
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 01 March 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1113659
This article is part of the Research Topic Advances in the Surgical Management of Gastric and Colorectal Cancers View all 35 articles
Purpose: The aim of this study was to evaluate the effect of carbon nanoparticles staining (CNS) on colorectal cancer (CRC) surgery, lymph node tracing and postoperative complications using propensity score matching (PSM).
Method: Patients who were diagnosed with CRC and underwent surgery were retrospectively collected from a single clinical center from Jan 2011 to Dec 2021. Baseline characteristics, surgical information and postoperative information were compared between the CNS group and the non-CNS group. PSM was used to eliminate bias.
Results: A total of 6,886 patients were enrolled for retrospective analysis. There were 2,078 (30.2%) patients in the CNS group and 4,808 (69.8%) patients in the non-CNS group. After using 1: 1 ratio PSM to eliminate bias, there were 2,045 patients left in each group. Meanwhile, all of their baseline characteristics were well matched and there was no statistical significance between the two groups (P > 0.05). In terms of surgical information and short-term outcomes, the CNS group had less intraoperative blood loss (P < 0.01), shorter operation time (P < 0.01), shorter postoperative hospital stay (P < 0.01), less metastatic lymph nodes (P = 0.013), more total retrieved lymph nodes (P < 0.01), more lymphatic fistula (P = 0.011) and less postoperative overall complications (P < 0.01) than the non-CNS group before PSM. After PSM, the CNS group had less intraoperative blood loss (P = 0.004), shorter postoperative hospital stay (P < 0.01) and more total retrieved lymph nodes (P < 0.01) than the non-CNS group. No statistical difference was found in other outcomes (P > 0.05).
Conclusion: Preoperative CNS could help the surgeons detect more lymph nodes, thus better determining the patient's N stage. Furthermore, it could reduce intraoperative blood loss and reduce the hospital stay.
Colorectal cancer (CRC) is one of the most common malignancies among both men and women and the second leading cause of cancer-related death in the world (1–3). Currently, there are about 185 million CRC patients worldwide (4). CRC increases the burden on world health, especially on the elderly (5). Although the treatment has developed, radical CRC surgery is still the main treatment at present (6, 7). The current mainstream surgical approach is usually performed laparoscopically or robotically assisted, and robotic assistance shows advantages (8). In recent years, carbon nanoparticles staining (CNS) technology has been widely used to improve surgical outcomes (9).
The applications of carbon nanoparticles were developing rapidly (10, 11). It was proved that carbon nanoparticles were safe and reliable tracers for CRC (9). Carbon nanoparticles could selectively penetrate lymphatic vessels rather than capillaries. When they entered the lymphatic vessels, they could be phagocytized by macrophages, and then stained the lymph nodes black (12). Thus, it facilitated the detection of lymph nodes during pathological examination. Meanwhile, a sufficient number of lymph nodes was crucial for accuracy of a patient's cancer stage (13). Studies reported that the number of lymph nodes dissected should be ≥ 12 for more accurate staging of the patient's N stage (14). Accurate cancer staging could guide post-operative treatment, furthermore, optimiz patients' short-term outcomes and improve patients' survival rates (14).
At present, CNS has been widely used in the localization of CRC and lymph node tracking (14–17). However, the number of metastatic lymph nodes detected remained controversial. The CNS group was considered to have more metastatic lymph nodes compared with the non-CNS group (5, 12). Other studies reported that there was no difference in the rate of metastatic lymph nodes between the two groups (18–20). Therefore, the aim of this study was to evaluate the effect of CNS on lymph node tracing and postoperative complications for CRC surgery.
Patients who were diagnosed with CRC and underwent surgery were collected from a single clinical center from Jan 2011 to Dec 2021, retrospectively. A total of 6,886 patients were enrolled. This study was approved by the Institutional Ethics Committee of our hospital (2021–536), and informed consents were obtained from all patients.
We included CRC patients who underwent radical surgery in a single clinical center (n = 8152). The exclusion criteria were as follows: 1, non-R0 resection (n = 22); 2, recurrent CRC surgery (n = 47); 3, incomplete baseline information (n = 148); and 4, incomplete information of carbon nanoparticles staining (n = 1049). Ultimately, a total of 6,886 patients were enrolled (Figure 1).
The baseline characteristics, operation information and short-term outcomes were collected from electronic medical records. The baseline characteristics were as follows: age, sex, body mass index (BMI), smoking, drinking, hypertension, type 2 diabetes mellitus (T2DM), coronary heart disease (CHD), surgical history, open surgery, tumor location, tumor nodes metastasis (TNM) stage, tumor size and CNS. The operation information included: intraoperative blood loss and operation time. The postoperative information included: postoperative hospital stay, metastatic lymph nodes, total retrieved lymph nodes, anastomotic fistula, lymphatic fistula, postoperative major complications and postoperative overall complications.
PSM was used to reduce the intergroup bias in this study. We conducted PSM method including age, sex, BMI, smoking, drinking, hypertension, T2DM, CHD, surgical history, open surgery, tumor location, TNM stage and tumor size. The CNS group was matched to the non-CNS group by using the nearest neighbor matching at a 1:1 ratio, and within a caliper of 0.01.
Patients in the CNS group were injected carbon nanoparticles (1 ml; 50 mg, Chongqing lummy Co.) into the submucosal layer before surgery by electronic colonoscopy. The carbon nanoparticles entered the lymphatic vessels rather than the blood vessels. Then, the tumor and lymph nodes would be stained black (Figure 2).
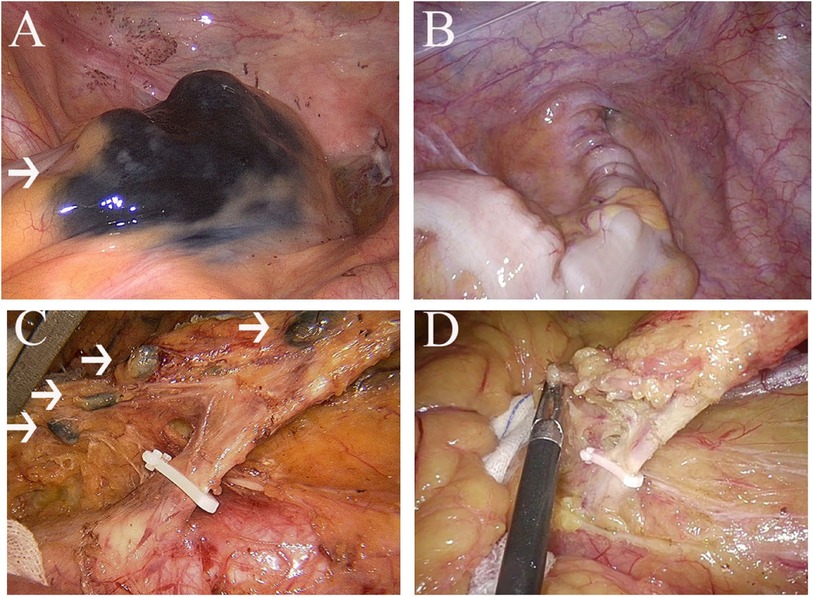
Figure 2. (A), nanocarbon stained tumour; (B), tumour without nanocarbon staining; (C), nanocarbon stained lymph nodes; (D), lymph nodes without nanocarbon staining.
The tumor node metastasis stage was diagnosed according to the AJCC 8th Edition (21). The complications were graded according to the Clavien-Dindo classification (22), and major complications were defined as ≥ III classification complications. The CNS group was defined as the patients who underwent injection of carbon nanoparticles before surgery, while the non-CNS group was defined as the patients who did not receive injection of carbon nanoparticles before surgery. R0 resection was defined as a negative margin on pathological examination.
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed as n (%). The Chi-square test was used to analyze categorical variables and the t-test was used to analyze continuous variables between the CNS group and the non-CNS group. Statistical analysis was performed by the SPSS (version 22.0) software. P-value of < 0.05 was considered statistically significant.
A total of 6,886 patients were included in this study according to the inclusion and exclusion criteria, and there were 2,078 (30.2%) patients in the CNS group and 4,808 (69.8%) patients in the non-CNS group. After 1:1 ratio PSM, there were 2,045 patients in each group (Figure 1). The mean age of the enrolled patients was 62.7 ± 12.4 years old. Meanwhile, 4,044 (58.7%) were males and 2,042 (41.3%) were females. In addition, more baseline characteristics including BMI, smoking, drinking, hypertension, T2DM, CHD, surgical history, open surgery, tumor location, TNM stage, tumor size, CNS, operation time, intraoperative blood loss and short-term outcomes were shown in Table 1.
The baseline characteristics before and after PSM were shown in Table 2. Before PSM, we found that the CNS group had a higher BMI (P < 0.01), a higher proportion of smoking (P = 0.037) and a higher proportion of open surgery (P < 0.01) than the non-CNS group. Meanwhile, significant difference was found in tumor location (P < 0.01) and TNM stage (P < 0.01). There was no difference in age, sex, drinking, hypertension, T2DM, CHD, surgical history or tumor size (P > 0.05). After PSM, all of these baseline characteristics were well matched and there was no statistical significance (P > 0.05).
The operation and postoperative characteristics of the two groups were compared before and after PSM, and the outcomes were shown in Table 3. The operation information included intraoperative blood loss and operation time. The postoperative information included postoperative hospital stay, metastatic lymph nodes, total retrieved lymph nodes, anastomotic fistula, lymphatic fistula, postoperative overall complications and postoperative major complications. Before PSM, intraoperative blood loss (P < 0.01), operation time (P < 0.01), postoperative hospital stay (P < 0.01), metastatic lymph nodes (P = 0.013), total retrieved lymph nodes (P < 0.01), lymphatic fistula (P = 0.011) and postoperative overall complications (P < 0.01) had significant differences in the two groups.
After PSM, the CNS group also had less intraoperative blood loss (P = 0.004), shorter postoperative hospital stay (P < 0.01) and more total retrieved lymph nodes than the Non-CNS group (P < 0.01).
There was a large sample size of 6,886 CRC patients in this study. After 1:1 ratio PSM, there were 2,045 patients left in each group. Before and after PSM, intraoperative blood loss, postoperative hospital stay and total retrieved lymph nodes were statistically significant. This suggested that CNS before surgery could help surgeons retrieve more lymph nodes, reduce intraoperative blood loss and reduce hospital stay. In term of total retrieved lymph nodes, we found that the CNS group retrieved more lymph nodes than the non-CNS group.
The guidelines of the European Society for Medical Oncology and the American Society of Clinical Oncology consider inadequate lymph nodes harvested to be one of the risk factors for stage II CRC (23, 24). Lymph nodes metastasis is an independent prognostic factor after radical resection for T1-2 CRC (25). Accurate TNM staging could guide postoperative chemoradiotherapy and improve the prognosis of CRC patients. However, it was required that the total number of lymph nodes detected exceeded twelve (26). Moreover, in order to accurately determine the pathological staging of patients with adenocarcinoma of esophagogastric junction, Zheng J et al. proposed that no less than 11 LNs must be resected in patients with stage T1-2 and no less than 16 LNs must be resected in patients with stage T3-4 (27). Meanwhile, it was found that the number of removed lymph nodes was positively correlated with the number of metastatic lymph nodes (28). Resection of more lymph nodes could improve the accuracy of postoperative pathological analysis. Lelin Pan et al. showed that the more lymph nodes were removed, the more accurate N-stage we obtained (18). However, a 2010 study indicated that lymph node detection rates for colorectal cancer remain low (29). A variety of lymph node staining were beginning to be used in clinical practice. Cawthorn et al. used xylene alcohol clearance technique to facilitate the identification of lymph nodes (30). Quadros et al. performed lymphoscintigraphy using technetium-99 m-phytate and patent blue to detect lymph nodes of rectal adenocarcinoma patients (31). However, these techniques are not widely used in clinical practice because they are time-consuming, labour-intensive and toxic to doctors (12). In this study, we used carbon nanoparticles for lymph node tracking and more total number of dissected lymph nodes in the CNS group were found than the non-CNS group. This could help surgeons obtain the accurate N stage of patients.
Although, the use of carbon nanoparticles increased the total number of lymph nodes detected, the number of metastatic lymph nodes detected remained controversial. Some studies reported that the rate of lymph node metastasis detected in the CNS group was higher than the non-CNS groups (5, 12). Other studies reported that there was no difference in the rate of metastatic lymph nodes between the two groups (18–20). The mechanism was not clear, but there were possible reasons as follows: first, CNS allowed for better anatomical clarity, making it more convenient for the operator to clear the lymph nodes. Second, it was found that there were many other factors that affected metastasis lymph node dissection, such as the patient's sex, age, tumor stage, type of surgery, and neoadjuvant chemotherapy (32–35). Although the information underlying the two groups was not statistically significant after PSM, we were still unable to determine their combined effect on metastatic lymph node dissection. We searched nine similar articles and listed some information in Table 4 (5, 12, 14, 18–20, 36–38). The main outcomes reported in these articles were the total or mean number of lymph nodes detected. Dissections of metastatic lymph nodes were reported in five articles (5, 12, 18–20). Two articles also reported that the dissections of microscopic lymph nodes in the CNS group was more than the non-CNS group (5, 12). Wang Q et al. reported the effect of CNS on intraoperative information (14). Complications and long-term survival without difference were reported by Wang LY et al. (20).
So far, CNS has been widely used. Studies reported that CNS could avoid aggressive axillary treatment of breast cancer (39). CNS was also reported to improve the outcomes of surgery for thyroid papillary carcinoma (40). In addition, CNS had a positive impact on the surgical results of early gastric cancer (41, 42). It was also reported that CNS played an essential role in lymph node dissection for non-small cell lung cancer (43). Fortunately, no significant adverse effects of CNS on patients have been identified at present. Van Tongeren MJ et al. did not demonstrate mutagenic or carcinogenic effects of carbon nanoparticles (44). Meanwhile, Magrez A et al. proved that CNS had no adverse effects on the central serious system, cardiovascular system and respiratory system (45). Expectantly, more systematic reactions to carbon nanoparticles are looking forward to be discovered. In the future, CNS may be widely used to improve patients' survival.
The application of CNS could also help surgeon obtain more convenience. Previous study found that CNS could help surgeons distinguish tissue structure (9). This finding could lead to less intraoperative blood loss and shorter postoperative hospital stay. Expectedly, it was found that the CNS group had less intraoperative blood loss and shorter postoperative hospital stay than the non-CNS group in this study. More benefits of CNS are waiting to be discovered by researchers.
To our knowledge, this was the first study to use PSM to analyze CNS on lymph node dissection, operation information and short-term outcomes for CRC. However, there were some limitations in this study. First, our data in this study came from a single clinical center. Second, we did not analyze the influence of other factors on lymph node dissection in detail. Third, there was no agreement on when patients should be injected carbon nanoparticles. Forth, lack of long-term patient outcomes. Therefore, prospective studies with a larger sample size were needed.
Preoperative CNS could help the surgeons detect more lymph nodes, thus better determining the patient's N stage. Furthermore, it could reduce intraoperative loss and reduce the hospital stay.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study was approved by the Institutional Ethics Committee of our hospital (2021–536), and informed consents were obtained from all patients.
All authors contributed to data collection. All the authors have agreed on the manuscript that will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
The authors are grateful to all the colleagues who helped in the preparation of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kang B, Liu XY, Li ZW, Yuan C, Zhang B, Wei ZQ, et al. The effect of the intraoperative blood loss and intraoperative blood transfusion on the short-term outcomes and prognosis of colorectal cancer: a propensity score matching analysis. Front Surg. (2022) 9:837545. doi: 10.3389/fsurg.2022.837545
2. Peng D, Liu XY, Cheng YX, Tao W, Cheng Y. Improvement of diabetes Mellitus after colorectal cancer surgery: a retrospective study of predictive factors for type 2 diabetes Mellitus remission and overall survival. Front Oncol. (2021) 11:694997. doi: 10.3389/fonc.2021.694997
3. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. (2020) 70(1):7–30. doi: 10.3322/caac.21590
4. Liu XY, Kang B, Cheng YX, Yuan C, Tao W, Zhang B, et al. The short-term and oncologic outcomes of younger VS older colorectal cancer patients undergoing primary surgery: a propensity score matching analysis. BMC Cancer. (2022) 22(1):153. doi: 10.1186/s12885-022-09246-4
5. Sun J, Zhang J. Assessment of lymph node metastasis in elderly patients with colorectal cancer by sentinel lymph node identification using carbon nanoparticles. J BUON. (2018) 23(1):68–72. PMID: 29552762
6. Cheng YX, Liu XY, Kang B, Tao W, Wei ZQ, Peng D. Comparison of surgical and oncologic outcomes in very elderly patients (≥ 80 years old) and elderly (65-79 years old) colorectal cancer patients: a propensity score matching. BMC Gastroenterol. (2022) 22(1):205. doi: 10.1186/s12876-022-02277-y
7. Jin Z, Wu Q, Deng X, Wang Z. The clinic factors in evaluating long-term outcomes of patients with stage I colorectal cancer. Asian J Surg. (2022) 45(11):2231–8. doi: 10.1016/j.asjsur.2021.11.055
8. Chen PJ, Su WC, Chang TK, Chen YC, Li CC, Yin TC, et al. Oncological outcomes of robotic-assisted total mesorectal excision after neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Asian J Surg. (2021) 44(7):957–63. doi: 10.1016/j.asjsur.2021.01.018
9. Liu P, Tan J, Tan Q, Xu L, He T, Lv Q. Application of carbon nanoparticles in tracing lymph nodes and locating tumors in colorectal cancer: a concise review. Int J Nanomedicine. (2020) 15:9671–81. doi: 10.2147/IJN.S281914
10. Dai LC, Yao X, Wang X, Niu SQ, Zhou LF, Fu FF, et al. In vitro and in vivo suppression of hepatocellular carcinoma growth by midkine-antisense oligonucleotide-loaded nanoparticles. World J Gastroenterol. (2009) 15(16):1966–72. doi: 10.3748/wjg.15.1966
11. Wu T, Zheng WL, Zhang SZ, Sun JH, Yuan H. Bimodal visualization of colorectal uptake of nanoparticles in dimethylhydrazine-treated mice. World J Gastroenterol. (2011) 17(31):3614–22. doi: 10.3748/wjg.v17.i31.3614
12. Cai HK, He HF, Tian W, Zhou MQ, Hu Y, Deng YC. Colorectal cancer lymph node staining by activated carbon nanoparticles suspension in vivo or methylene blue in vitro. World J Gastroenterol. (2012) 18(42):6148–54. doi: 10.3748/wjg.v18.i42.6148
13. Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg. (2006) 244(4):602–10. doi: 10.1097/01.sla.0000237655.11717.50
14. Wang Q, Chen E, Cai Y, Chen C, Jin W, Zheng Z, et al. Preoperative endoscopic localization of colorectal cancer and tracing lymph nodes by using carbon nanoparticles in laparoscopy. World J Surg Oncol. (2016) 14(1):231. doi: 10.1186/s12957-016-0987-1
15. Gill A, Brunson A, Lara P Jr, Khatri V, Semrad TJ. Implications of lymph node retrieval in locoregional rectal cancer treated with chemoradiotherapy: a California cancer registry study. Eur J Surg Oncol. (2015) 41(5):647–52. doi: 10.1016/j.ejso.2015.01.037
16. Li Z, Ao S, Bu Z, Wu A, Wu X, Shan F, et al. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: a prospective randomized trial. World J Surg Oncol. (2016) 14:88. doi: 10.1186/s12957-016-0835-3
17. Liu CL, Yang TL, Chen BF. Sentinel lymph node mapping with emulsion of activated carbon particles in patients with pre-mastectomy diagnosis of intraductal carcinoma of the breast. J Chin Med Assoc. (2003) 66(7):406–10. PMID: 14509402
18. Pan L, Ye F, Liu JJ, Ba XQ, Sheng QS. A study of using carbon nanoparticles to improve lymph nodes staging for laparoscopic-assisted radical right hemicolectomy in colon cancer. Int J Colorectal Dis. (2018) 33(8):1131–4. doi: 10.1007/s00384-018-3050-6
19. Ge W, Li Q, Liu WJ, Zhang XQ, Fan XS, Shao LH, et al. Carbon nanoparticle suspension could help get a more accurate nodal staging for patient with rectal cancer. Sci Rep. (2021) 11(1):9933. doi: 10.1038/s41598-021-89541-5
20. Wang LY, Li JH, Zhou X, Zheng QC, Cheng X. Clinical application of carbon nanoparticles in curative resection for colorectal carcinoma. Onco Targets Ther. (2017) 10:5585–9. doi: 10.2147/OTT.S146627
21. Weiser MR. AJCC 8th Edition: colorectal cancer. Ann Surg Oncol. (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
22. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
23. Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. (2004) 22(16):3408–19. doi: 10.1200/JCO.2004.05.063
24. Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: eSMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2013) 24(Suppl 6):vi64–72. doi: 10.1093/annonc/mdt354
25. Huang X, Liu H, Liao X, Xiao Z, Huang Z, Li G. Prognostic factors for T1-2 colorectal cancer after radical resection: lymph node distribution is a valuable predictor of its survival. Asian J Surg. (2021) 44(1):241–6. doi: 10.1016/j.asjsur.2020.06.013
26. Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2017) 15(3):370–98. doi: 10.6004/jnccn.2017.0036
27. Zheng J, Yan Q, Hu W, Luo B, Li Y. Minimum number of lymph nodes necessary for the accurate staging of adenocarcinoma of esophagogastric junction. Asian J Surg. (2022) S1015- 9584(22):01147–2. doi: 10.1016/j.asjsur.2022.08.034
28. Kotake K, Honjo S, Sugihara K, Hashiguchi Y, Kato T, Kodaira S, et al. Number of lymph nodes retrieved is an important determinant of survival of patients with stage II and stage III colorectal cancer. Jpn J Clin Oncol. (2012) 42(1):29–35. doi: 10.1093/jjco/hyr164
29. Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. (2005) 97(3):219–25. doi: 10.1093/jnci/dji020
30. Cawthorn SJ, Gibbs NM, Marks CG. Clearance technique for the detection of lymph nodes in colorectal cancer. Br J Surg. (1986) 73(1):58–60. doi: 10.1002/bjs.1800730124
31. Quadros CA, Lopes A, Araújo I. Retroperitoneal and lateral pelvic lymphadenectomy mapped by lymphoscintigraphy for rectal adenocarcinoma staging. Jpn J Clin Oncol. (2010) 40(8):746–53. doi: 10.1093/jjco/hyq060
32. Chou JF, Row D, Gonen M, Liu YH, Schrag D, Weiser MR. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer. (2010) 116(11):2560–70. doi: 10.1002/cncr.25032
33. Ng SK, Lu CT, Pakneshan S, Leung M, Siu S, Lam AK. Harvest of lymph nodes in colorectal cancer depends on demographic and clinical characteristics of the patients. Int J Colorectal Dis. (2018) 33(1):19–22. doi: 10.1007/s00384-017-2927-0
34. Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. (2005) 41(2):272–9. doi: 10.1016/j.ejca.2004.10.010
35. Li J, Jia S, Wang Y, Zhang Y, Kong L, Cao Y, et al. Long-term tracing and staining of carbon nanoparticles for axillary lymph nodes in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy. Asian J Surg. (2022) 45(1):89–96. doi: 10.1016/j.asjsur.2021.03.020
36. Tang L, Sun L, Zhao P, Kong D. Effect of activated carbon nanoparticles on lymph node harvest in patients with colorectal cancer. Colorectal Dis. (2019) 21(4):427–31. doi: 10.1111/codi.14538
37. Wang R, Mo S, Liu Q, Zhang W, Zhang Z, He Y, et al. The safety and effectiveness of carbon nanoparticles suspension in tracking lymph node metastases of colorectal cancer: a prospective randomized controlled trial. Jpn J Clin Oncol. (2020) 50(5):535–42. doi: 10.1093/jjco/hyaa011
38. Zhang XM, Liang JW, Wang Z, Kou JT, Zhou ZX. Effect of preoperative injection of carbon nanoparticle suspension on the outcomes of selected patients with mid-low rectal cancer. Chin J Cancer. (2016) 35:33. doi: 10.1186/s40880-016-0097-z
39. Du J, Zhang Y, Ming J, Liu J, Zhong L, Liang Q, et al. Evaluation of the tracing effect of carbon nanoparticle and carbon nanoparticle-epirubicin suspension in axillary lymph node dissection for breast cancer treatment. World J Surg Oncol. (2016) 14(1):164. doi: 10.1186/s12957-016-0925-2
40. Liu Y, Li L, Yu J, Fan YX, Lu XB. Carbon nanoparticle lymph node tracer improves the outcomes of surgical treatment in papillary thyroid cancer. Cancer Biomark. (2018) 23(2):227–33. doi: 10.3233/CBM-181386
41. Yan J, Zheng X, Liu Z, Yu J, Deng Z, Xue F, et al. A multicenter study of using carbon nanoparticles to show sentinel lymph nodes in early gastric cancer. Surg Endosc. (2016) 30(4):1294–300. doi: 10.1007/s00464-015-4358-8
42. Zhang L, Huang Y, Yang C, Zhu T, Lin Y, Gao H, et al. Application of a carbon nanoparticle suspension for sentinel lymph node mapping in patients with early breast cancer: a retrospective cohort study. World J Surg Oncol. (2018) 16(1):112. doi: 10.1186/s12957-018-1414-6
43. He J, Li S, Shao W, Wang D, Chen M, Yin W, et al. Activated carbon nanoparticles or methylene blue as tracer during video-assisted thoracic surgery for lung cancer can help pathologist find the detected lymph nodes. J Surg Oncol. (2010) 102(6):676–82. doi: 10.1002/jso.21684
44. Van Tongeren MJ, Kromhout H, Gardiner K. Trends in levels of inhalable dust exposure, exceedance and overexposure in the European carbon black manufacturing industry. Ann Occup Hyg. (2000) 44(4):271–80. doi: 10.1016/S0003-4878(99)00109-X
Keywords: carbon nanoparticles, colorectal cancer, surgery, lymph nodes tracing, propensity score matching
Citation: Liu F, Peng D, Liu X, Liu X, Li Z, Wei Z and Wang C (2023) The effect of carbon nanoparticles staining on lymph node tracking in colorectal cancer: A propensity score matching analysis. Front. Surg. 10:1113659. doi: 10.3389/fsurg.2023.1113659
Received: 1 December 2022; Accepted: 13 February 2023;
Published: 1 March 2023.
Edited by:
Bo Zhang, Sichuan University, ChinaReviewed by:
Alfonso De Stefano, G. Pascale National Cancer Institute Foundation (IRCCS), Italy© 2023 Liu, Peng, Liu, Liu, Li, Wei and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Yi Wang MTk2Mjk4OTg1M0BxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.