nomogram, DCA was used to evaluate its usefulness in clinical practice. Finally, we performed internal validation using 1,000 bootstrap samples to assess the stability of the prognostic nomogram.
Results
A total of 236 patients were enrolled in this study and completed the 12-month follow-up according to the inclusion and exclusion criteria. Thirty patients were included in the LBP group (VAS ≥ 3.5), and the incidence of residual LBP following PKP was 12.7%. Another 206 patients with a low back pain VAS score <3.5 were included as the non-LBP group. Both groups experienced significant improvement in VAS scores compared with the preoperative scores at the end of follow-up (P < 0.01), but significant differences existed between the two groups (P < 0.01), as shown in Table 1.
Univariate and multivariate analysis
Univariate analysis results of baseline demographic characteristics, baseline clinical and radiological factors, and intraoperative factors are shown in Tables 2. The two groups differed significantly in terms of depression (P = 0.01), intravertebral vacuum cleft (P < 0.01), anti-osteoporosis treatment (P < 0.01), cement volume (P = 0.01), and cement distribution (P = 0.01). None of the other variables differed significantly between the two groups. Multivariate analysis indicates that depression (OR = 3.56, 95% CI: 1.19–10.64, P = 0.02), intravertebral vacuum cleft (OR = 4.41, 95% CI: 1.57–12.36, P = 0.01), no anti-osteoporosis treatment (OR = 9.63, 95% CI: 3.50–28.33, P < 0.01), insufficient cement distribution in the fractured area (OR = 3.66, 95% CI: 1.36–9.86, P = 0.01), and cement volume <3 ml (OR = 4.62, 95% CI: 1.26–16.91, P = 0.02) were independent risk factors for residual LBP, as shown in Table 3.
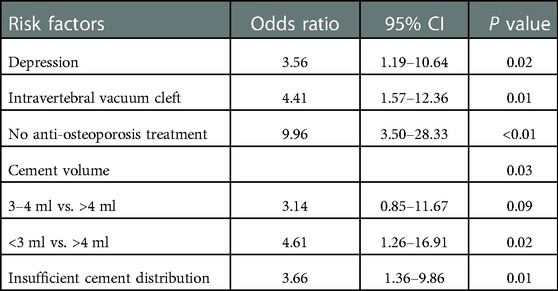
Table 3. Multivariate logistic regression analysis of independent risk factors for residual low back pain.
Development and validation of a nomogram for residual LBP
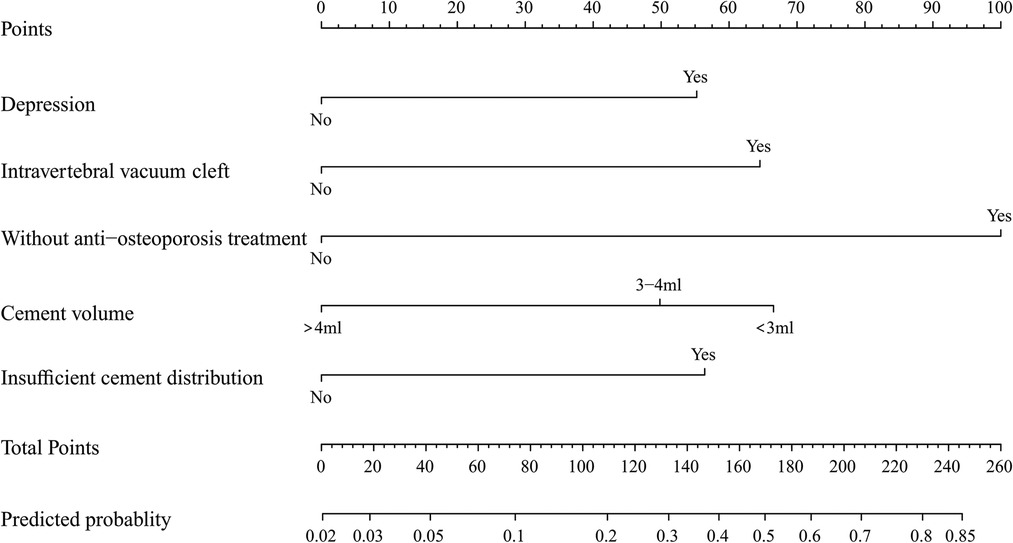
To predict residual LBP, we developed a nomogram using the predictive factors identified in multivariate analysis (Figure 5). The predictive score of each factor was added together in the nomogram to calculate the total score, from which the probability of residual LBP occurrence was calculated (Figure 5). The model has high predictive accuracy and discrimination with an AUC of 0.83 (0.74–0.93) and a corrected AUC of 0.83 (0.73–0.92) based on bootstrapping validation, as shown in Figure 6. Furthermore, the calibration curve of the nomogram demonstrated an excellent consistency between the actual diagnosis of residual LBP and the predicted likelihood (Figure 7). Similarly, the DCA curves indicate that the nomogram would provide a high net benefit when applied to the clinic (Figure 8).
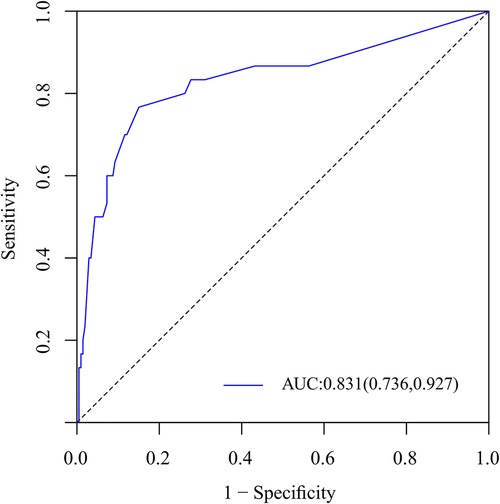
Figure 6. ROC curves of the nomogram for the assessment of capable of accurate prediction and discrimination.
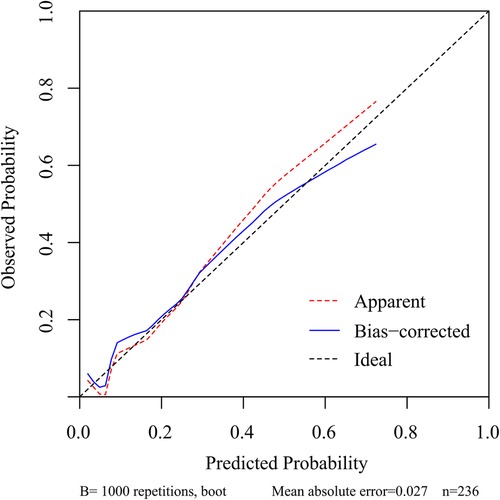
Figure 7. Calibration curve of the nomogram for the assessment of the consistency between the actual diagnosed residual LBP and the projected likelihood of residual LBP.
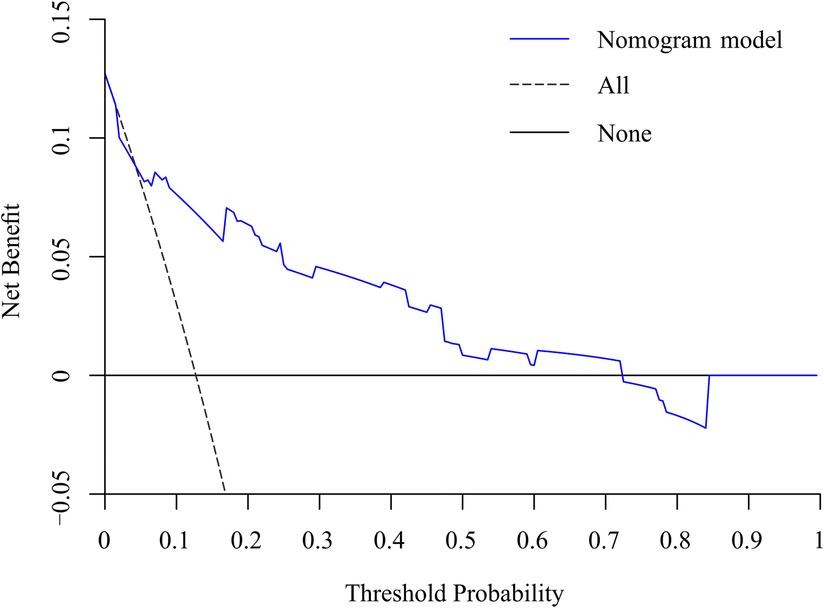
Figure 8. DCA curve of the nomogram for the assessment of clinical practice based on the net benefit and threshold probabilities.
Discussion
In this study, we analyzed 236 patients with osteoporotic vertebral fractures to identify baseline impact factors for postoperative residual LBP and developed and validated a predictive model to predict the risk of LBP occurrence following PKP. We found that 12.7% of patients experienced residual LBP postoperatively, which is similar to that reported in previous studies (5, 9). Multifactorial logistic regression analysis identified depression, intravertebral vacuum cleft, no anti-osteoporosis treatment, insufficient cement distribution in the fractured area, and cement volume <3 ml as key predictive factors related to residual LBP. The above factors were included in the nomogram.
Several studies have shown that depression can affect pain and function following surgery for musculoskeletal disorders (15, 16). The Mental Component Score (MCS) of the Short Form-36 (SF-36) has been used in numerous studies as an indicator of depression or depressive tendencies to predict surgical outcomes for lumbar degenerative diseases (17–19). It was found that patients with depressive symptoms based on their MCS experienced worse performance postoperatively in health-care-related quality of life (HRQOL) than patients without depression (18). Kimura et al. (13) found that the presence of depression (SF-36 MCS < 50) was associated with persistent neck pain following cervical laminoplasty and significantly worse functional outcomes. According to this study, depressed patients may experience residual low back pain after PKP. These results suggest that the presence of depression affects not only clinical outcomes following spinal fusion surgery but also those following percutaneous kyphoplasty.
The pathogenesis of the intravertebral vacuum cleft is unclear. Most studies (20, 21) tend to indicate avascular necrosis of trabecular bone. On T2-weighted or STIR images, IVC typically showed high or low signal intensity, depending on whether fluid or gas fills the cleft (22, 23). Many scholars agree that a strong correlation exists between IVC and residual low back pain, vertebral recollapse, and instability (24). A retrospective study of at least two years (25) found that low back pain VAS scores in patients with IVC were higher than those without IVC, and the height loss of the vertebral body in the IVC group was greater than that noted in the non-IVC group at the final follow-up. A meta-analysis by Yu et al. (26) also found that improvement in VAS back pain scores was limited in patients with IVC. Similar to these earlier studies, our results revealed that IVC is a vital contributor to residual low back pain.
The pathological basis of OVCF is osteoporosis, and PKP can help patients relieve pain and regain self-care early. However, the procedure only treats fractured vertebrae and has no therapeutic effect on systemic osteoporosis. Previous reports (27) have noted a 19.2% incidence of new vertebral fractures in the year following treatment in those who had vertebral fractures. Several studies (28, 29) have found that the application of bisphosphonate anti-osteoporosis therapy after PKP reduced vertebral refractures or new fractures and reduced low back pain VAS scores. During the follow-up period of our study, we identified refractures or new fractures using spinal MR images in combination with physical examination, but we lacked an effective means to identify microfractures of the vertebral body. More importantly, because patients can tolerate this low level of chronic low back pain, they do not go to the hospital for treatment. Several studies (30, 31) have shown that postoperative anti-osteoporotic treatment can prevent height loss after vertebral PKP, and we believe that this finding may serve as indirect evidence indicating that anti-osteoporotic treatment can reduce vertebral microfractures because the most visual manifestation of microfractures is vertebral height loss. Therefore, standardized anti-osteoporosis drug therapy should be actively taken after surgery to relieve pain, inhibit acute bone loss, increase bone strength, improve bone quality, and reduce fractures or new fractures.
Cement volume and cement distribution in the fractured area are independent predictors of residual low back pain that may be related to the inadequate restoration of vertebral stiffness and strength (32). With at least 6 months of follow-up, Christoph (33) found only a 40% chance of attaining a minimum 41-point pain alleviation when the cement volumes were less than 4.5 ml, and the most suitable fill volume for PKP seems to be between 4.5–7.5 ml. Fu et al. (34) demonstrated that cement dosage is positively correlated with pain alleviation. Therefore, the authors recommended that the vertebral body should be injected with as much cement as possible when performing the PKP procedure. More importantly, the cement volume can be changed intraoperatively. Sufficient bone cement diffusion in the fractured area after PKP stabilizes micromotion in the fracture area, limiting local nerve ending stimulation and relieving fracture-induced pain (35, 36). Furthermore, we hypothesize that inadequate cement volume and insufficient distribution of cement in the fractured area were associated with vertebral deformity in the coming later. Although there was no significant difference in vertebral body height or kyphosis angle between the LBP and non-LBP groups measured 1 day after surgery, inadequate recovery of vertebral body strength and stiffness resulting from inadequate cement volume or insufficient distribution in the fracture area may result in progressive vertebral kyphosis deformity over time. Multiple studies have demonstrated that the presence of vertebral kyphosis is a critical risk factor for long-term persistent low back pain after osteoporotic vertebral fractures (37, 38). Future prospective studies are needed to clarify the relationship between cement volume, cement distribution, and progressive vertebral kyphosis following PKP.
After analyzing potential risk factors, such as demographic characteristics, clinical and radiological factors, and intraoperative factors, we developed a nomogram for predicting residual LBP. Intraoperative interventions can be performed to modify these risk factors in patients, such as the amount and distribution of cement. Additionally, this study emphasizes the need for standardized anti-osteoporosis treatment for OVCF patients after PKP.
Our research also has some limitations. (1). Although we collected mean VAS scores for low back pain in patients at the 12th month postoperatively, it is still possible that this low back pain is incidental; therefore, studies with long follow-up periods and multiple follow-up nodes are needed to further validate the findings. (2). Patients have different ranges of pain tolerance, leading to overestimating or underestimating the residual LBP rate. (3). As this was a retrospective study, some confounding factors were unavoidable, such as the patient's preoperative low back muscle mass, daily activities that may contribute to the sensation of low back pain, comorbidity with other underlying conditions, differences in philosophy and technique between surgeons, and the patient's socioeconomic status. (4). We exclusively administered bisphosphonates for anti-osteoporosis treatment, and the effects anti-osteoporosis drugs with different mechanisms of action (e.g., denosumab, teriparatide, estrogen replacement therapy) on postoperative residual low back pain remain unclear. (5). This study is a single-center study from China, and future multicenter studies in different hospitals, regions, or countries are needed to further validate the findings of this study.
Conclusion
Our study found that depression, intravertebral vacuum cleft, no anti-osteoporosis treatment, insufficient cement distribution in the fractured area, and cement volume <3 ml are independent risk factors for residual LBP after PKP treated for OVFs. The nomogram containing the above five predictors can accurately predict the risk of residual LBP after surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Tianjin Union Medical Centre. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJXZDXK-064B)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. (2006) 17(12):1726–33. doi: 10.1007/s00198-006-0172-4
2. Hinde K, Maingard J, Hirsch JA, Phan K, Asadi H, Chandra RV. Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Radiology. (2020) 295(1):96–103. doi: 10.1148/radiol.2020191294
3. Luthman S, Widén J, Borgström F. Appropriateness criteria for treatment of osteoporotic vertebral compression fractures. Osteoporos Int. (2018) 29(4):793–804. doi: 10.1007/s00198-017-4348-x
4. Long Y, Yi W, Yang D. Advances in vertebral augmentation systems for osteoporotic vertebral compression fractures. Pain Res Manag. (2020) 2020:3947368. doi: 10.1155/2020/3947368
5. Lin CC, Shen WC, Lo YC, Liu YJ, Yu TC, Chen IH, et al. Recurrent pain after percutaneous vertebroplasty. AJR Am J Roentgenol. (2010) 194(5):1323–9. doi: 10.2214/AJR.09.3287
6. Yang JS, Liu JJ, Chu L, Li J, Chen C, Chen H, et al. Causes of residual back pain at early stage after percutaneous vertebroplasty: a retrospective analysis of 1,316 cases. Pain Physician. (2019) 22(5):E495–e503. doi: 10.36076/ppj/2019.22.E495
7. Dohm M, Black CM, Dacre A, Tillman JB, Fueredi G. A randomized trial comparing balloon kyphoplasty and vertebroplasty for vertebral compression fractures due to osteoporosis. AJNR Am J Neuroradiol. (2014) 35(12):2227–36. doi: 10.3174/ajnr.A4127
8. Li Y, Huang M, Chen J, Wu Y, Wang X. The impact of facet joint violation on clinical outcomes after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. World Neurosurg. (2018) 119:e383–8. doi: 10.1016/j.wneu.2018.07.170
9. Li Y, Yue J, Huang M, Lin J, Huang C, Chen J, et al. Risk factors for postoperative residual back pain after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Eur Spine J. (2020) 29(10):2568–75. doi: 10.1007/s00586-020-06493-6
10. Li Q, Shi L, Wang Y, Guan T, Jiang X, Guo D, et al. A nomogram for predicting the residual back pain after percutaneous vertebroplasty for osteoporotic vertebral compression fractures. Pain Res Manag. (2021) 2021:3624614. doi: 10.1155/2021/3624614
11. McCarthy J, Davis A. Diagnosis and management of vertebral compression fractures. Am Fam Physician. (2016) 94(1):44–50. doi: 10.1016/j.amjmed.2022.02.035
12. Boonstra AM, Schiphorst Preuper HR, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. (2014) 155(12):2545–50. doi: 10.1016/j.pain.2014.09.014
13. Kimura A, Shiraishi Y, Inoue H, Endo T, Takeshita K. Predictors of persistent axial neck pain after cervical laminoplasty. Spine. (2018) 43(1):10–5. doi: 10.1097/BRS.0000000000002267
14. Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. (2004) 23(13):2109–23. doi: 10.1002/sim.1802
15. Trief PM, Ploutz-Snyder R, Fredrickson BE. Emotional health predicts pain and function after fusion: a prospective multicenter study. Spine. (2006) 31(7):823–30. doi: 10.1097/01.brs.0000206362.03950.5b
16. Carreon LY, Djurasovic M, Dimar JR 2nd, Owens RK 2nd, Crawford CH 3rd, Puno RM, et al. Can the anxiety domain of EQ-5D and mental health items from SF-36 help predict outcomes after surgery for lumbar degenerative disorders? J Neurosurg Spine. (2016) 25(3):352–6. doi: 10.3171/2016.2.SPINE151472
17. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. (2007) 370(9590):851–8. doi: 10.1016/S0140-6736(07)61415-9
18. Stull JD, Divi SN, Goyal DKC, Bowles DR, Reyes AA, Bechay J, et al. Preoperative mental health component scoring is related to patient reported outcomes following lumbar fusion. Spine. (2020) 45(12):798–803. doi: 10.1097/BRS.0000000000003399
19. Held U, Burgstaller JM, Deforth M, Steurer J, Pichierri G, Wertli MM. Association between depression and anxiety on symptom and function after surgery for lumbar spinal stenosis. Sci Rep. (2022) 12(1):2821. doi: 10.1038/s41598-022-06797-1
20. Kolb AR, Patsch JM, Vogl WD, Benca E, Stelzeneder D, Windhager R, et al. The role of the subchondral layer in osteonecrosis of the femoral head: analysis based on HR-QCT in comparison to MRI findings. Acta Radiol. (2019) 60(4):501–8. doi: 10.1177/0284185118786070
21. Naul LG, Peet GJ, Maupin WB. Avascular necrosis of the vertebral body: mR imaging. Radiology. (1989) 172(1):219–22. doi: 10.1148/radiology.172.1.2740507
22. Wang Q, Wang C, Fan S, Zhao F. Pathomechanism of intravertebral clefts in osteoporotic compression fractures of the spine: basivertebral foramen collapse might cause intravertebral avascular necrosis. Spine J. (2014) 14(6):1090–1. doi: 10.1016/j.spinee.2014.01.051
23. Yu CW, Hsu CY, Shih TT, Chen BB, Fu CJ. Vertebral osteonecrosis: MR imaging findings and related changes on adjacent levels. AJNR Am J Neuroradiol. (2007) 28(1):42–7. PMID: 17213422
24. Ito Y, Hasegawa Y, Toda K, Nakahara S. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J. (2002) 2(2):101–6. doi: 10.1016/S1529-9430(01)00165-6
25. Ha KY, Lee JS, Kim KW, Chon JS. Percutaneous vertebroplasty for vertebral compression fractures with and without intravertebral clefts. J Bone Joint Surg Br. (2006) 88(5):629–33. doi: 10.1302/0301-620X.88B5.17345
26. Yu W, Liang D, Yao Z, Qiu T, Ye L, Jiang X. The therapeutic effect of intravertebral vacuum cleft with osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Int J Surg. (2017) 40:17–23. doi: 10.1016/j.ijsu.2017.02.019
27. Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. Jama. (2001) 285(3):320–3. doi: 10.1001/jama.285.3.320
28. Wu XG, Zhang DY, Zhu BQ, Li AM. Efficacy of zoledronic acid with percutaneous kyphoplasty/vertebroplasty in the treatment of osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2020) 24(23):12358–67. doi: 10.26355/eurrev_202012_24030
29. Tian P, Liu Y, Li ZJ, Xu GJ, Ma XL. Zoledronic acid in osteoporotic vertebral compression fractures treated with percutaneous kyphoplasty: a meta-analysis. Front Surg. (2021) 8:668551. doi: 10.3389/fsurg.2021.668551
30. Lu K, Yin Y, Li C, Jin Y, Shan HQ. Efficacy of annual zoledronic acid in initial percutaneous kyphoplasty patients with osteoporotic vertebral compression fractures: a 3-year follow-up study. Osteoporos Int. (2021) 32(7):1429–39. doi: 10.1007/s00198-020-05816-z
31. Alvarez L, Pérez-Higueras A, Granizo JJ, de Miguel I, Quiñones D, Rossi RE. Predictors of outcomes of percutaneous vertebroplasty for osteoporotic vertebral fractures. Spine. (2005) 30(1):87–92. doi: 10.1097/00007632-200501010-00016
32. Leeson MC, Lippitt SB. Thermal aspects of the use of polymethylmethacrylate in large metaphyseal defects in bone. A clinical review and laboratory study. Clin Orthop Relat Res. (1993) 295:239–45. doi: 10.1097/00003086-199310000-00035
33. Röder C, Boszczyk B, Perler G, Aghayev E, Külling F, Maestretti G. Cement volume is the most important modifiable predictor for pain relief in BKP: results from SWISSspine, a nationwide registry. Eur Spine J. (2013) 22(10):2241–8. doi: 10.1007/s00586-013-2869-3
34. Fu Z, Hu X, Wu Y, Zhou Z. Is there a dose-response relationship of cement volume with cement leakage and pain relief after vertebroplasty? Dose Response. (2016) 14(4):1559325816682867. doi: 10.1177/1559325816682867
35. Liang D, Ye LQ, Jiang XB, Yang P, Zhou GQ, Yao ZS, et al. Biomechanical effects of cement distribution in the fractured area on osteoporotic vertebral compression fractures: a three-dimensional finite element analysis. J Surg Res. (2015) 195(1):246–56. doi: 10.1016/j.jss.2014.12.053
36. Chen B, Li Y, Xie D, Yang X, Zheng Z. Comparison of unipedicular and bipedicular kyphoplasty on the stiffness and biomechanical balance of compression fractured vertebrae. Eur Spine J. (2011) 20(8):1272–80. doi: 10.1007/s00586-011-1744-3
37. Iwata A, Kanayama M, Oha F, Shimamura Y, Hashimoto T, Takahata M, et al. Is bone nonunion, vertebral deformity, or spinopelvic malalignment the best therapeutic target for amelioration of low back pain after osteoporotic vertebral fracture? Spine. (2020) 45(13):E760–e767. doi: 10.1097/BRS.0000000000003422
Keywords: low back pain, osteoporotic vertebral fractures, percutaneous kyphoplasty, risk factors, nomogram
Citation: Yu H, Luo G, Wang Z, Yu B, Sun T and Tang Q (2023) Predictors of residual low back pain in patients with osteoporotic vertebral fractures following percutaneous kyphoplasty. Front. Surg. 10:1119393. doi: 10.3389/fsurg.2023.1119393
Received: 8 December 2022; Accepted: 2 January 2023;
Published: 3 February 2023.
Edited by:
Qingquan Kong, Sichuan University, China© 2023 Yu, Luo, Wang, Yu, Sun and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianwei Sun YmlsbHN1bnR3QDE2My5jb20=
Specialty Section This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Hongwei Yu
Hongwei Yu Gan Luo
Gan Luo Ziqi Wang1
Ziqi Wang1 Bin Yu
Bin Yu Tianwei Sun
Tianwei Sun