- Department of Orthopaedics, China-Japan Union Hospital of Jilin University, Changchun, China
Background: Extensive spinal epidural abscess (SEA) is an exceptional and threatening condition that requires prompt recognition and proper management to avoid potentially disastrous complications. We aimed to find key elements of early diagnosis and rational treatments for extensive SEA.
Case presentation: A 70-year-old man complained of intense pain in the cervical-thoracic-lumbar spine that radiated to the lower extremity. Laboratory test results revealed a marked increase in all indicators of infection. The spinal magnetic resonance imaging (MRI) revealed a ventral SEA extending from C2 to L4. Owing to the patient's critical condition, laminectomy, drainage, and systemic antibiotic therapy were administered. And the multidrug-resistant Staphylococcus epidermidis was detected in the purulent material from this abscess.
Results: Postoperative MRI revealed diminished epidural abscess, and the clinical symptoms were dramatically and gradually relieved after two rounds of surgery and systemic antibiotic therapy involving the combination of ceftriaxone, linezolid, and rifampicin.
Conclusions: A comprehensive emergency assessment based on neck or back pain, neurological dysfunctions, signs of systemic infection, and MRI are important for early diagnosis of extensive SEA. Further, the combination of laminectomy, drainage, and systemic antibiotic therapy may be a rational treatment choice for patients with SEA, especially for extensive abscess or progressive neurological dysfunction.
1. Introduction
Spinal epidural abscess (SEA), i.e., an accumulation of purulent contents in the epidural space of the spinal canal, can lead to spinal medullary ischemia upon compression, resulting in progressive neurological dysfunction, systemic inflammation, and even death (1–4). A recent report indicated that SEA-associated hospitalization has increased to nearly 1/1,000 (5, 6). This trend may be due to the growing population of immunocompromised patients with cancer and diabetes, and the increasing number of invasive spinal surgeries. The mean age of SEA is 50 years with most cases occurring between the ages of 30 and 70 years, though children may be rarely affected (1, 2, 7, 8). Although typical SEA symptoms include fever, back pain, and progressive neurological dysfunction (9), approximately 50% of patients have either back pain or other non-specific manifestations (10, 11), leading to a high rate of misdiagnosis and inevitably missed diagnosis. In addition to diagnosis, the therapy and prognosis of SEA are important and can directly determine the survival rate of patients. A neurosurgeon, Dandy (12) first used laminectomy and performed direct drainage of the abscess for SEA surgery. Moreover, the emergence of antibiotics effectively reduced mortality. Therefore, a combination of these two is the rational treatment for symptomatic SEA, especially for extensive spinal cord compression and progressive neurological dysfunction (13, 14). However, the prognosis of SEA remains not optimistic, with 4%–22% of patients having severe neurologic disability such as irreversible paraplegia or other deficits occurring, and less than half of patients return to baseline neurologic status (15–19). Mortality rates range from 2% to 20% (1, 2, 15, 16, 19, 20).
According to Reihsaus et al. (10), only 1% patients presented with extensive SEA spanning the cervical-thoracic-lumbar region. For the treatment of extensive SEA, the majority of neurosurgeons and spine surgeons favor aggressive surgical approaches. The techniques include single- or multi-segment laminectomy, followed by irrigation or sufficient epidural drainage to remove purulent secretions (4, 21, 22). Determining the scope of surgery and minimizing surgical trauma with proper drainage are crucial for extensive SEA treatment.
In August 2022, a case of extensive SEA spanning the cervical-thoracic-lumbar region caused by Staphylococcus epidermidis was successfully treated at our department. Herein, we analyze and discuss the diagnosis and treatment experience.
2. Case presentation
A 70-year-old man was admitted to the hospital with a visual analog scale (VAS) score of 7 and a body temperature of 38.5°C due to acute discomfort in the cervical-thoracic-lumbar spine that radiated to the lower extremity. He had back and neck pain for two weeks, which restricted the spine's range of motion, affected his lower limbs, and caused physical weakness. He reported a history of diabetes mellitus and underwent lumbar discectomy and transpedicular screw fixation (L4/5) 4 months previously.
On admission, the muscle power was 3/5 in the left lower extremity but normal in the other extremities. Reflexes were reduced in the quadriceps and ankle jerks. The left straight leg raise test result was positive at 30°. Negative pathological reflexes were observed in the upper and lower extremities along with neck stiffness and positive Brudzinski signs.
Laboratory test results showed a marked increase in white blood cell (WBC; 13.99 × 109/l), erythrocyte sedimentation rate (ESR; 78.00 mm/h), and C-reactive protein (CRP; 123.96 mg/l) levels, suggesting a severe infection. Blood culture results were negative. Magnetic resonance imaging (MRI) confirmed extensive SEA from C2 to L4 and a spondylodiscitis at the L3/4 segment (Figure 1).
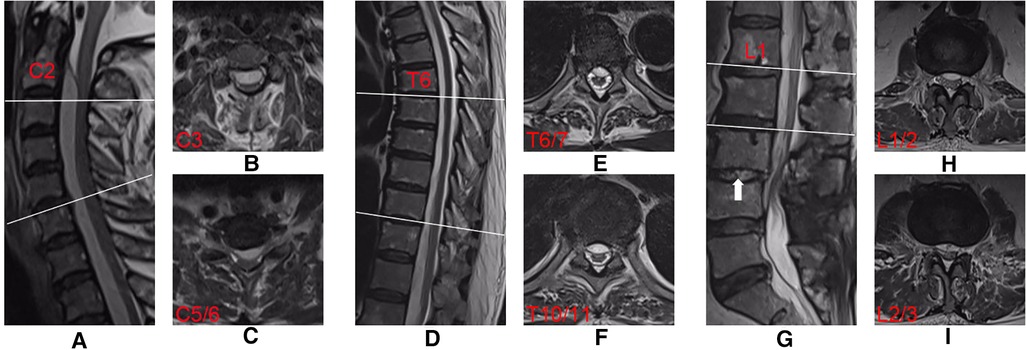
Figure 1. Preoperative T2-weighted MRI sequences obtained from an extensive SEA in a 70-year-old man. Midsagittal views showing the SEA (A,D,G). Axial imaging at C-3 (B), C-5/6 (C), T-6/7 (E), T-10/11 (F), L-1/2 (H) and L-2/3 (I) showing extensive ventral collection. Mixed-high signals (white arrow) on T2-weighted imaging indicate the spondylodiscitis at the L3/4 segment (G).
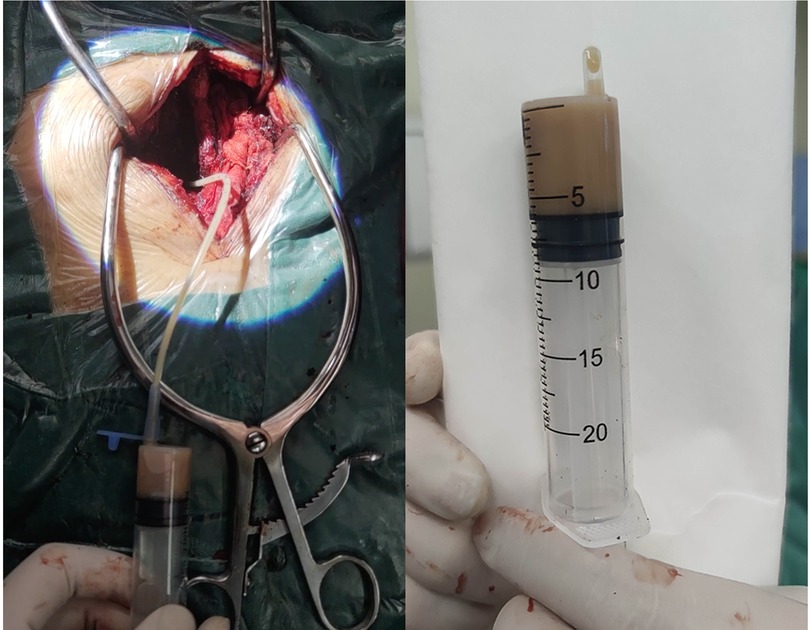
The patient's general condition was critical, with progressive weakness of the left lower extremity. The extensive SEA was the ultimate cause of this weakness. Therefore, laminectomy and abscess drainage were performed urgently. During the surgery, the internal fixations in L4 and L5 were taken out firstly. Then, L3/4 left hemilaminectomy was performed to break through the posterior longitudinal ligament, and a pale-yellow purulent fluid was observed. A 3-mm-diameter ventricular drainage tube was gently and gradually guided cephalad along the ventral side, and the operating table was tilted (reverse Trendelenburg) for drainage (Figure 2). In total, about 20 ml of purulent fluid was drained. The collected samples were sent for Gram staining and bacteriological culturing. Irrigation and evacuation procedures were repeated until no abscess or purulent material was observed. The incision was repeatedly and carefully douched with iodine and saline with vancomycin. Finally, the incisions were closed after the negative pressure drainage vessel was retained. As the patient was allergic to vancomycin, empirical intravenous antibiotics with ceftriaxone (2.0 g/day) were administered on the right postoperative day. After surgery, the patient was placed on the bed with his head elevated and his foot lowered and drainage was continued. The patient's blood and drained pus cultures were positive for multidrug-resistant S. epidermidis. Antibiotic therapy was, therefore, changed to linezolid (0.6 g/day) and rifampicin (0.6 g/day) based on the antibiotic sensitivity profile of the bacteria. Seven days after surgery, the patient's lower back pain persisted, with a VAS score of 6, and the muscle power was 4/5 in the left lower extremity. Twelve days after surgery, re-examination of the spinal MRI showed that the abscess volume was slightly reduced and the pressure in the purulent cavity was relieved. However, mixed-high signals appeared in the lumbar incision on T2-weighted imaging, indicating aggravation of local infection (Figures 3A–I).
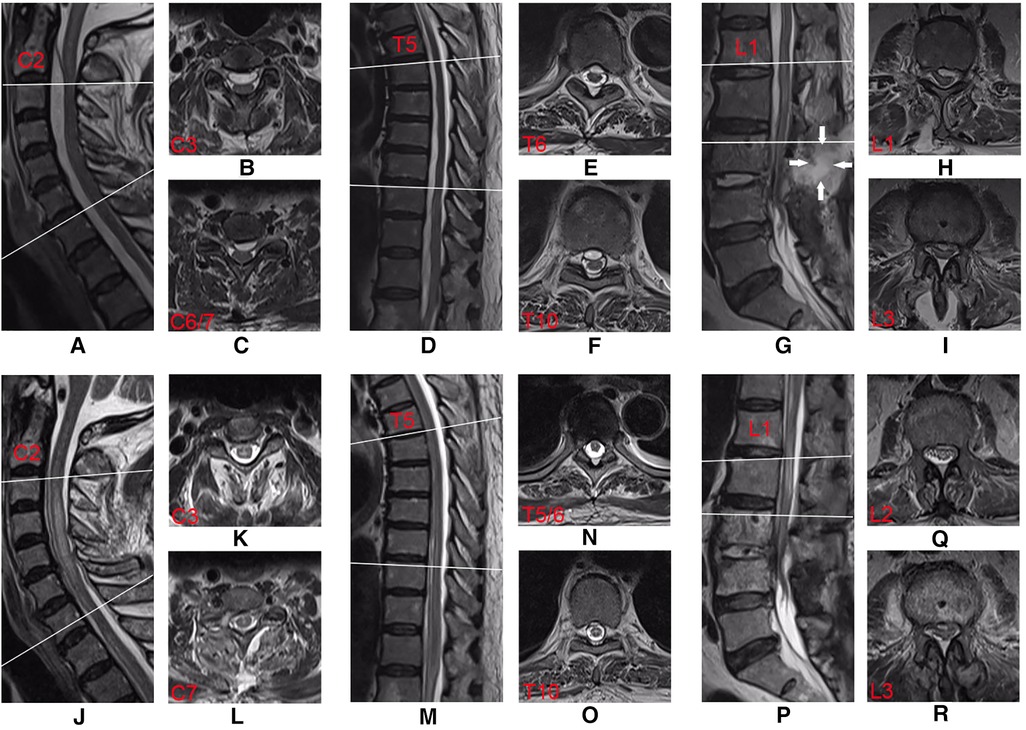
Figure 3. Postoperative T2-weighted MRI sequences of the first surgery (A–I) and second surgery (J–R). Comparing with pre-operation, the midsagittal view (A,D,G) illustrated slightly reduction of epidural abscess at cervical, thoracic, and lumbar levels. Mixed-high signals (white arrows) on T2-weighted imaging indicate that the lumbar incision infection was aggravated (G). The midsagittal view (J,M,P) and axial view (K,L,N,O,Q,R) showed the absence of epidural abscess, and the compression has significantly relieved after the second surgery.
To further relieve spinal cord compression and clean up local infection and necrotic tissue of the original lumbar incision, hemilaminectomy was performed again at the center of C6/7 and L3/4 on the 14th day after the first surgery. During surgery, part of the C6/7 lamina was removed, the posterior longitudinal ligament was broken through, a ventricular drainage tube was inserted into the caudal sides along the ventral side, and about 3 ml of purulent fluid was drained. The abscess was repeatedly irrigated with saline. The incision was repeatedly and carefully douched with iodine solution and saline with vancomycin, and a negative-pressure drainage vessel was retained outside the epidural space. Thereafter, the original lumbar incision was opened again, and the local necrotic tissue was cleaned. L3/4 right hemilaminectomy was performed, and a ventricular drainage tube was guided cephalad along the ventral side. Approximately 6 ml of purulent fluid was drained, and the purulent cavity was repeatedly irrigated with saline. The intervertebral infection and lesion tissues were cleaned again; gentamicin sulfate (4 ml) and vancomycin-loaded calcium sulfate (10 ml) were added to sterile water (6 ml); the mixture was converted into artificial bone granules, approximately 3 mm in size, and implanted into the L3/4 intervertebral space. Finally, the incisions were closed after the negative pressure drainage vessel was retained.
After surgery, linezolid (0.6 g/day) and rifampicin (0.6 g/day) were continued for anti-infection therapy. Blood and drained pus cultures were negative three days after the second surgery. Twelve days after the second surgery, pain was relieved, with a VAS score of 2, and the muscle power remained 4/5 in the left lower extremity. Re-examination of the spinal MRI showed that the abscess in the spinal canal had almost disappeared, and the compression was significantly relieved (Figures 3J–R). The schema of antibiotics application and time course for CRP, WBC, and ESR are shown in Figure 4. Thirty days after the second surgery, almost all neurological symptoms disappeared and the muscle power of the extremities was 4–5/5.
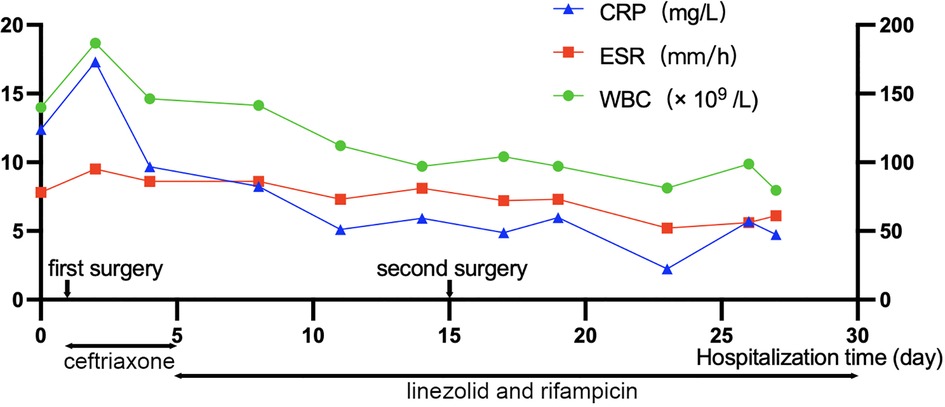
Figure 4. Schema of antibiotics application and time course for C-reactive protein (CRP), white blood cell (WBC), and erythrocyte sedimentation rate (ESR). The horizontal axis indicates the days of hospitalization, and the vertical axis indicates the levels of CRP, WBC, and ESR. The two-way arrows below the graph indicate the intravenous antibiotic treatment.
3. Discussion
SEA can be secondary to bacteremia caused by any factor (15), of which diabetes is a common complication, occurring in 21%–43% of patients with SEA (8). The placement of an epidural catheter is also an important risk factor for SEA, with an incidence rate of 0.5%–3.0% (23, 24). Other potential risk factors include intravenous drug injection, HIV infection, trauma, tattooing, acupuncture, and adjacent bone or soft tissue infection. Herein, the patient had a history of diabetes mellitus and lumbar internal fixation surgery, which was followed by insertion of an epidural drainage tube. We believe that this patient had a delayed infection after surgery, which caused extensive SEA, and diabetes mellitus was one of the aggravating factors.
Over 70% of SEA instances, according to Reihsaus et al. (10), are caused by systemic or localized inflammatory disorders or immunodeficiency diseases, and about 22% occur postoperatively. Methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) are responsible for about 70% of infections. Escherichia coli, Pseudomonas aeruginosa, S. epidermidis, and other pathogens have also been discovered (25). S. epidermidis infection is extremely rare (26, 27). In recent years, S. epidermidis gradually evolved into an important opportunistic pathogen, mainly causing medical device-related infections (28, 29). It is the primary pathogen behind catheter-related infections, early newborn sepsis, joint prosthesis infections, prosthetic valve endocarditis, and other infections linked to biomedical devices (30–33). A more serious problem with medical device-related infections is that they are often caused by multidrug-resistant bacteria, which produce adhesion factors and capsules on the surface of implanted devices that reduce the efficacy of antibacterial treatment and may even require removal of implants to completely eliminate the infection (28).
Early symptoms of SEA, including fever and fatigue, are usually non-specific. The classic triad of diagnostic significance is fever, back pain, and neurological dysfunction (9). Unfortunately, <8% patients exhibit these three manifestations simultaneously (8). For severe localized back pain and fever, SEA or spinal osteomyelitis should be considered. However, when patients seek medical advice for back pain only, they are rarely considered to have SEA. Nearly 75% of patients ultimately diagnosed with SEA are believed to have diagnostic delays, several trips to the emergency room, hospitalizations without a diagnosis, or time-lag >24 h before a conclusive diagnostic test (9, 34). We believe that patients with back pain who may be suspected with SEA should undergo a comprehensive physical examination; ESR, CRP levels, and blood culture should be checked immediately. Blood culture results are often consistent with subsequent pus culture results; thus, pathogens can be determined in advance, and these results can guide early intervention and treatment. If ESR is significantly elevated, MRI should be performed immediately. Currently, MRI is a powerful tool for diagnosing SEA (35). Through standard and enhanced scanning, we can understand the condition of the vertebral body and surrounding soft tissues of the spine, the scope and composition of the abscess, the compression of the dural sac, and the degree of spinal cord injury. Moreover, through re-examination, MRI can be used as an indicator to evaluate efficacy. Additionally, noninvasive MRI can avoid the risk of infection of subarachnoid space through injection of contrast agent or puncture and aspiration; for patients with complete spinal canal obstruction, it can also prevent the aggravation of nerve injury by injection of contrast agent.
Currently, there are no randomized controlled trials comparing the results of conservative treatment (non-surgical treatment) and surgical treatment for SEA. Most reports are retrospective, single-center case studies, or case reports. Many retrospective studies have selection bias, i.e., patients in the conservative treatment group have smaller abscesses and better initial neurological functional statuses than those in the surgical treatment group (36). There is no clear consensus on the treatment of SEA, and disagreement persists between non-surgical and surgical treatments. Early literatures suggest surgical decompression for all patients with SEA, while a recent systematic retrospective study shows that an increasing number of patients with only back pain but no neurological symptoms received non-surgical treatment; there was no statistically significant difference between the results of the surgical treatment group and those of the non-surgical treatment group (25). However, for neurological symptoms or extensive compression in patients with SEA, early intervention is required to avoid irreversible neurological damage. SEA treatment completely eliminates pathogenic microorganisms and suppress purulent secretions. Early surgical decompression and drainage improve the final prognosis (37). Surgeons prefer laminectomy and decompression; however, the scope of laminectomy remains uncertain. Schultz et al. (38) suggested selective laminectomies at the rostral and caudal poles of the abscess with subsequent drainage, whereas others performed laminectomies focused at the apices of natural spinal lordosis and kyphosis of the cervical spine at C-4, C-5, or C-6, in the thoracic spine at T-6, T-7, or T-8, and in the lumbar spine at L-3 or L-4 (21). There is also a report on the surgical treatment of extensive SEA with whole spinal laminectomies (39). However, extensive multi-segment laminectomies may not be performed for the following reasons: severe or critical patient condition, risk of mechanical instability, long times of surgery, and high operative blood loss (40–42).
We reviewed the extensive SEA cases from 2010 to 2022. The electronic database PubMed was searched, and the search terms included “extensive spinal epidural abscess” or “holocord spinal epidural abscess”. 21 cases (Table 1), which the abscess covered three or more spinal regions, were ultimately selected with a wide range age, including infant (8-month-old) and older patient (81-year-old). Notably, 10 of the 21 patients had diabetes mellitus, and five patients had soft tissue infection in other parts. In 14 of the 21 patients, the abscess was dorsal to the dural sac, in (only) two, it was ventral; and in the remaining five, it was peripheral to the dural sac; 12 cases were infected by S. aureus (including MSSA and MRSA), and three were infected by Streptococcus. In total, 19 patients chose laminectomy and epidural catheter irrigation, but internal fixators were not used to stabilize their spines. Only two patients chose abscess puncture irrigation and drainage exclusively. Ultimately, most patients' extremity power significantly improved. Herein, most patients underwent multi-segment laminectomies and epidural catheter irrigation and drainage. Catheter irrigation reduced severity of the infection, stopped the spread of the inflammation, and successfully released spinal cord compression (22). In all cases of laminectomies, surgery at the cervicothoracic, thoracolumbar, or lumbosacral connections is never performed during laminectomies to minimize instability (6, 60, 61).
Eroshkin et al. (46) reported two cases of extensive SEA and successfully inserted a drainage tube throughout the spinal canal. The abscess was then fully drained and irrigated. However, in our case, it was very difficult to insert the drainage tube during surgery, and the drainage was insufficient. The main considerations were: (1) possible separations in the abscess, which hindered insertion of the drainage tube; (2) negative pressure in the abscess made suction and drainage difficult; and (3) the abscess, being a non-Newtonian fluid was highly viscous, impeding suction and drainage. During surgery, samples for bacterial culture and drug sensitivity tests indicated the presence of sensitive antibiotics. For patients with a preliminary diagnosis of SEA, the empirical scheme used was vancomycin + cefotaxime/ceftriaxone/cefepime/ceftazidime, and antibiotic treatment generally lasted for 4–8 weeks (62). Because of the severe gastrointestinal reaction after vancomycin administration, this patient only empirically selected ceftriaxone. The postoperative drug sensitivity test results suggested that the patient was treated with linezolid and rifampicin for 3 weeks. Re-examination showed that the abscess in the spinal canal had disappeared, and spinal cord compression was relieved. We removed the internal fixations, performed laminectomy, decompressed the vertebral canal, drained the abscess, removed the intervertebral infection and lesion tissues, and implanted vancomycin-loaded calcium sulfate. The main reasons are as follows: (1) internal fixations may be the source of infection, so we removed them, and (2) artificial disc or interbody fusion cage and other implants cannot be planted into the infected intervertebral disc, and the vancomycin-loaded calcium sulfate implantation can achieve long-term interbody fusion, stabilize the spine, and prevent infection.
Because of the rarity of this disease, the acuteness, and the varied range of lesions, there were also deficiencies and irregularities in the diagnosis and treatment: (1) lack of experienced clinicians prevented timely diagnosis and treatment, causing rapid progression of the disease and the spread of abscess; (2) in the first operation, only single-segment laminectomy and abscess drainage were performed, and drainage was insufficient; (3) during the surgery, laminectomy was performed and the pedicle screws were removed, and vancomycin-loaded calcium sulfate was implanted in the intervertebral disc. It remained unclear if instability of the spine in the later period would still require long-term follow-up observation; (4) the abscess was not continuously irrigated with antibiotics after surgery; and (5) the patient was placed on bed after surgery with his head elevated and his foot lowered to utilize gravity for further drainage, but this also caused pus accumulation in the lumbar incision aggravating the infection at that site. Therefore, the case can be used as a reference for early diagnosis, improving the treatment plan, and avoiding future issues.
4. Conclusion
Early diagnosis is the key to treating extensive SEA. For patients with back pain who may be suspected with this disease, a comprehensive emergency assessment should be performed immediately. For patients with neck or back pain, spinal cord degeneration, neurological dysfunction, or signs of systemic infection, ESR, CRP, blood culture, and MRI should be performed immediately. Based on neurological tests and medication therapy failure, surgical techniques should be considered if SEA is discovered during imaging. For extensive SEA with clear diagnosis, decompression treatment should be performed at an early stage, when neurological symptoms, spinal cord signal changes, or extensive compression occur. The mode and scope of decompression should follow the principles of fully relieving spinal cord compression and draining purulent secretions with minimal trauma. For patients without neurological symptoms or those with known pathogens who respond to antibiotic treatment, surgical treatment may not be required, but this approach must be undertaken cautiously because neurological dysfunction may be aggravated or become fatal at any time. The treatment duration should be comprehensively evaluated, combining results from the patient's clinical manifestations, laboratory indicators (WBC, ESR, and CRP levels), and imaging examination. Administering broad-spectrum intravenous antibiotics immediately and ongoing medical attention are crucial part for treating extensive SEA. All patients with acute exacerbation should have early surgical intervention for extensive SEA as an adjuvant therapeutic option.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-WP wrote the draft of the manuscript and participated in the follow-up examination of the patient and clinical material. Y-WP, YG, and D-JZ participated in the surgical and medical treatment and followed up the patient. Y-XT and L-MJ have been involved in drafting the manuscript or revising it critically. J-JJ and D-XZ performed the surgery, coordinated and helped to draft and finalize the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Jilin University Research Program: Development of spine surgery image recognition software (2019220101002305).
Acknowledgments
We are thankful for the patient supplying personal and imaging information and thank all colleagues involved in the study for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tetsuka S, Suzuki T, Ogawa T, Hashimoto R, Kato H. Spinal epidural abscess: a review highlighting early diagnosis and management. JMA J. (2020) 3(1):29–40. doi: 10.31662/jmaj.2019-0038
2. Sharfman ZT, Gelfand Y, Shah P, Holtzman AJ, Mendelis JR, Kinon MD, et al. Spinal epidural abscess: a review of presentation, management, and medicolegal implications. Asian Spine J. (2020) 14(5):742–59. doi: 10.31616/asj.2019.0369
3. Pradilla G, Ardila GP, Hsu W, Rigamonti D. Epidural abscesses of the CNS. Lancet Neurol. (2009) 8(3):292–300. doi: 10.1016/s1474-4422(09)70044-4
4. Rosc-Bereza K, Arkuszewski M, Ciach-Wysocka E, Boczarska-Jedynak M. Spinal epidural abscess: common symptoms of an emergency condition. A case report. Neuroradiol J. (2013) 26(4):464–8. doi: 10.1177/197140091302600411
5. Artenstein AW, Friderici J, Holers A, Lewis D, Fitzgerald J, Visintainer P. Spinal epidural abscess in adults: a 10-year clinical experience at a tertiary care academic medical center. Open Forum Infect Dis. (2016) 3(4):ofw191. doi: 10.1093/ofid/ofw191
6. Adogwa O, Karikari IO, Carr KR, Krucoff M, Ajay D, Fatemi P, et al. Spontaneous spinal epidural abscess in patients 50 years of age and older: a 15-year institutional perspective and review of the literature: clinical article. J Neurosurg Spine. (2014) 20(3):344–9. doi: 10.3171/2013.11.Spine13527
7. Schwab JH, Shah AA. Spinal epidural abscess: diagnosis, management, and outcomes. J Am Acad Orthop Surg. (2020) 28(21):e929–38. doi: 10.5435/jaaos-d-19-00685
8. Long B, Carlson J, Montrief T, Koyfman A. High risk and low prevalence diseases: spinal epidural abscess. Am J Emerg Med. (2022) 53:168–72. doi: 10.1016/j.ajem.2022.01.008
9. Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. (2004) 26(3):285–91. doi: 10.1016/j.jemermed.2003.11.013
10. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. (2000) 23(4):175–204; discussion 5. doi: 10.1007/pl00011954
11. Curry WT Jr, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. (2005) 63(4):364–71; discussion 71. doi: 10.1016/j.surneu.2004.08.081
12. Dandy W. Abscesses and inflammatory tumors in the spinal epidural space (so-called pachymeningitis Externa). Arch Surg. (1926) 13:477–94. doi: 10.1001/archsurg.1926.01130100021002
13. Karikari IO, Powers CJ, Reynolds RM, Mehta AI, Isaacs RE. Management of a spontaneous spinal epidural abscess: a single-center 10-year experience. Neurosurgery. (2009) 65(5):919–23; discussion 23–4. doi: 10.1227/01.Neu.0000356972.97356.C5
14. Grieve JP, Ashwood N, O’Neill KS, Moore AJ. A retrospective study of surgical and conservative treatment for spinal extradural abscess. Eur Spine J. (2000) 9(1):67–71. doi: 10.1007/s005860050012
15. Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. QJM. (2008) 101(1):1–12. doi: 10.1093/qjmed/hcm100
16. Farber SH, Murphy KR, Suryadevara CM, Babu R, Yang S, Feng L, et al. Comparing outcomes of early, late, and non-surgical management of intraspinal abscess. J Clin Neurosci. (2017) 36:64–71. doi: 10.1016/j.jocn.2016.10.035
17. Epstein NE. Timing and prognosis of surgery for spinal epidural abscess: a review. Surg Neurol Int. (2015) 6(Suppl 19):S475–86. doi: 10.4103/2152-7806.166887
18. Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). (2018) 160(3):487–96. doi: 10.1007/s00701-018-3467-2
19. Eltorai AEM, Naqvi SS, Seetharam A, Brea BA, Simon C. Recent developments in the treatment of spinal epidural abscesses. Orthop Rev (Pavia). (2017) 9(2):7010. doi: 10.4081/or.2017.7010
20. Du JY, Schell AJ, Kim CY, Trivedi NN, Ahn UM, Ahn NU. 30-day mortality following surgery for spinal epidural abscess: incidence, risk factors. Predictive Algorithm, and Associated Complications. Spine (Phila Pa 1976). (2019) 44(8):E500–9. doi: 10.1097/brs.0000000000002875
21. Abd-El-Barr MM, Bi WL, Bahluyen B, Rodriguez ST, Groff MW, Chi JH. Extensive spinal epidural abscess treated with “apical laminectomies” and irrigation of the epidural space: report of 2 cases. J Neurosurg Spine. (2015) 22(3):318–23. doi: 10.3171/2014.11.Spine131166
22. Smith GA, Kochar AS, Manjila S, Onwuzulike K, Geertman RT, Anderson JS, et al. Holospinal epidural abscess of the spinal axis: two illustrative cases with review of treatment strategies and surgical techniques. Neurosurg Focus FOC. (2014) 37(2):E11. doi: 10.3171/2014.5.Focus14136
23. Sethna NF, Clendenin D, Athiraman U, Solodiuk J, Rodriguez DP, Zurakowski D. Incidence of epidural catheter-associated infections after continuous epidural analgesia in children. Anesthesiology. (2010) 113(1):224–32. doi: 10.1097/ALN.0b013e3181de6cc5
24. Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the third national audit project of the royal college of anaesthetists. Br J Anaesth. (2009) 102(2):179–90. doi: 10.1093/bja/aen360
25. Lt A, Quach E, Nguyen V, Chang D, Sukul V, Kim BS. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus. (2014) 37(2):E4. doi: 10.3171/2014.6.Focus14127
26. Schein M, Fulcher W. Primary peritonitis with Staphylococcus Epidermidis following prosthetic spinal surgery. Surg Infect (Larchmt). (2005) 6(1):93–4. doi: 10.1089/sur.2005.6.93
27. Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion: a retrospective analysis of eight cases. Spine. (1997) 22(20):2444–50. doi: 10.1097/00007632-199710150-00023
28. Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. (2014) 27(4):870–926. doi: 10.1128/cmr.00109-13
29. Widerström M, Wiström J, Sjöstedt A, Monsen T. Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus Epidermidis and Staphylococcus Saprophyticus. Eur J Clin Microbiol Infect Dis. (2012) 31(1):7–20. doi: 10.1007/s10096-011-1270-6
30. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American heart association. Circulation. (2015) 132(15):1435–86. doi: 10.1161/cir.0000000000000296
31. Rosenthal VD, Maki DG, Mehta Y, Leblebicioglu H, Memish ZA, Al-Mousa HH, et al. International nosocomial infection control consortium (INICC) report, data summary of 43 countries for 2007-2012. Device-associated module. Am J Infect Control. (2014) 42(9):942–56. doi: 10.1016/j.ajic.2014.05.029
32. Kahl BC, Becker K, Löffler B. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin Microbiol Rev. (2016) 29(2):401–27. doi: 10.1128/cmr.00069-15
33. Sgro M, Shah PS, Campbell D, Tenuta A, Shivananda S, Lee SK. Early-onset neonatal sepsis: rate and organism pattern between 2003 and 2008. J Perinatol. (2011) 31(12):794–8. doi: 10.1038/jp.2011.40
34. Bhise V, Meyer AND, Singh H, Wei L, Russo E, Al-Mutairi A, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med. (2017) 130(8):975–81. doi: 10.1016/j.amjmed.2017.03.009
35. Teman AJ. Spinal epidural abscess. Early detection with gadolinium magnetic resonance imaging. Arch Neurol. (1992) 49(7):743–6. doi: 10.1001/archneur.1992.00530310091017
36. Tuchman A, Pham M, Hsieh PC. The indications and timing for operative management of spinal epidural abscess: literature review and treatment algorithm. Neurosurg Focus. (2014) 37(2):E8. doi: 10.3171/2014.6.Focus14261
37. Alton TB, Patel AR, Bransford RJ, Bellabarba C, Lee MJ, Chapman JR. Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J. (2015) 15(1):10–7. doi: 10.1016/j.spinee.2014.06.010
38. Schultz KD J, Comey CH, Haid RW Jr. Technical note. Pyogenic spinal epidural abscess: a minimally invasive technique for multisegmental decompression. J Spinal Disord. (2001) 14(6):546–9. doi: 10.1097/00002517-200112000-00015
39. Gorchynski J, Hwang J, McLaughlin T. A methicillin-resistant Staphylococcus aureus-positive holospinal epidural abscess. Am J Emerg Med. (2009) 27(4):514.e7–9. doi: 10.1016/j.ajem.2008.07.041
40. Martin-Benlloch JA, Maruenda-Paulino JI, Barra-Pla A, Laguia-Garzaran M. Expansive laminoplasty as a method for managing cervical multilevel spondylotic myelopathy. Spine (Phila Pa 1976). (2003) 28(7):680–4. doi: 10.1097/01.Brs.0000051913.55259.5f
41. Tai CL, Hsieh PH, Chen WP, Chen LH, Chen WJ, Lai PL. Biomechanical comparison of lumbar spine instability between laminectomy and bilateral laminotomy for spinal stenosis syndrome - an experimental study in porcine model. BMC Musculoskelet Disord. (2008) 9:84. doi: 10.1186/1471-2474-9-84
42. Oda I, Abumi K, Lü D, Shono Y, Kaneda K. Biomechanical role of the posterior elements, costovertebral joints, and rib cage in the stability of the thoracic spine. Spine (Phila Pa 1976). (1996) 21(12):1423–9. doi: 10.1097/00007632-199606150-00005
43. Tracz J, Judy B, Witham T. Management of holospinal epidural abscess. World Neurosurg. (2022) 166:49–51. doi: 10.1016/j.wneu.2022.07.024
44. Lee CY, Chen PC, Wu MH, Huang TJ, Chang CC, Wang PY, et al. Minimally invasive surgical treatment of extensive spinal epidural abscess with unilateral laminotomy for bilateral decompression using an ultrasonic bone curette: a technique note. World Neurosurg. (2022) 168:111–9. doi: 10.1016/j.wneu.2022.09.089
45. Koyama K, Aoki Y, Inoue M, Kubota G, Watanabe A, Nakajima T, et al. Skip decompression surgeries in the treatment of holospinal epidural abscess: a case report. Spinal Cord Ser Cases. (2021) 7(1):38. doi: 10.1038/s41394-021-00401-w
46. Eroshkin A, Romanukha D, Voitsekhovskyi S. Surgical management of an extensive spinal epidural abscess: illustrative cases. J Neurosurg Case Lessons. (2021) 1(2):Case2050. doi: 10.3171/case2050
47. Roberti F. Tailored minimally invasive tubular laminectomies for the urgent treatment of rare holocord spinal epidural abscess: case report and review of technique. J Spine Surg. (2020) 6(4):729–35. doi: 10.21037/jss-20-603
48. Fujii M, Shirakawa T, Shime N, Kawabata Y. Successful treatment of extensive spinal epidural abscess with fluoroscopy-guided percutaneous drainage: a case report. JA Clin Rep. (2020) 6(1):4. doi: 10.1186/s40981-020-0309-z
49. Supreeth S, Al Ghafri K. Ventral holocord spinal epidural abscess managed surgically in a critical setting. Surg Neurol Int. (2019) 10:248. doi: 10.25259/sni_306_2019
50. Saito K, Fukazawa R, Ogura S, Kasai T, Mizuno T. A case of extensive epidural abscess concomitant with intracranial involvement due to Staphylococcus Aureus successfully treated with ceftriaxone in combination with linezolid and rifampin. eNeurologicalSci. (2019) 14:1–3. doi: 10.1016/j.ensci.2018.11.025
51. Thomson C. Spinal cord compression secondary to epidural abscess: the importance of prompt diagnosis and management. BMJ Case Rep. (2018) 2018. doi: 10.1136/bcr-2017-220694
52. Dang V, Rajkumar A. Spinal epidural abscess caused by a community acquired extended spectrum beta lactamase producing klebsiella pneumonia. IDCases. (2018) 13:e00438. doi: 10.1016/j.idcr.2018.e00438
53. Kurudza E, Stadler JA 3rd., Pediatric holocord epidural abscess treated with apical laminotomies with catheter-directed irrigation and drainage. Cureus. (2019) 11(9):e5733. doi: 10.7759/cureus.5733
54. Okada N, Nishiyama T, Kurihara M, Nishimura Y, Nishimura Y, Ando Y, et al. A case of panspinal epidural abscess that presented with meningeal irritation. Acute Med Surg. (2017) 4(3):363–6. doi: 10.1002/ams2.294
55. Sinatra PM, Alander DH. Lemierre disease: a case with multilevel epidural abscess and aggressive neurological weakness: case report and literature review. J Pediatr Orthop. (2017) 37(1):e58–61. doi: 10.1097/bpo.0000000000000652
56. Xiang H, Ma X, Shen N, Yue B, Zhang G, Chen B. Holocord spinal epidural abscess: case report and literature review. Orthop Traumatol Surg Res. (2016) 102(6):821–5. doi: 10.1016/j.otsr.2016.05.004
57. Oh GS, Abou-Al-Shaar H, Arnone GD, Barks AL, Hage ZA, Neckrysh S. Spinal epidural abscess in a patient with Piriformis pyomyositis. Surg Neurol Int. (2016) 7(Suppl 38):S911–3. doi: 10.4103/2152-7806.194518
58. Verma R, Chaudhari TS, Lachuriya G. Spontaneous extensive spinal epidural abscess presenting as acute quadriparesis. BMJ Case Rep. (2014) 2014, doi: 10.1136/bcr-2014-204892
59. Smith C, Kavar B. Extensive spinal epidural abscess as a complication of Crohn’s disease. J Clin Neurosci. (2010) 17(1):144–6. doi: 10.1016/j.jocn.2009.02.038
60. Patel A, Alton T, Bransford R, Lee M, Bellabarba C, Chapman J. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. (2014) 14:326–30. doi: 10.1016/j.spinee.2013.10.046
61. Carpenter W, Afshar N, Mihara K. Spinal epidural abscess with discitis and vertebral osteomyelitis. J Gen Intern Med. (2012) 27:1560. doi: 10.1007/s11606-012-2066-9
Keywords: spinal epidural abscess (SEA), staphylococcus epidermidis, laminectomy, drainage, antibiotic therapy
Citation: Pi Y, Gong Y, Jiang J, Zhu D, Tong Y, Jiang L and Zhao D (2023) Extensive spinal epidural abscess caused by Staphylococcus epidermidis: A case report and literature review. Front. Surg. 10:1114729. doi: 10.3389/fsurg.2023.1114729
Received: 2 December 2022; Accepted: 20 February 2023;
Published: 8 March 2023.
Edited by:
Siegmund Lang, University Medical Center Regensburg, GermanyReviewed by:
Vadim Byvaltsev, Irkutsk State Medical University, RussiaJonas Krückel, University Medical Center Regensburg, Germany
Soumaya Boussaid, Hôpital La Rabta, Tunisia
© 2023 Pi, Gong, Jiang, Zhu, Tong, Jiang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-xu Zhao ZHh6aGFvQGpsdS5lZHUuY24=
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Yang-wei Pi
Yang-wei Pi Yan Gong
Yan Gong Yue-xin Tong
Yue-xin Tong Dong-xu Zhao
Dong-xu Zhao