- 1Servicio de Cirugía General y Digestiva, Hospital General Universitario Morales Meseguer, Murcia, Spain
- 2Grupo de Investigación Quirurgica en Area de Salud, Instituto Murciano de Investigación Biosanitaria Pascual Parrilla, Murcia, Spain
Background: anastomosis leak still being a handicap in colorectal surgery. Bowel mechanical preparation and oral antibiotics are not a practice recommended in many clinical practice guides. The aim is to analyse the decrease in frequency and severity of postoperative complications, mainly related to anastomotic leak, after the establishment of a bundle.
Methods: Single-center, before-after study. A bundle was implemented to reduce anastomotic leaks and their consequences. The Bundle group were matched to Pre-bundle group by propensity score matching. Mechanical bowel preparation, oral and intravenous antibiotics, inflammatory markers measure and early diagnosis algorithm were included at the bundle.
Results: The bundle group shown fewer complications, especially in Clavien Dindós Grade IV complications (2.3% vs. 6.2% p < 0.01), as well as a lower rate of anastomotic leakage (15.5% vs. 2.2% p < 0.01). A significant decrease in reinterventions, less intensive unit care admissions, a shorter hospital stay and fewer readmissions were also observed. In multivariate analysis, the application of a bundle was an anastomotic leakage protective factor (OR 0.121, p > 0.05)
Conclusions: The implementation of our bundle in colorectal surgery which include oral antibiotics, mechanical bowel preparation and inflammatory markers, significantly reduces morbidity adjusted to severity of complications, the anastomotic leakage rate, hospital stay and readmissions.
Register study: The study has been registered at clinicaltrials.gov Code: nct04632446.
Introduction
The safety of patients undergoing colorectal surgery has significantly improved during the past 50 years due to the progress in preoperative preparation, surgical technique and postoperative treatment. Even so, there are still postsurgical complications, with a current morbidity of close to 40% in elective surgery (1).
Among the complications of colorectal surgery, Surgical Site Infection (SSI) is the most important one, reaching up 20% (2) and represents the highest rates in all major abdominal surgery. This is probably due to the influence of Organ-Space Infection, which includes anastomosis leak (AL) and whose origin seems to differ from Incisional SSI. Organ-space SSI alone accounts for 23% of re-hospitalizations, 60% of reoperations and 29% of admissions to Intensive Care Units (ICU), trebling hospital stay (3). The incidence of AL varies between authors, from 2 to 14% in colon surgery and from 2 to 29% in rectal surgery (4).
Due to the frequency and severity of SSI in elective colorectal surgery, specific guidelines have been prepared in order to reduce this type of complications by using bundles or a series of measures aimed at improving postoperative results. Today, there is not just one bundle, but different groups (5–7) and societies (8) who have implemented different measures succeeding in significantly reducing SSI. Mechanical bowel preparation (MBP) and oral antibiotic prophylaxis have been two of the most frequently used measures. Although there is a broad consensus that antibiotic prophylaxis is essential before colorectal surgery, there is still controversy about whether antibiotics should be administered intravenously, orally, or combined. On the other hand, the role of MBP alone or with oral antibiotics has been extensively discussed.
The purpose of this study is to evaluate the improved frequency and severity of complications and the morbidity associated with anastomosis leak after de use of a bundle in patients undergoing elective colorectal surgery.
Methods
We conducted a study before and after implementing a bundle with 5 new measures. The Pre-bundle cohort consisted of 95 consecutive patients undergoing elective colorectal surgery with anastomosis, from October 1, 2017 to May 30, 2018. The incidence of complications of these patients was recorded and their C-Reactive Protein (CRP) reference levels were obtain as a marker for early diagnosis of anastomotic dehiscence by applying the ROC curves and calculating the pathological reference value using the Youden's index (>15 mg/dl on the third postoperative day, >10 mg/dl on the fourth postoperative day and >9 mg/dl on the fifth postoperative day) (9). These values were used in the bundle for early diagnosis of complications.
The sample size of the patients in the Bundle group was calculated for a decrease in serious complications (grades IV and V of the Clavien-Dindo Classification) to 6%, having the Pre-bundle group as a reference.
The inclusion criteria were: patients over 18 years old, signed the informed consent and underwent elective colorectal surgery due to malignant or benign neoplasia with anastomosis during surgery. Patients who required transfer to another center or those with fatal evolution (death) before the third postoperative day, were excluded from the study. Finally, the Bundle group consisted of 139 patients.
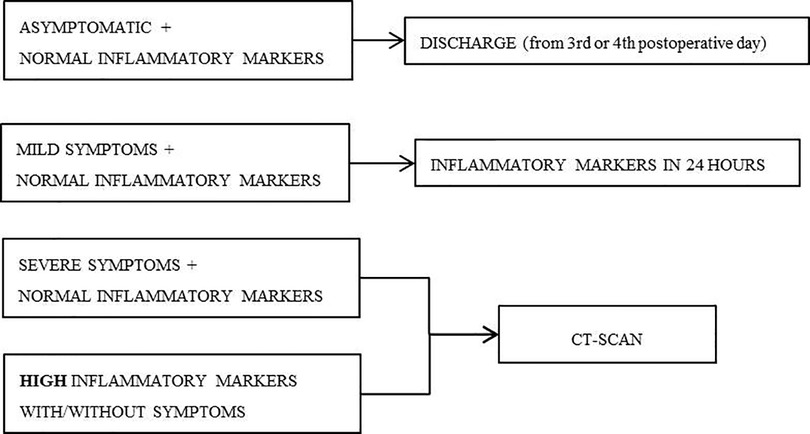
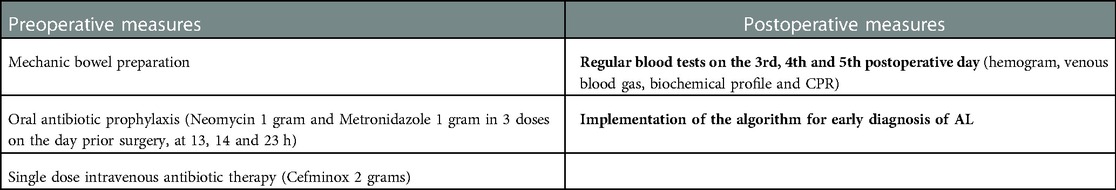
The bundle consisted on preoperative and postoperative measures (Table 1) and included an algorithm for the early diagnosis of anastomosis leak (Figure 1). All our patients are assessed daily by a coloproctology unit surgeon, since the first postoperative day, and a CRP were measured ant 3rd, 4th and 5th postoperative day.
The asymptomatic patients with CRP below the calculated cut-off point were discharged on the third or fourth postoperative day. Patients with mild symptoms (such as feeding intolerance, absence of intestinal transit or abdominal discomfort) and markers within normal ranges, had another blood test performed after 24 h. Patients with serious symptoms (like fever, hemodynamic instability or peritoneal irritation signs) and normal markers had an abdominal and pelvic Computed Tomography scan (CT-scan) with double or triple contrast performed. Patients with high inflammatory markers had a CT-scan performed, whether they had symptoms or not. All the patients of the Pre-Bundle group received IV Cefminox 2gr within the hour prior to surgery and oral MBP, with sodium picosulfate.
The study was single-center. Patient selection, data collection, and later follow-up were conducted prospectively during the first 30 days after surgery. The preoperative, intraoperative and postoperative variables of all patients were collected.
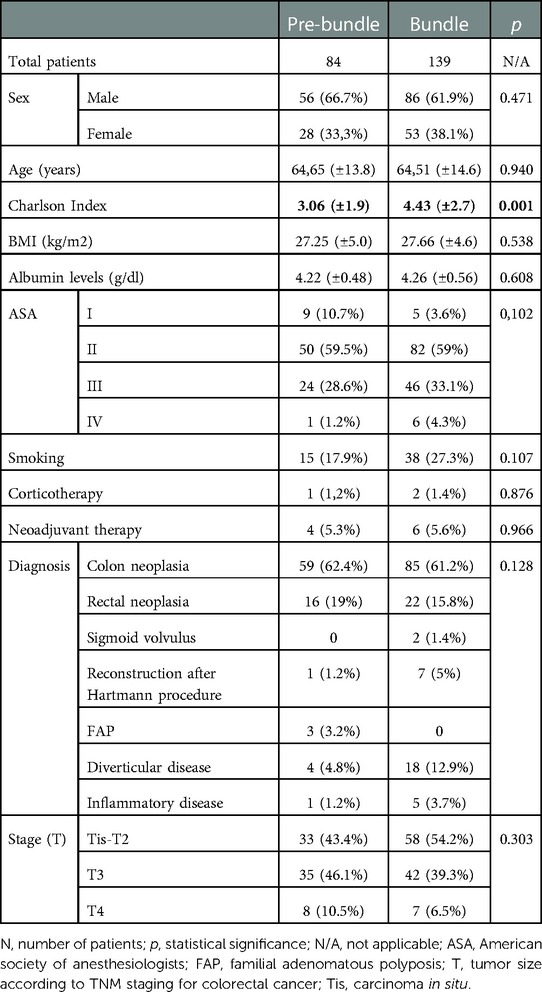
Data processing and statistical analysis were performed using SPSS 24.0. In order to obtain two comparable homogeneous groups, a propensity score matching analysis was performed. Confounding variables used to set the propensity score were age, sex, Charlson index, American Society of Anaesthesiologists (ASA) classification, preoperative steroids and surgical approach. On the basis of multi-factor logistic analysis, the propensity score was calculated with a caliper width of 0.2, obtaining two groups with 84 patients in the Pre-bundle Group and 139 in the Bundle group (Figure 2). At first, a univariate analysis was performed to compare the groups by using chi-squared distribution for discrete variables (considering standardized residual in tables bigger than 2 × 2); and Students' t-distribution for continuous variables (using Levene's test to assess the distribution of variances). For the purpose to avoid any bias, a subgroup analysis was performed according the colon condition (benign or malignant).
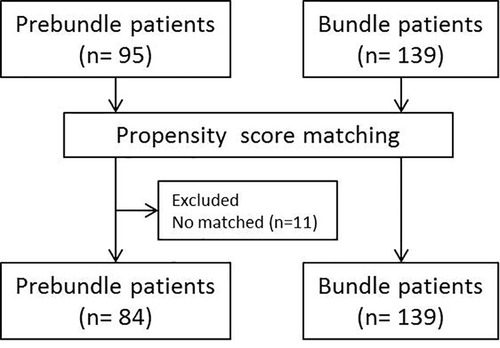
Figure 2. Study population and flowchart showing patient group before and after propensity score matching.
In order to assess the possible prognostic factors of the severity of morbidity, ICU stay and presence of anastomosis leak, a multivariate analysis was conducted using backward stepwise logistic regression to describe the significant variables for our study.
The study has been approved by the appropriate institutional ethics committee and have been performed in accordance with the ethical standards as laid down in the 1,964 Declaration of Helsinki and its later amendments or comparable ethical standards. Patients have consented to participate and to publication.
Results
The 84 patients of the Pre-bundle group were compared with a cohort of 139 patients subjected to the bundle (Bundle group), operated between March 2019 and May 2020. Adherence to the bundle was 99.3% for mechanic preparation and 95.7% for oral antibiotic prophylaxis. However, the adherence to the implementation of the diagnostic algorithm for postoperative complications was 87%.
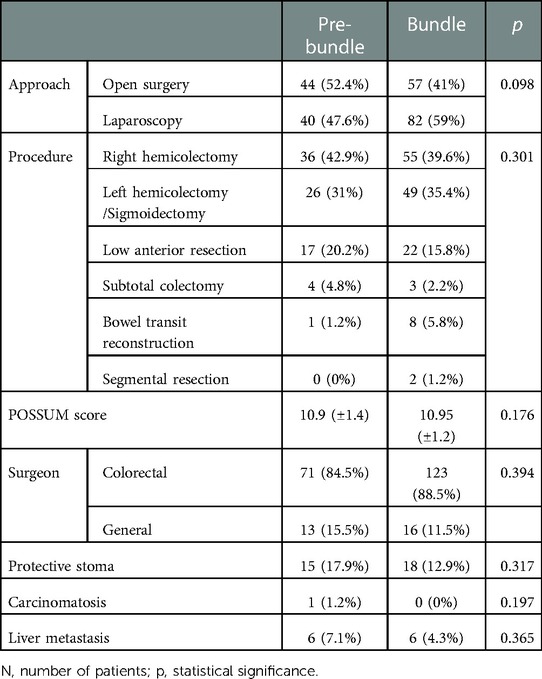
Both groups were homogeneous (sex, age and BMI, among others), although the Bundle group presented with higher Charlson Index (Table 2). Both were also homogeneous regarding surgical approach, procedure, surgical team, ostomy confection, metastasis, carcinomatosis and Possum score (Table 3).
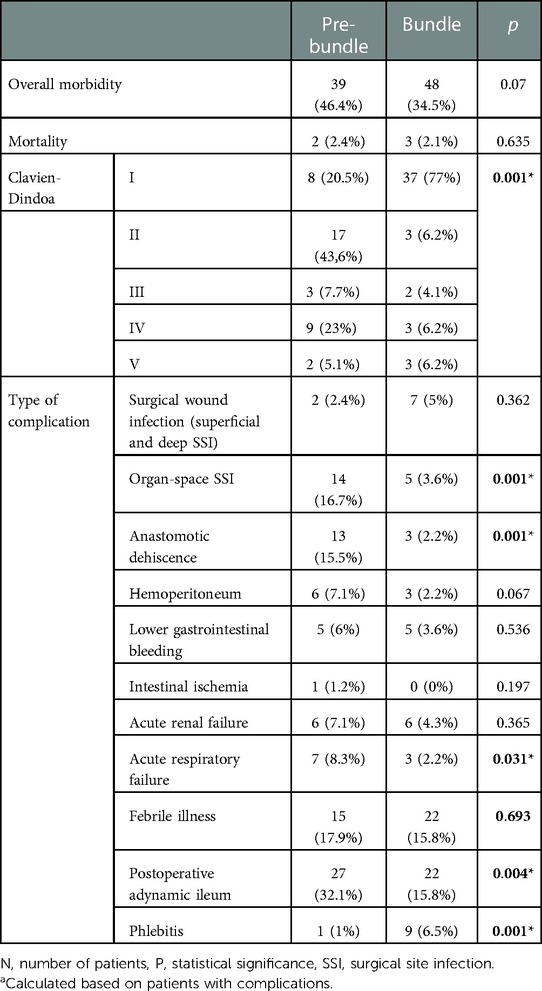
The percentage of patients who presented with complications in the Bundle group was lower than in the Pre-bundle group (34.5% vs. 46.4%), without reaching statistical significance, but when analyzing the distribution of the complications according to their gravity, we observed that the Bundle group mainly presented with mild complications, grade I of Clavien-Dindo classification (77% vs. 20.5% p = 0.001), whereas in the Pre-bundle group the rate of severe complications, grade IV of Clavien-Dindo classification, was significantly higher (23% vs. 6.2%, p = 0.001). Moreover, the incidences of Organ-space SSI (and therefore of AL), acute respiratory failure and dynamic ileus were lower in the Bundle group, p < 0.05. Mortality was similar in both series (2.4% in the Pre-bundle group vs. 2.1% in the Bundle group) (Table 4).
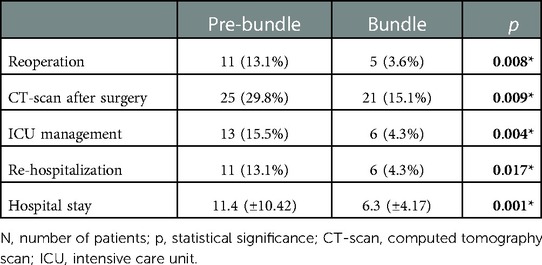
Hospital stay for the Bundle group was shorter (6.3 vs. 11.4 days, p = 0.001), as well as the need for ICU (4.3% vs. 15.5%, p = 0.004), the re-hospitalization rate (4.3% vs. 13.1%, p = 0.017) and the need for imaging tests during the postoperative period, despite complying with the diagnostic algorithm (15.1% vs. 29.8%, p = 0.009), were significantly lower (Table 5).
On the other hand, the laparoscopic approach was associated with less incidence of complications (18.8% vs. 47.5%, p = 0.001) and of severe complications (grades IV and V of Clavien-Dindo classification) than open surgery (4.1% vs. 11.9%, p = 0.001). However, these differences were not significant regarding AL, whose incidence was similar in both approaches (Table 6).
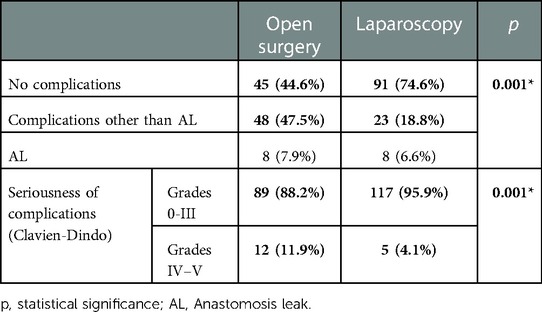
Table 6. Comparative analysis of the approach in relation to the postoperative complications and their seriousness.
When analyzing the variables of the subgroups according to the condition (benign vs. malignant), we have obtained that both are also homogeneous with no differences in the preoperative and operative variables. Moreover, the morbidity, mortality, all complications and postoperative results were similar to the overall series both in benign and malignant subgroups.
The multivariate analysis included the variables related to AL occurrence: sex, age, ASA, Charlson Index, BMI, surgical approach and bundle implementation. The bundle itself was a protective factor for AL occurrence [OR 0.121,—CI 95% (0.033–0.446)]. Moreover, male sex was associated with a significantly higher risk of AL (OR 9.350, CI 95% 1.190–73.488).
Discussion
Due to the high risk and repercussion of SSI and AL in colorectal surgery, many have been the strategies used throughout history to try to reduce them. In 1934, Poth (10) concluded that MBP on its own did not succeed in reducing the bacterial content in the colon; therefore, oral non-absorbable antibiotics were introduced (11, 12). Later on, with the detection of anaerobic microorganisms in the colon (13), an anaerobicidal agent, such as metronidazole, was added to neomycin, which, in combination with MBP, succeeded in reducing aerobic and anaerobic bacteria outgrowth in the sample (14), and reduced the incidence of SSI and AL (15), thus consolidating the principles of bowel preparation.
This trend has continued in the United States and Canada since the 80s (5–7, 16–31) with good results regarding SSI decrease. But this is not the case in Europe (32–36), where the ERAS® program (37) and the guidelines of the British National Institution of Health and Clinical Excellence 2008 (38) reject MBP and advocate the superiority of intravenous prophylaxis for SSI prevention, reporting an increased incidence of pseudomembranous colitis and antibiotic resistance associated with oral prophylaxis (39).
Due to the high morbidity resulting from the AL and the disparity of the results of the published works regarding how to avoid it, we decided to monitor the complication rate in our unit, which resulted in an incidence of infection of the surgical wound (superficial and deep) of 2.4% and an AL rate of 15.5%. Not only the overall incidence of complications but also their grade of severity was high, with 23% of grade IV complications according to Clavien-Dindo classification and 5.1% of grade V. Moreover, the mean hospital stay was 11 days with 13.1% of re-hospitalizations. After being aware of these figures, we created a bundle that allowed for decreasing the incidence of such complications and reducing their severity and repercussion on the patient.
With the implementation of the new bundle, we obtained a decrease in morbidity from 46.4% to 34.5%, although without reaching significant values. However, the severity of complications did change considerably in both groups. Most of the complications in the Bundle group were grade I of Clavien-Dindo classification (77% vs. 20.5% in the Pre-bundle group), and grade IV complications of Clavien-Dindo classification were significantly higher in the Pre-bundle group (23% vs. 6.2% in the Bundle group). Therefore, we can say that the implementation of the new measures drastically reduced the severe complications of elective colorectal surgery. The most relevant difference was the incidence of organ-space infection (16.7% to 3.6%) and particularly the incidence of anastomosis leak, which significantly decreased from 15.5% to 2.2% in the Bundle group (p = 0.001).
Other authors have published similar results on the decrease of SSI after the implementation of bundles. Lutfiya et al. (5) who, after implementing the measures of the American College of Surgeons “ACS NSQIP” (8), obtained a decrease in overall SSI at the expense of superficial and deep incisional infection (21.15% to 6.67%, p = 0.001). Weiser et al. (40) in 2018 conducted a study before and after the implementation of a bundle, in which they divided the patients according to their risk of SSI. The incidence of SSI decreased from 11% to 4.1% at the expense of the groups with intermediate or high risk of SSI. These differences were significant in the superficial and deep incisional infections. A much smaller range of measures than “ACS NSQIP” (8) was implemented in our study, thus facilitating compliance (7).
Studies such as Gorgun et al. (22) also found a decrease in overall SSI when implementing their bundle (11.8% to 6.6%, p = 0.001), associated with decreased organ-space infection (5.5% vs. 1.7%, p = 0.001). Likewise, Mulder et al. (24) also succeeded in significantly reducing overall SSI and AL, thus reducing hospital stay from 8 to 7 days. Like in our study, a laparoscopic approach was most frequently used in the group after the bundle implementation. In this line, we also observed that a laparoscopic approach yielded a lower complication rate, particularly severe complications (grade IV and V of Clavien-Dindo classification), than an open approach (4.1%vs.11.9%). It is worth noting that Mulder et al. administered oral antibiotic prophylaxis and intravenous prophylaxis, without mechanical bowel preparation. In our study, we opted for a combination of antibiotics and MBP because we found little evidence in favor of the use of oral antibiotics without mechanical bowel preparation. Hoang et al. (23) also implemented a bundle including mechanical preparation and dual antibiotic therapy together, which resulted in a significant decrease in overall SSI. We found striking that Hoang's study included patients undergoing emergency surgeries, in which cases it is difficult to administer mechanical bowel preparation and oral antibiotic therapy.
In our study, besides the decreased infectious complications, there was also a significant decrease in other medical complications such as respiratory failure (8.3%vs.2.2%) and adynamic ileus (32.1%to15.8%). Other studies obtained similar results (25, 28), as opposed to the ERAS® protocols (37), which recommended against mechanical preparation because they considered that it provided no benefits but posed a greater risk of paralytic ileum after surgery.
In addition to trying to reduce SSI with preoperative measures, we included in our bundle some postoperative measures that allowed us for an early diagnosis of severe intra-abdominal infectious complications. After confirming the usefulness of CRP as a biologic marker for the early diagnosis of AL in the Pre-bundle group, we created an algorithm to facilitate the early detection of this complication and proceed accordingly, and to be able to early and safely discharge those patients who had that marker below the pre-established values.
Although we performed CT-scan based mostly on the results of blood tests, the number of them performed was significantly lower than in the Pre-bundle group (29.8% vs. 15.1%); therefore, our measures not only do they decreases the AL rate but allowed for a better selection of patients who required a CT-scan during the postoperative period also. Besides, we succeeded in significantly reducing the number of reoperations from 13.1% to 3.6% (p = 0.008), the need of ICU management from 15.5% to 4.3% (p = 0.004) and re-hospitalizations from 13.1% to 4.3% (p = 0.017), which resulted in a 5-day decrease in hospital stays (11 vs. 6 days p = 0.001). These results show that the implementation of a bundle also decrease healthcare costs. Other studies such as the one by Keenan et al. (6) show similar results.
In our study, after conducting the multivariate comparative analysis, we found that the implementation of our bundle proved to be a protective factor from the most important complication in colorectal surgery.
Limitations
A potential limitation of our study is to be a before-after, single-centre study, rather than a randomized and multicenter one which would provide more reliable results. A larger sample size would have allowed to find more relations between risk factors and complications. Finally, it is a heterogeneous sample, since it encompasses a range of pathologies in colorectal surgery as colorectal cancer, diverticulosis and inflammatory disease, but we wanted to have a clinically representative population sample.
Conclusions
The implementation of our bundle significantly reduces morbidity adjusted to the severity of complications, the AL rate, hospital stay and re-hospitalizations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository and accession number can be found below: 10.6084/m9.figshare.17009111<.
Ethics statement
The studies involving human participants were reviewed and approved by CEI-CEIm Hospital General Universitario José María Morales Meseguer. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All the authors have contributed substantially to the design of the study, acquisition, analysis and interpretation of data. Likewise, everyone has revised critically the draft and approval the final document. Specifically: BM, VN, PF and AA: have designed the study. BM, SA, MB, GM and BB: have collect all the data. BM and VN: have made the statistical analysis. All the authors have analyzed and interpreted the data, discussed the results and approved the paper. All authors contributed to the article and approved the submitted version.
Funding
This study has been founding by Carlos III Health Institute grant number PI19/00902
Acknowledgments
Carlos III Health Institute Grant PI19/00902. To Alfonso Perez-Martinez MD for his support in the laboratory determinations. To Enrique Girela-Baena MD for his support in performing the imaging tests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ruiz-Tovar A, Morales-Castiñeiras V, Lobo-Martínez E. Postoperative complications of colon surgery. Cir Cir. (2010) 78(3):283–91.
2. Pujol M, Limön E, Löpez-Contreras J, Sallés M, Bella F, Gudiol F. Surveillance of surgical site infections in elective colorectal surgery. Results of the VINCat program (2007–2010). Enferm Infecc Microbiol Clin. (2012) 30(Suppl 3):20–5. doi: 10.1016/S0213-005X(12)70092-7
3. Shaw E, Badia J, Piriz M, Escofet R, Limön E, Gudiol F, et al. What surgical site infection rates in colorectal surgery should be considered for benchmarking standards? Antimicrob Resist Infect Control. (2013) 2(Suppl1):O53. doi: 10.1186/2047-2994-2-S1-O53
4. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. (2009) 208(2):269–78. doi: 10.1016/j.jamcollsurg.2008.10.015
5. Lutfiyya W, Parsons D, Breen J A. Colorectal “care bundle” to reduce surgical site infections in colorectal surgeries: a single-center experience. Perm J. (2012) 16(3):10–6. doi: 10.7812/TPP/12.968
6. Keenan JE, Speicher PJ, Thacker JKM, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. (2014) 149(10):1045–52. doi: 10.1001/jamasurg.2014.346
7. Waits SA, Fritze D, Banerjee M. Developing an argument for bundled interventions to reduce surgical site infection in colorectal surgery. Surgery. (2014) 155(4):602–6. doi: 10.1016/j.surg.2013.12.004
8. Cima R, Dankbar E, Lovely J, Pendlimari R, Aronhalt K, Nehring S, et al. Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program-driven multidisciplinary single- institution experience. J Am Coll Surg. (2013) 216(1):23–33. doi: 10.1016/j.jamcollsurg.2012.09.009
9. Baeza-Murcia M, Valero-Navarro G, Pellicer-Franco E, Soria-Aledo V, Mengual-Ballester M, Garcia-Marin JA, et al. Early diagnosis of anastomotic leakage in colorectal surgery: prospective observational study of the utility of inflammatory markers and determination of pathological levels. Updates Surg. (2021) 73(6):2103–11. doi: 10.1007/s13304-021-01082-8
11. Goldring J, Mcnaught W, Scott A, Gillespie G. Prophylactic oral antimicrobial agents in elective colonic surgery. A controlled trial. Lancet. (1975) 306(7943):997–1000. doi: 10.1016/S0140-6736(75)90289-5
12. Clarke JS, Condon RE, Barlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg. (1977) 3(186):251–9. doi: 10.1097/00000658-197709000-00003
13. Bentley DW, Nichols RL, Condon RE, Gorbach SL. The microflora of the human ileum and intraabdominal colon: results of direct needle aspiration at surgery and evaluation of the technique. J Lab Clin Med. (1972) 79(3):421–9.4551448
14. Smith MB, Goradia VK, Holmes JW, McCluggage SG, Smith JW, Nichols RL. Suppression of the human mucosal-related colonic microflora with prophylactic parenteral and/or oral antibiotics. World J Surg. (1990) 14(5):636–41. doi: 10.1007/BF01658812
15. Coppa GF, Eng K, Gouge TH, Ranson JHC, Localio SA. Parenteral and oral antibiotics in elective colon and rectal surgery. A prospective, randomized trial. Am J Surg. (1983) 145(1):62–5. doi: 10.1016/0002-9610(83)90167-8
16. Lewis RT, Bs MB. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg. (2002) 45(3):173–80.12067168
17. Sadahiro S, Suzuki T, Tanaka A, Okada K. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery. (2014) 155(3):493–503. doi: 10.1016/j.surg.2013.06.002
18. Anjum N, Ren J, Wang G, Li G, Wu X, Dong H, et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum. (2017) 60(12):1291–8. doi: 10.1097/DCR.0000000000000927
19. European Society of Coloproctology (ESCP) collaborating group. Association of mechanical bowel preparation with oral antibiotics and anastomotic leak following left sided colorectal resection: an international, multi-centre, prospective audit. Colorectal Dis. (2018) 20:15–32. doi: 10.1111/codi.14362
20. Ambe PC, Zarras K, Stodolski M, Wirjawan I, Zirngibl H. Routine preoperative mechanical bowel preparation with additive oral antibiotics is associated with a reduced risk of anastomotic leakage in patients undergoing elective oncologic resection for colorectal cancer. World J Surg Oncol. (2019) 20:1–6. doi: 10.1186/s12957-019-1563-2
21. Lei P, Ruan Y, Yang X, Wu J, Hou Y, Wei H, et al. Preoperative mechanical bowel preparation with oral antibiotics reduces surgical site infection after elective colorectal surgery for malignancies: results of a propensity matching analysis. World J Surg Oncol. (2020) 18(1):35. doi: 10.1186/s12957-020-1804-4
22. Gorgun E, Rencuzogullari A, Ozben V, Stocchi L, Fraser T, Benlice C, et al. An effective bundled approach reduces surgical site infections in a high- outlier colorectal unit. Dis Colon Rectum. (2018) 61(1):89–98. doi: 10.1097/DCR.0000000000000929
23. Hoang SC, Klipfel AA, Roth LA, Vrees M, Schechter S. The American journal of surgery colon and rectal surgery surgical site infection reduction bundle: to improve is to change. Am J Surg. (2019) 217(1):40–5. doi: 10.1016/j.amjsurg.2018.07.008
24. Mulder T, Crolla RMPH, Bergh MFQK den, Mourik MSM van, Romme J. Preoperative oral antibiotic prophylaxis reduces surgical site infections after elective colorectal surgery: results from a before—after study. Clin Infect Dis. (2019) 2019(1):93–9. doi: 10.1093/cid/ciy839
25. Kiran RP, Murray ACA, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak and ileus after colorectal surgery. Ann Surg. (2015) 262(3):416–25. doi: 10.1097/SLA.0000000000001416
26. Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg. (2015) 261(6):1034–40. doi: 10.1097/SLA.0000000000001125
27. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection. An analysis of colectomy-targeted ACS NSQIP. Ann Surg. (2015) 262(2):331–7. doi: 10.1097/SLA.0000000000001041
28. Garfinkle R, Abou-Khalil J, Morin N, Ghitulescu G, Vasilevsky MDS, Gordon MDP, et al. Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis Colon Rectum. (2017) 60:729–37. doi: 10.1097/DCR.0000000000000851
29. Midura EF, Jung AD, Hanseman DJ, Dhar V, Shah SA, Rafferty JF, et al. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery. (2018) 163(3):528–34. doi: 10.1016/j.surg.2017.10.023
30. Ohman KA, Wan L, Guthrie T, Johnston B, Leinicke JA, Glasgow SC, et al. Combination of oral antibiotics and mechanical bowel preparation reduces surgical site infection in colorectal surgery. J Am Coll Surg. (2017) 225(4):465–71. doi: 10.1016/j.jamcollsurg.2017.06.011
31. Khorasani S, Dossa F, McKechnie T, Englesakis M, Brar MS, van Overstraeten A. Association between preoperative oral antibiotics and the incidence of postoperative Clostridium difficile infection in adults undergoing elective colorectal resection: a systeatic review and meta- analysis. Dis Colon Rectum. (2020) 63(4):545–61. doi: 10.1097/DCR.0000000000001619
32. Bucher P, Gervaz P, Soravia C, Mermillod B, Erne M, Morel P. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg. (2005) 92(4):409–14. doi: 10.1002/bjs.4900
33. Espín-Basany E, Sanchez-Garcia JL, Lopez-Cano M, Lozoya-Trujillo R. Prospective, randomised study on antibiotic prophylaxis in colorectal surgery. Is it really necessary to use oral antibiotics? Int J Colorectal Dis. (2005) 20(6):542–6. doi: 10.1007/s00384-004-0736-8
34. Contant CME, Hop WCJ, Pieter H, Sant V, Oostvogel HJM, Smeets HJ, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet. (2007) 370(9605):2112–7. doi: 10.1016/S0140-6736(07)61905-9
35. Cao F, Li J, Li F. Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta-analysis. Int J Colorectal Dis. (2012) 27(6):803–10. doi: 10.1007/s00384-011-1361-y
36. Güenaga KF, Matos DW-JP. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. (2011) 23(8):783–5. doi: 10.1002/14651858.CD001544.pub4
37. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. (2013) 37(2):259–84. doi: 10.1007/s00268-012-1772-0
38. National Institute for Clinical Excelence (2019) Surgical site infections: prevention and treatment NICE guideline. Available at: www.nice.org.uk/guidance/ng125
39. Keighley MRB, Arabi Y, Alexander-Williams J, Youngs D, Burdon DW. Comparison between systemic andoral antimicrobial prophylaxis in colorectal surgery. Lancet. (1979) 313(8122):894–7. doi: 10.1016/S0140-6736(79)91373-4
Keywords: bundle, anastomosis leakage, colorectal surgery complicatios, bowel mechanical preparation, inflammatory marker
Citation: Baeza-Murcia M, Valero-Navarro G, Pellicer-Franco E, Soria-Aledo V, Mengual-Ballester M, Garcia-Marin JA, Betoret-Benavente L and Aguayo-Albasini JL (2023) Bundles reduce anastomosis leak in patients undergoing elective colorectal surgery. A propensity score-matched study. Front. Surg. 10:1119236. doi: 10.3389/fsurg.2023.1119236
Received: 8 December 2022; Accepted: 1 February 2023;
Published: 27 February 2023.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Monica Ortenzi, Università Politecnica delle Marche, ItalyMichele Manigrasso, University of Naples Federico II, Italy
© 2023 Baeza-Murcia, Valero-Navarro, Pellicer-Franco, Soria-Aledo, Mengual-Ballester, Garcia-Marin, Betoret-Benavente and Aguayo-Albasini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: G Valero-Navarro Z3JhY2llbGEudmFsZXJvQHVtLmVz
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 M Baeza-Murcia
M Baeza-Murcia G Valero-Navarro
G Valero-Navarro E Pellicer-Franco1,2
E Pellicer-Franco1,2