- Department of Orthopaedics and Tumor Orthopaedics, University Hospital Muenster, Muenster, Germany
The two-stage revision arthroplasty is a common treatment option for chronic periprosthetic infection (PJI). The time to reimplantation (TTR) reported in the literature varies substantially from a few days to several hundred days. It is hypothesized that longer TTR could be associated with worse infection control after second stage. A systematic literature search was performed according to Preferred Reporting items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, in Pubmed, Cochrane Library and Web of Science Core Collection in clinical studies published until January 2023. Eleven studies investigating TTR as a potential risk factor for reinfection met the inclusion criteria (ten retrospective and one prospective study, published 2012–2022). Study design and outcome measures differed notably. The cutoff points above which TTR was regarded as “long” ranged from 4 to 18 weeks. No study observed a benefit for long TTR. In all studies, similar or even better infection control was observed for short TTR. The optimal TTR, however, is not yet defined. Larger clinical studies with homogeneous patient populations and adjustment for confounding factors are needed.
Introduction
Periprosthetic joint infection (PJI) is a feared complication in orthopedic surgery that requires complex surgical procedures and long systemic treatments aiming at infection control. This is an enormous burden for affected patients and results in high costs for the health care system (1). The infection risk after primary total hip or knee arthroplasty is 1%–2% (2), but the risk for recurrence of infection can reach up to 50% in complex cases after multiple revisions (3–6). The current gold standard for chronic PJI is the two-stage revision arthroplasty (7, 8). A temporary polymethyl methacrylate (PMMA) spacer fills the debrided joint space, bridges bony defects, stabilizes the joint and ideally maintains the length of the extremity. In addition, local anti-infective substances mixed in the PMMA are released into the surrounding, reaching very high local concentrations, with little risk of systemic side effects (9). However, surgeons in clinical practice are confronted with the issue of timing second stage reimplantation surgery. From a patient's perspective, a short interval appears preferable to regain the ability to use the affected limb in everyday life. Yet, various factors such as comorbidities, clinical examination, laboratory results and organizational factors influence the time to reimplantation (TTR) (10). A widely adopted classification by Trampuz and Zimmerli defines intervals of two to four weeks (short interval) and six to eight weeks (long interval) until reimplantation (11). Other authors suggest four to six weeks (12), or nine weeks between the stages (13). However, spacer intervals reported in clinical studies often exceeded the time periods of guideline recommendations. They range from a few days to several hundred days, but mostly an average interval around 80 to 100 days is reported (4, 7, 14–22),. This heterogeneity in clinical practice indicates that an optimal interval period between the stages, has not been conclusively defined. In this study, we systematically searched the literature for studies that described two-stage revision arthroplasty of the hip and knee and analyzed the outcome “reinfection” in relation to the TTR.
Methods
The preferred reporting items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook for Systematic Reviews of Interventions were followed (23, 24).
Data sources
Electronic searches were performed in the databases PubMed (including MEDLINE; 1970 to 2023), Cochrane Library (1970 to 2023), and Web of Science Core Collection (1970 to 2023) to identify relevant studies. For PubMed and the Cochrane Library, index terms (MeSH-terms) were included and combined with free text words to search in title, abstract, and keywords. We used four concepts (1. Arthroplasty, 2. Infection, 3. Treatment, 4. Humans). These four concepts were combined with the Boolean operator “AND”. The operator “NOT” was used to exclude case reports and reviews. The search was performed on January 1, 2023. The full search strategy is available in the Supplementary Material.
Study selection
After identification of 6,010 publications, duplicates were removed and eligible studies were selected by the authors in three phases, resulting in eleven included studies (Figure 1). Eligibility criteria were set as follows: 1) population: Adult humans with chronic PJI of hip and knee, 2) intervention: treatment with completed two-stage revision arthroplasty 3) outcome: reinfection after the second stage; and 4) study design – retrospective cohort studies, prospective cohort studies and Randomized Controlled Trials (RCT). Technical notes were excluded. Only studies providing information on the time to reimplantation (TTR) after the first stage of a completed two-stage revision arthroplasty were included. We excluded the following studies: studies of paediatric patients; studies not involving endoprostheses of the hip and knee; treatment of septic arthritis of native joints, treatment of PJI with one-stage revision arthroplasty, DAIR procedure (Debridement, antibiotics, implant retention) or only partial removal of prosthesis components; studies that did not provide sufficient information on the surgery, experimental or animal studies; and studies written in languages other than English. After removal of duplicates, 5,659 titles and abstracts were screened. A total of 65 clinical studies evaluated the outcome of two-stage revision arthroplasty and reported on the time to reimplantation (TTR). The full-text analysis lead to the exclusion of 54 articles. Eleven studies met the inclusion criteria and were included in the analysis (Figure 1).
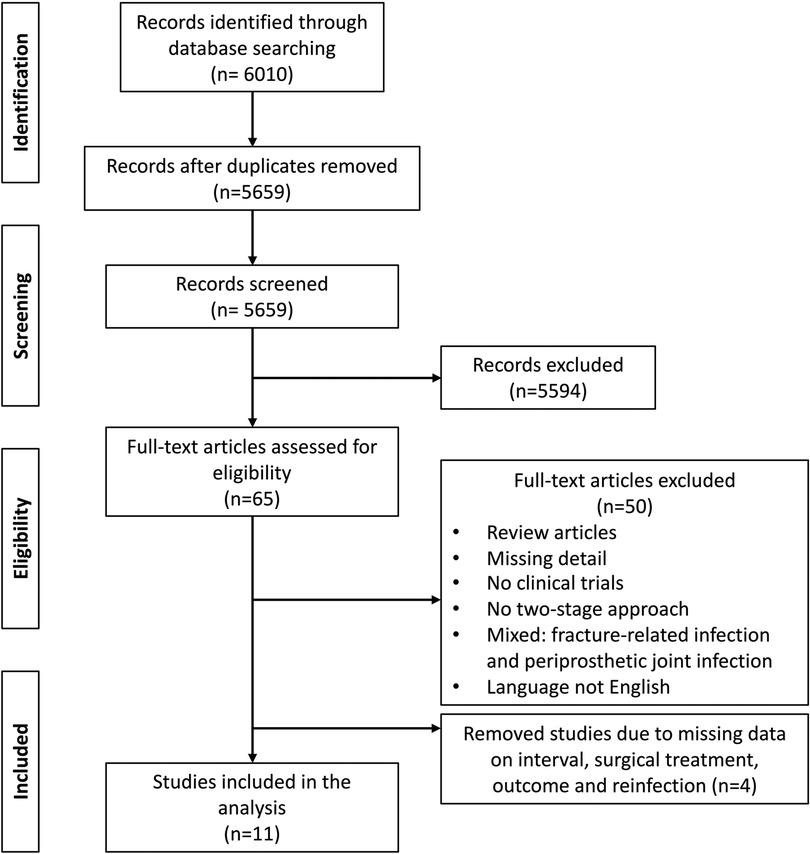
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram: eligibility assessment.
Data analysis
A descriptive analysis was performed by comparing the risk of reinfection in the observation period after completed second stage, in relation to the time to reimplantation (TTR: time interval between first and second stage). In addition, potential sources for bias were identified.
Results
Study characteristics
The included studies and their main characteristics are summarized in Table 1. All were published between 2012 and 2022 and reported on a total of 1,552 patients treated between 1996 and 2019. Ten studies were retrospective, and one was a prospective cohort study.
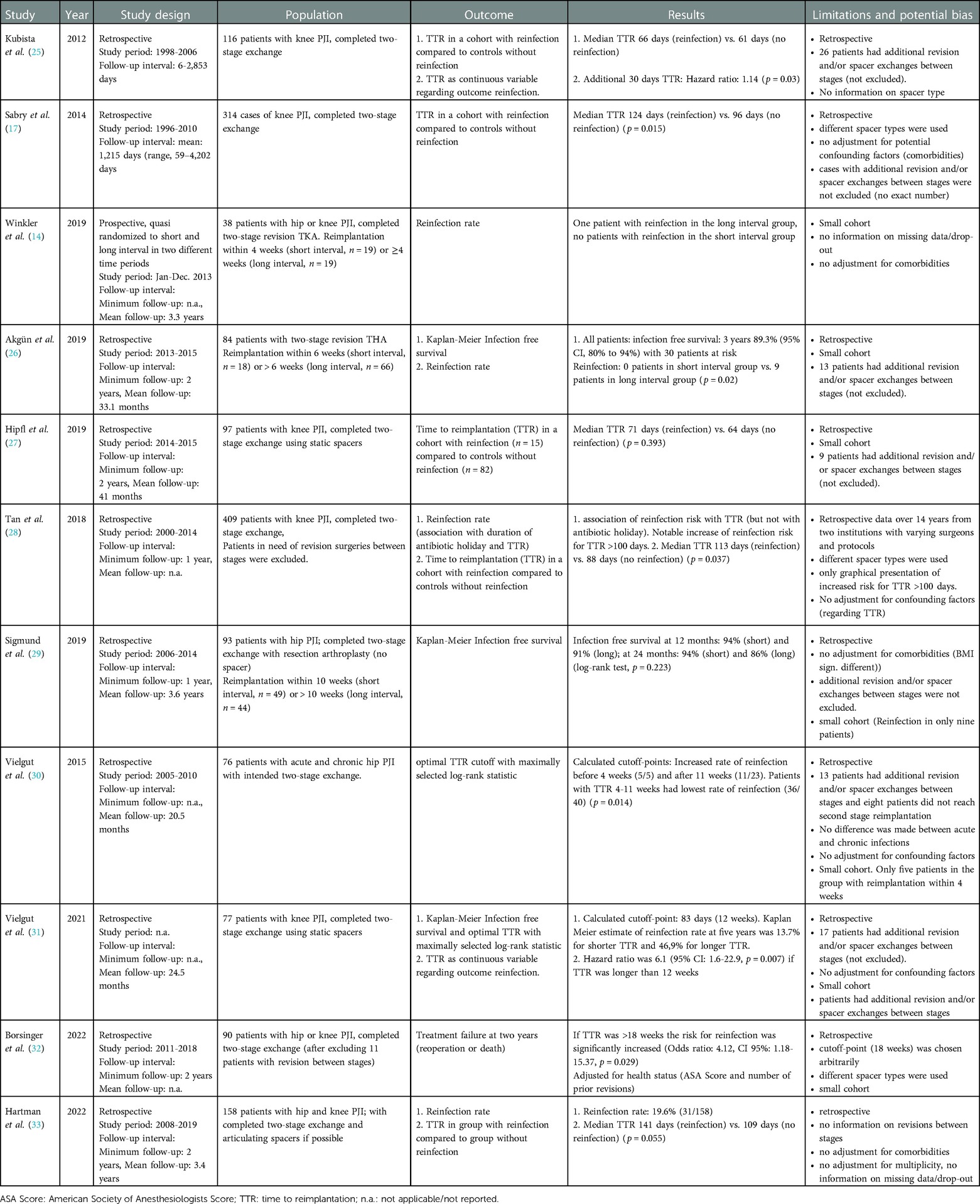
Table 1. Overview of eleven studies assessing the association of the time to reimplantation (TTR) and the risk of reinfection after two-stage revision arthroplasty.
Reinfection after two-stage revision arthroplasty and time to reimplantation (TTR)
Kubista et al. compared risk factors from 58 patients with reinfections after two-stage exchange of total knee arthroplasty (TKA) with 58 patients they randomly selected from a cohort without reinfection (25). The median TTR in their study was 66 days in the reinfected group and 61 days in control group. They also considered TTR as a continuous variable and calculated a hazard ratio for additional 30 days TTR of 1.14 (p = 0.03). However, they included a relevant proportion of patients that required additional revision and spacer exchanges before reimplantation (n = 26, 22%; n = 17 in the reinfected group and n = 9 in the group without reinfection p = 0.01). This could be a confounding factor as these revisions likely prolonged the TTR and are considered themselves a risk factor for reinfection (34–36).
Sabry et al. identified TTR as an independent risk factor among 314 patients with knee PJI undergoing a two-stage exchange with a median of 124 days until reimplantation in the reinfected group vs. 96 days in the group without reinfection (p = 0.015) (17). Again, patients requiring a spacer exchange in between the stages were not excluded from the analysis.
Winkler et al. published a small series of patients with hip and knee PJI receiving reimplantation either within four weeks (n = 19) or thereafter (n = 19) (14). Cases with difficult to treat microorganisms and patients with critical soft tissues were excluded. On average the short interval group had a mean of 17.9 days compared to 63 days in the long interval group. Only one reinfection was observed in this cohort in the long interval group, therefore the authors suggested that the shorter interval might at least achieve similar infection control compared to longer intervals.
Akgün et al. from the same group published a cohort of 18 patients with hip PJI in 2019 with an interval of less than 6 weeks and 66 patients with a longer interval (26). Mean time interval of all patients between stages was reported 60.9 days (8.7 weeks, range: 1–25). Girdlestone resection arthroplasty without the use of cement spacers was the preferred treatment approach. Thirteen patients required revision surgery between the stages due to infection persistence and were kept in the analysis. Reinfection was observed in none of the patients in the shorter interval group and in nine patients in the longer interval group, however this difference was not significant.
Hipfl et al. reviewed 97 cases of knee PJI with static spacers and reported an average TTR of 66 days for all patients (mean ± Standard Deviation: 9.4 ± 3.5 weeks) (27). Fifteen patients had a reinfection and their average TTR was 71 days (10.2 ± 4.0 weeks) compared to 64 days (9.2 ± 4.0 weeks) in uninfected patients, however this difference was not significant (p = 0.393). The lack of statistical validation may be due to the considerably small number of patients.
Tan et al. investigated the association of the antibiotic holiday with the risk for reinfection after two-stage revision in a large retrospective cohort of 409 patients in two institutions over 14 years from 2000 to 2014 (28). All patients that had additional surgery in the interim period between the stages were excluded. No association with the duration of the antibiotic holiday was found, but with TTR. When graphed alongside the treatment failure rate a steep increase of the treatment failure rate was observed after 100 days TTR. The average TTR for patients without treatment failure in their study was reported 87.9 days and 112.8 days for patients with treatment failure (p = 0.037).
Sigmund et al. in 2019 defined ten weeks as a cutoff between a short and a long TTR interval in a retrospective cohort of 93 patients with hip PJI (29). The infection free survival after one year amounted to 94% for the group with the short interval and 91% in the long interval group. At 24 months the survival was 94% (short interval) and 86% (long interval). However, these differences were not significantly different (log-rank test, p = 0.223), potentially due to the small number of only nine patients with observed reinfections.
Vielgut et al. analyzed 76 patients with acute and chronic hip PJI that were treated with two-stage exchange arthroplasty from 2005 to 2010 (30). Most patients in their cohort received spacers that consisted of a femoral stem with metal head, wrapped in antibiotic-loaded cement. Reimplantation of a prosthesis was planned once the infection was considered eradicated. This required a regular clinical and laboratory examination, three negative joint aspirates and a normal leukocyte scintigraphy. Intraoperative frozen sections and local status at the second stage determined, whether an endoprosthesis was reimplanted or the spacer was exchanged. Thirteen cases required spacer exchange. On average TTR amounted to 12.6 weeks. A TTR-threshold was calculated using the maximally selected log-rank statistic by Hothorn and Lausen (37). This method calculates a cutoff where the survival data yields the biggest difference between two groups. A significantly higher reinfection rate was observed when TTR was less than four weeks or more than eleven weeks. The authors concluded that the optimal TTR, therefore, lies within this timeframe. However, the <4 weeks group contained only five patients, that were all reinfected during the observation period, thus limiting validity. In addition, eight patients that were not fit for second stage surgery due to other preconditions and thirteen patients that required spacer exchange were not excluded from the analysis. Therefore, the authors conclude that the association of TTR with reinfection might be biased by worse overall health condition in the group with longer TTR.
A more recent publication of the same group from 2021 analyzed 77 patients with knee PJI (31). Using a similar methodology, they calculated an optimal cutoff of 83 days (11.8 weeks) for this cohort. The risk for reinfection after the second stage was increased sixfold for patients with a longer interval. In contrast to the patient cohort with hip PJI no second cutoff was identified. Again, patients with spacer exchanges in the interval period were not excluded and no adjustment for the host status was performed, although both factors were identified as significant predictors for reinfection.
In 2022 Borsinger et al. reported an increased rate of reinfection after two years for patients with TTR of more than 18 weeks [Odds ratio, CI 95%: 4.12 (1.18–15.37)] (32). Adjustment for comorbidities and previous revision surgeries was done in a cohort of 90 patients with hip and knee PJI (after excluding eleven patients with spacer exchange or Girdlestone resection arthroplasty in the spacer interval). Another group (TTR: 12–18 weeks) had higher odds of treatment failure compared to a group with TTR <12 weeks (odds ratio, CI 95%: 1.89 (0.67–5.77), although not significantly different. The cutoffs at 12 and 18 weeks were defined arbitrarily resulting in groups of similar group size. The calculation of an optimal cutoff with the method by Hothorn and Lausen (37) and additionally a consideration of TTR as a continuous variable would have been interesting. The patient cohort was heterogenous as hip and knee PJI was reported together and the type of knee spacer was inconsistent (static and mobile, prefabricated and handmade, some containing polyethylene tibial components in the PMMA).
Hartman et al. in 2022 reported on a retrospective cohort of 158 patients with hip and knee PJI that underwent both stages with mainly articulating spacers (33). The overall reinfection rate was reported as 19.6% (31/158) and the median TTR in the group with reinfection was 141 days compared to 109 days in the group without reinfection, although not statistically significant (p = 0.055). No information on potential revision surgeries between stages was reported.
Discussion
Few studies have systematically analyzed the potential association of outcomes with TTR in the concept of two-stage revision arthroplasty. However, this topic has recently received increasing attention. This is reflected by the fact that seven of the included eleven studies were published after 2019. The identified studies showed that shorter intervals can achieve comparable or even better infection control compared to longer TTR. In Borsinger's study, this difference was still significant even after adjustment for potential confounding factors and exclusion of all patients with additional surgeries in the interim phase (32).
In chronic PJI, pathogens had long time to penetrate deep into tissue and form mature biofilms on surface areas. Recent findings have shown that S. aureus is able to invade deep into the bone via the osteocyte lacuno-canalicular network (38). This highlights the need for a radical debridement during the first stage in order to reduce the bacterial load. However, it is difficult to clearly identify infiltrated bone and define “clean” resection margins (39). In the concept of the two-stage exchange arthroplasty, any remaining bacteria after the first stage should be completely eradicated by antibiotic therapy. In addition to systemic therapy, the use of local antibiotics is well established. In many cases antibiotic loaded temporary cement spacers are a preferred treatment concept for chronic PJI (7, 8). The spacer has the task of filling the dead space, stabilizing the joint, maintaining the length of the extremity and releasing local anti-infective substances. Nevertheless, elution decreases over time and the amount of this decrease depends on various factors such as surface size, dosage, mixing technique and choice of antibiotic among other factors (40–43). Without relevant antibiotic elution the spacer acts as a foreign body that could be recolonized by remaining bacteria as observed after sonication of retrieved spacers (21, 44, 45). To avoid this situation, it seems reasonable to keep TTR as short as possible. Additional modern drug delivery systems are commercially available, such as calcium sulfate, that can deliver antibiotics over the time the carrier substance is resorbed (46). Other drug delivery systems such as anti-infective microspheres with high bone affinity are currently being investigated (47).
Another possible explanatory approach for the phenomenon of increased risk of reinfection after long TTR could be the following. The interim phase before reimplantation often means immobilization for elderly patients, especially if static spacers are used and weight bearing is not recommended. Immobilization promotes major complications, including pressure ulcers, pneumonia, urinary tract infection and thromboembolic events (48). Besides a significant reduction of muscle mass in elderly patients (49), negative effects of bed rest are also observed for the immune system (50, 51). It therefore seems plausible that patients with a deteriorated immune system after long immobilization periods could be more prone to reinfection.
These considerations suggest that there is a strong case for shorter spacer intervals. Following this line of reasoning, one could question the value of the two-stage exchange compared to the increasingly propagated one-stage exchange (52, 53). However, It has become accepted that certain conditions are regarded as contraindication for the one-step exchange, such as severe immunocompromise, significant soft-tissue or bony compromise and acute sepsis (54). Therefore, a certain minimum duration of TTR seems justified, but it is still not clear whether this is in the range of 2–4 weeks or longer. The optimal TTR probably depends on various patient specific factors. This circumstance demands a great deal of experience from the surgeons, which confirms that septic revision arthroplasty should be performed at specialised centres with a high caseload.
The question arises why, in clinical trials with large patient cohorts, the reported TTR has so far been significantly longer than known guidelines recommend (4, 7, 14–22). An important factor currently preventing the introduction of short spacer intervals seems to be rules in hospital payment systems (55–57). Many countries, including the United States, Germany and the United Kingdom, have introduced rules that make another surgery for the same diagnosis financially unattractive within a certain period after discharge, which is usually 30 days (58, 59). These measures, which were supposed to improve quality of care by penalizing inappropriately early discharges, have the potential of nudging surgeons to schedule second stage reimplantation later. The consideration of the second stage as a separate case becomes evident in economic analyses, where the second stage reimplantation is classified as an “aseptic” revision case (56, 57). This leads to the situation that the second stage competes for scarce capacity with other surgeries considered “elective”. In the context of a general shortage of hospital capacity, aggravated by the Covid-19 pandemic it is to be expected that implementing shorter TTR will become even more difficult (60, 61). The potential future increase in waiting times for the second stage reimplantation should be closely monitored in registries. The interpretation of the second stage reimplantation as an “aseptic” elective revision case appears inappropriate and should rather be considered as “ongoing infection treatment” that ends only after the antibiotics have been completed after reimplantation. A reasonable consideration to address this barrier seems to be for insurers and health policy makers to provide financial incentives for reimplantation to occur during one inpatient stay or shortly thereafter, as this could reduce the societal costs associated with long-term immobilized patients (62) and could achieve, at least, similar infection control.
This systematic review has substantial limitations. Thus, the results should be interpreted with caution. The most important limitation is the compromised comparability of the studies due to different study designs, small sample size, different definition of treatment success and statistical approaches. Most studies did not evaluate TTR as the primary outcome. Rather, it was one parameter among many to identify potential risk factors as part of an exploratory approach. Although the studies report a measure of the overall health status of patients, it is certainly possible that other factors that were not considered in most studies, such as the virulence of microorganisms, soft tissue condition, nutritional status, wound healing, treatment adherence, or other patient-specific factors, had a relevant impact on TTR and infection control. Spacer exchanges or wound revisions in the interim period prolonged the TTR and this is considered a risk factor for reinfection. But most studies did not exclude these cases. In addition, patients who a surgeon believes might be at a higher likelihood of treatment failure based on clinical experience may have been monitored longer before reimplantation in order to detect persisting infection or reinfection. Only the study by Winkler et al. included patients in two different time periods, quasi randomized, to longer or shorter TTR, however the cohort of 38 patients was small and reinfection was observed only once in the whole cohort (14). Because of this variety, a meta-analysis of the results is currently not possible. We suggest for future studies to exclude all patients that require surgery between the stages and to perform adequate adjustment for confounding factors.
Conclusion
The optimal time to reimplantation within the concept of two-stage revision arthroplasty is not yet defined conclusively. Current evidence suggests that short time to reimplantation might be associated with similar or even better infection control compared to long intervals, although cohorts in the existing literature are still rather small and inhomogeneous. This hypothesis should be investigated in larger clinical studies with standardized outcome parameters and adequate adjustment for potential confounding factors.
Author contributions
All authors conceptualized this systematic review, analyzed current literature, drafted the initial manuscript and revised the final version. All authors contributed to the article and approved the submitted version.
Funding
The open access publication fund of the University of Muenster covers the publication fee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1113006/full#supplementary-material.
References
1. Kamath AF, Ong KL, Lau E, Chan V, Vail TP, Rubash HE, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. (2015) 30(9):1492–7. doi: 10.1016/j.arth.2015.03.035
2. Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the medicare population. J Arthroplasty. (2018) 33(10):3238–45. doi: 10.1016/j.arth.2018.05.042
3. Theil C, Stock ME, Gosheger G, Moellenbeck B, Schwarze J, Schmidt-Braekling T. Gastrocnemius muscle flaps for soft tissue coverage in periprosthetic knee joint infection. J Arthroplasty. (2020) 35(12):3730–6. doi: 10.1016/j.arth.2020.06.074
4. Dieckmann R, Schmidt-Braekling T, Gosheger G, Theil C, Hardes J, Moellenbeck B. Two stage revision with a proximal femur replacement. BMC Musculoskelet Disord. (2019) 20(1):58. doi: 10.1186/s12891-019-2442-2
5. Parvizi J, Tarity TD, Slenker N, Wade F, Trappler R, Hozack WJ, et al. Proximal femoral replacement in patients with non-neoplastic conditions. J Bone Joint Surg Am. (2007) 89(5):1036–43. doi: 10.2106/00004623-200705000-00016
6. Theil C, Schneider KN, Gosheger G, Schmidt-Braekling T, Ackmann T, Dieckmann R, et al. Revision TKA with a distal femoral replacement is at high risk of reinfection after two-stage exchange for periprosthetic knee joint infection. Knee Surg Sports Traumatol Arthrosc. (2022) 30(3):899–906. doi: 10.1007/s00167-021-06474-2
7. Gomez MM, Tan TL, Manrique J, Deirmengian GK, Parvizi J. The fate of spacers in the treatment of periprosthetic joint infection. J Bone Joint Surg Am. (2015) 97(18):1495–502. doi: 10.2106/JBJS.N.00958
8. Petis SM, Perry KI, Pagnano MW, Berry DJ, Hanssen AD, Abdel MP. Retained antibiotic spacers after total hip and knee arthroplasty resections: high complication rates. J Arthroplasty. (2017) 32(11):3510–8. doi: 10.1016/j.arth.2017.05.053
9. Theil C, Riegel RF, Gosheger G, Schwarze J, Schmidt-Braekling T, Moellenbeck B. Acute renal failure after the first stage of a 2-stage exchange for periprosthetic joint infection. J Arthroplasty. (2021) 36(2):717–21. doi: 10.1016/j.arth.2020.08.028
10. Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. (2009) 467(7):1699–705. doi: 10.1007/s11999-009-0742-9
11. Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep. (2008) 10(5):394–403. doi: 10.1007/s11908-008-0064-1
12. Warth LC, Hadley CJ, Grossman EL. Two-Stage treatment for total knee arthroplasty infection utilizing an articulating prefabricated antibiotic spacer. J Arthroplasty. (2020) 35(3s):S57–s62. doi: 10.1016/j.arth.2019.10.049
13. Cooper HJ, Della Valle CJ. The two-stage standard in revision total hip replacement. Bone Joint J. (2013) 95-b(11 Suppl A):84–7. doi: 10.1302/0301-620X.95B11.32906
14. Winkler T, Stuhlert MGW, Lieb E, Müller M, von Roth P, Preininger B, et al. Outcome of short versus long interval in two-stage exchange for periprosthetic joint infection: a prospective cohort study. Arch Orthop Trauma Surg. (2019) 139(3):295–303. doi: 10.1007/s00402-018-3052-4
15. Vasarhelyi E, Sidhu SP, Somerville L, Lanting B, Naudie D, Howard J. Static vs articulating spacers for two-stage revision total knee arthroplasty: minimum five-year review. Arthroplasty Today. (2022) 13:171–5. doi: 10.1016/j.artd.2021.10.010
16. Aali Rezaie A, Goswami K, Shohat N, Tokarski AT, White AE, Parvizi J. Time to reimplantation: waiting Longer confers No added benefit. J Arthroplasty. (2018) 33(6):1850–4. doi: 10.1016/j.arth.2018.01.073
17. Sabry FY, Buller L, Ahmed S, Klika AK, Barsoum WK. Preoperative prediction of failure following two-stage revision for knee prosthetic joint infections. J Arthroplasty. (2014) 29(1):115–21. doi: 10.1016/j.arth.2013.04.016
18. Hart WJ, Jones RS. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J Bone Joint Surg Br. (2006) 88(8):1011–5. doi: 10.1302/0301-620X.88B8.17445
19. Babis GC, Sakellariou VI, Pantos PG, Sasalos GG, Stavropoulos NA. Two-Stage revision protocol in multidrug resistant periprosthetic infection following total hip arthroplasty using a long interval between stages. J Arthroplasty. (2015) 30(9):1602–6. doi: 10.1016/j.arth.2015.04.004
20. Choi HR, von Knoch F, Zurakowski D, Nelson SB, Malchau H. Can implant retention be recommended for treatment of infected TKA? Clin Orthop Relat Res. (2011) 469(4):961–9. doi: 10.1007/s11999-010-1679-8
21. Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res. (2014) 472(7):2208–14. doi: 10.1007/s11999-014-3571-4
22. Petis SM, Perry KI, Mabry TM, Hanssen AD, Berry DJ, Abdel MP. Two-Stage exchange protocol for periprosthetic joint infection following total knee arthroplasty in 245 knees without prior treatment for infection. J Bone Joint Surg Am. (2019) 101(3):239–49. doi: 10.2106/JBJS.18.00356
23. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken, New Jersey: John Wiley & Sons (2019).
24. Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
25. Kubista B, Hartzler RU, Wood CM, Osmon DR, Hanssen AD, Lewallen DG. Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int Orthop. (2012) 36(1):65–71. doi: 10.1007/s00264-011-1267-x
26. Akgün D, Müller M, Perka C, Winkler T. High cure rate of periprosthetic hip joint infection with multidisciplinary team approach using standardized two-stage exchange. J Orthop Surg Res. (2019) 14(1):78. doi: 10.1186/s13018-019-1122-0
27. Hipfl C, Winkler T, Janz V, Perka C, Müller M. Management of chronically infected total knee arthroplasty with severe bone loss using static spacers with intramedullary rods. J Arthroplasty. (2019) 34(7):1462–9. doi: 10.1016/j.arth.2019.03.053
28. Tan TL, Kheir MM, Rondon AJ, Parvizi J, George J, Higuera CA, et al. Determining the role and duration of the “antibiotic holiday” period in periprosthetic joint infection. J Arthroplasty. (2018) 33(9):2976–80. doi: 10.1016/j.arth.2018.04.019
29. Sigmund IK, Winkler T, Onder N, Perka C, Renz N, Trampuz A. Complications of resection arthroplasty in two-stage revision for the treatment of periprosthetic hip joint infection. J Clin Med. (2019) 8(12):2224. doi: 10.3390/jcm8122224
30. Vielgut I, Sadoghi P, Wolf M, Holzer L, Leithner A, Schwantzer G, et al. Two-stage revision of prosthetic hip joint infections using antibiotic-loaded cement spacers: when is the best time to perform the second stage? Int Orthop. (2015) 39(9):1731–6. doi: 10.1007/s00264-015-2751-5
31. Vielgut I, Schwantzer G, Leithner A, Sadoghi P, Berzins U, Glehr M. Successful two-stage exchange arthroplasty for periprosthetic infection following total knee arthroplasty: the impact of timing on eradication of infection. Int J Med Sci. (2021) 18(4):1000–6. doi: 10.7150/ijms.47655
32. Borsinger TM, Resnick CT, Werth PM, Schilling PL, Moschetti WE. Does time to reimplantation after explant for prosthetic joint infection influence the likelihood of successful outcomes at 2 years? J Arthroplasty. (2022) 37(6):1173–9. doi: 10.1016/j.arth.2022.02.025
33. Hartman CW, Daubach EC, Richard BT, Lyden ER, Haider H, Kildow BJ, et al. Predictors of reinfection in prosthetic joint infections following two-stage reimplantation. J Arthroplasty. (2022) 37(7s):S674-s7. doi: 10.1016/j.arth.2022.03.017
34. Klemt C, Smith EJ, Tirumala V, Bounajem G, van den Kieboom J, Kwon YM. Outcomes and risk factors associated with 2-stage reimplantation requiring an interim spacer exchange for periprosthetic joint infection. Journal of Arthroplasty. (2021) 36(3):1094–100. doi: 10.1016/j.arth.2020.09.012
35. Kozaily E, Tan TL, Yacovelli S, Anis H, Higuera C, Piuzzi NS, et al. Interim spacer exchange for treatment of periprosthetic joint infection: almost half the patients subsequently fail. Journal of Arthroplasty. (2022) 37(1):150–5. doi: 10.1016/j.arth.2021.08.028
36. Jaiben G, Evan MM, Gannon LC, Alison KK, Wael KB, Michael AM, et al. Success of two-stage reimplantation in patients requiring an interim spacer exchange. J Arthroplasty. (2018) 33(7S):S228–32. doi: 10.1016/j.arth.2018.03.038
37. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. (2003) 43(2):121–37. doi: 10.1016/S0167-9473(02)00225-6
38. Masters EA, Salminen AT, Begolo S, Luke EN, Barrett SC, Overby CT, et al. An in vitro platform for elucidating the molecular genetics of S. aureus invasion of the osteocyte lacuno-canalicular network during chronic osteomyelitis. Nanomedicine. (2019) 21:102039. doi: 10.1016/j.nano.2019.102039
39. Malone M, Fritz BG, Vickery K, Schwarzer S, Sharma V, Biggs N, et al. Analysis of proximal bone margins in diabetic foot osteomyelitis by conventional culture, DNA sequencing and microscopy. Apmis. (2019) 127(10):660–70. doi: 10.1111/apm.12986
40. Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from Simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res. (2006) 24(8):1615–21. doi: 10.1002/jor.20214
41. Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. (1998) 13(3):331–8. doi: 10.1016/S0883-5403(98)90179-6
42. Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. (2009) 80(2):193–7. doi: 10.3109/17453670902884700
43. Fink B, Vogt S, Reinsch M, Büchner H. Sufficient release of antibiotic by a spacer 6 weeks after implantation in two-stage revision of infected hip prostheses. Clin Orthop Relat Res. (2011) 469(11):3141–7. doi: 10.1007/s11999-011-1937-4
44. Sambri A, Maso A, Storni E, Donati ME, Pederzoli A, Dallari D, et al. Is sonication of antibiotic-loaded cement spacers useful in two-stage revision of prosthetic joint infection? J Microbiol Methods. (2019) 156:81–4. doi: 10.1016/j.mimet.2018.12.006
45. Mariconda M, Ascione T, Balato G, Rotondo R, Smeraglia F, Costa GG, et al. Sonication of antibiotic-loaded cement spacers in a two-stage revision protocol for infected joint arthroplasty. BMC Musculoskelet Disord. (2013) 14:193. doi: 10.1186/1471-2474-14-193
46. Razvan E, Mihai N, Dragos E, Adrian C, Catalin C. Review of calcium-sulphate-based ceramics and synthetic bone substitutes used for antibiotic delivery in PJI and osteomyelitis treatment. EFORT Open Reviews. (2021) 6(5):297. doi: 10.1302/2058-5241.6.200083
47. Stijn GR, Keith T, Dirk WG, Robert GR, Thomas FM, David E, et al. Development of bone seeker-functionalised microspheres as a targeted local antibiotic delivery system for bone infections. J Orthop Translat. (2020) 21:136–45. doi: 10.1016/j.jot.2019.07.006
48. Wu X, Li Z, Cao J, Jiao J, Wang Y, Liu G, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLoS One. (2018) 13(10):e0205729. doi: 10.1371/journal.pone.0205729
49. Moreira JB, Wohlwend M, Åmellem I, Jannig PR. Age-dependent effects of bed rest in human skeletal muscle: exercise to the rescue. J Physiol. (2016) 594(2):265–6. doi: 10.1113/JP271758
50. Hoff P, Belavý DL, Huscher D, Lang A, Hahne M, Kuhlmey AK, et al. Effects of 60-day bed rest with and without exercise on cellular and humoral immunological parameters. Cell Mol Immunol. (2015) 12(4):483–92. doi: 10.1038/cmi.2014.106
51. Schmitt DA, Schaffar L. Isolation and confinement as a model for spaceflight immune changes. J Leukoc Biol. (1993) 54(3):209–13. doi: 10.1002/jlb.54.3.209
52. Jenny JY, Barbe B, Gaudias J, Boeri C, Argenson JN. High infection control rate and function after routine one-stage exchange for chronically infected TKA. Clin Orthop Relat Res. (2013) 471(1):238–43. doi: 10.1007/s11999-012-2480-7
53. Thakrar RR, Horriat S, Kayani B, Haddad FS. Indications for a single-stage exchange arthroplasty for chronic prosthetic joint infection: a systematic review. Bone Joint J. (2019) 101-B(1_Supppl_A):19. doi: 10.1302/0301-620X.101B1.BJJ-2018-0374.R1
54. Pangaud C, Ollivier M, Argenson JN. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. (2019) 4(8):495–502. doi: 10.1302/2058-5241.4.190003
55. Müller M, Trampuz A, Winkler T, Perka C. The economic challenge of centralised treatment of patients with periprosthetic infections. Z Orthop Unfall. (2018) 156:407–13. doi: 10.1055/s-0044-100732
56. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J. (2015) 97-b(2):197–201. doi: 10.1302/0301-620X.97B2.33707
57. Vanhegan IS, Malik AK, Jayakumar P, Ul Islam S, Haddad FS. A financial analysis of revision hip arthroplasty: the economic burden in relation to the national tariff. J Bone Joint Surg Br. (2012) 94(5):619–23. doi: 10.1302/0301-620X.94B5.27073
58. Or Z, Häkkinen U. DRGs and quality: for better or worse. Diagnosis-Related Groups in Europe: Moving Towards Transparency, Efficiency and Quality in Hospitals. (2011) p. 115–129. Berkshire, England: Open University Press McGraw-Hill Education.
59. Kristensen SR, Bech M, Quentin W. A roadmap for comparing readmission policies with application to Denmark, England, Germany and the United States. Health Policy. (2015) 119(3):264–73. doi: 10.1016/j.healthpol.2014.12.009
60. Fulin P, Daniel M, Walder J, Grzelecki D, Pokorny D. Impact of the COVID-19 pandemic on orthopaedic and traumatological care in Prague, the capital of the Czech republic. PLoS One. (2022) 17(6):e0269164. doi: 10.1371/journal.pone.0269164
61. Haffer H, Schömig F, Rickert M, Randau T, Raschke M, Wirtz D, et al. Impact of the COVID-19 pandemic on orthopaedic and trauma surgery in university hospitals in Germany: results of a nationwide survey. J Bone Joint Surg Am. (2020) 102(14):e78. doi: 10.2106/JBJS.20.00756
Keywords: periprosthetic joint infection, two-stage exchange, revision arthroplasty, time to reimplantation, spacer interval, TKA, THA
Citation: Puetzler J, Schulze M, Gosheger G, Schwarze J, Moellenbeck B and Theil C (2023) Is long time to reimplantation a risk factor for reinfection in two-stage revision for periprosthetic infection? A systematic review of the literature. Front. Surg. 10:1113006. doi: 10.3389/fsurg.2023.1113006
Received: 30 November 2022; Accepted: 31 January 2023;
Published: 17 February 2023.
Edited by:
Markus Rupp, University Medical Center Regensburg, GermanyReviewed by:
Anita Jacombs, Macquarie University Hospital, AustraliaJoerg Mika, Friedrich Schiller University Jena, Germany
© 2023 Puetzler, Schulze, Gosheger, Schwarze, Moellenbeck and Theil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Puetzler amFuLnB1ZXR6bGVyQHVrbXVlbnN0ZXIuZGU=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Jan Puetzler
Jan Puetzler Martin Schulze
Martin Schulze