- 1Department of Pediatric Surgery, Binzhou Medical University Hospital, Binzhou, China
- 2Department of General Surgery, Boxing People's Hospital, Boxing, China
- 3Department of Anorectal Surgery, Binzhou Medical University Hospital, Binzhou, China
- 4Department of Surgery, Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai, China
- 5Department of Pediatric Surgery, Qilu Hospital of Shandong University Dezhou Hospital, Dezhou, China
Background: Anastomotic leakage is a life-threatening complication. Improvement of the anastomosis technique is needed, especially in patients with an inflamed edematous intestine. The aim of our study was to evaluate the safety and efficacy of an asymmetric figure-of-eight single-layer suture technique for intestinal anastomosis in pediatric patients.
Methods: A total of 23 patients underwent intestinal anastomosis at the Department of Pediatric Surgery of Binzhou Medical University Hospital. Demographic characteristics, laboratory parameters, anastomosis time, duration of nasogastric tube placement, day of first postoperative bowel movement, complications, and length of hospital stay were statistically analyzed. The follow-up was conducted for 3–6 months after discharge.
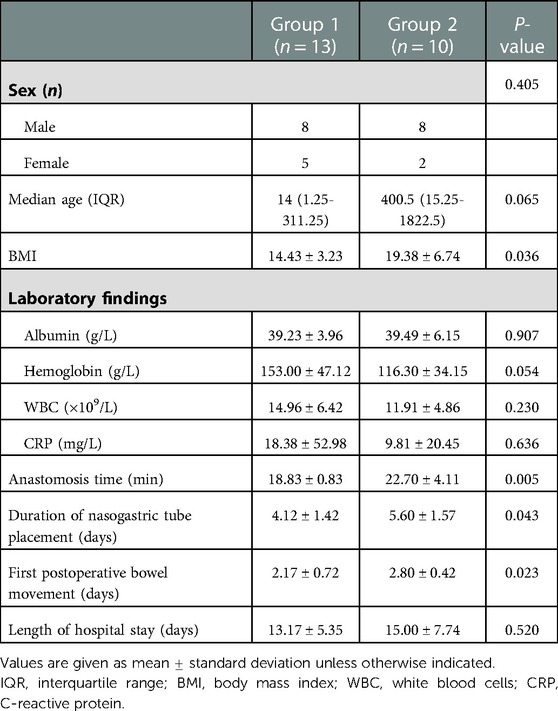
Results: Patients were divided into two groups: the single-layer asymmetric figure-of-eight suture technique (group 1) and the traditional suture technique (group 2). Body mass index in group 1 was lower than in group 2 (14.43 ± 3.23 vs. 19.38 ± 6.74; P = 0.036). The mean intestine anastomosis time in group 1 (18.83 ± 0.83 min) was less than that in group 2 (22.70 ± 4.11 min; P = 0.005). Patients in group 1 had an earlier first postoperative bowel movement (2.17 ± 0.72 vs. 2.80 ± 0.42; P = 0.023). The duration of nasogastric tube placement in group 1 was shorter than that in group 2 (4.12 ± 1.42 vs. 5.60 ± 1.57; P = 0.043). There was no significant difference in laboratory variables, complication occurrence, and length of hospital stay between the two groups.
Conclusion: The asymmetric figure-of-eight single-layer suture technique for intestinal anastomosis was feasible and effective. More studies are needed to compare the novel technique with the traditional single-layer suture.
Introduction
Intestinal anastomosis is a basic technique to restore gut continuity. Several intestinal anastomosis techniques, including hand-sewn, stapled, laparoscopic, robotic, and sutureless anastomoses, are used with varied outcomes (1–8). The hand-sewn intestinal anastomosis technique is divided into single-layer and double-layer suture techniques (9–12). Interrupted single-layer anastomosis is the gold standard owing to the occurrence of fewer complications (13). However, the smaller diameter of the intestinal wall in the pediatric population and a pathological status, including the presence of edema, ischemia, and inflammation, may make implementing the single-layer suture technique difficult. Moreover, the higher occurrence of postoperative complications may encourage surgeons to change their strategy to temporary intestinal stoma and two-stage procedures, among others (12–15). Thus, some patients may have to face several possible complications arising from multiple procedures. Herein, we modified an asymmetric figure-of-eight single-layer suture technique on the basis of an abdominal wall closure technique described by Höllwarth (16) and an in vitro porcine experimental finding (17). The asymmetric figure-of-eight single-layer suture technique has some advantages; it is easy to perform, is time-saving, and has better mucosal apposition (17). We piloted the modified suture technique by performing intestinal anastomoses, especially in the bowels with a pathological status in pediatric patients. We also assessed its feasibility and efficacy.
Methods
Data collection
Between January 2020 and October 2022, 23 pediatric patients underwent intestinal anastomoses at the Department of Pediatric Surgery, Binzhou Medical University Hospital. The inclusion criteria were as follows: (1) age <18 years; (2) undergoing emergent or elective intestine anastomosis; (3) normal renal and liver function; and (4) no contraindications for intestinal anastomosis, such as being hemodynamically unstable, having a severe intraperitoneal abscess, and having severe shock. The exclusion criteria included the following: (1) patients who underwent anastomosis in the stomach or distal part of the rectum; and (2) with any of the above contraindications.
Variables included age, sex, body mass index (BMI), laboratory data (white blood cell count and hemoglobin, albumin, and C-reactive protein levels), length of postoperative hospital stay, time of anastomosis, duration of nasogastric tube placement, time of first postoperative bowel movement, and postoperative complications, including anastomotic bleeding, leakage, stricture, intra-abdominal abscess, pelvic collection, and abdominal distension. The time taken for the anastomosis (in minutes) was recorded from the beginning of the first stitch placement to the end of cutting extra suture material from the last stitch.
An anastomotic leak was defined as follows: (1) body temperature >38°C, persistent abdominal pain, and tachycardia; (2) signs of peritonitis or intra-abdominal abscess confirmed by abdominal ultrasound; (3) non-absorbable material draining from a wound after oral administration; (4) elevated leucocyte count; and (5) visible disruption of the suture line during re-exploration (18, 19).
Procedures
Patients were divided into two groups: the single-layer asymmetric figure-of-eight suture technique group (group 1) and the traditional suture technique, i.e., single-layer interrupted sutures, group (group 2) (9, 10, 20, 21). The single-layer asymmetric figure-of-eight suture technique has been described in detail by Liu et al. (17). To summarize, an absorbable surgical suture (4–0 or 5–0 Ethicon) was used for anastomosis. The first suture and withdrawal were conducted by taking a bite of 2 mm apart from the cut end and assured to include all layers of the intestine. The needle was obliquely inserted into the serosa, muscularis, and submucosa without mucosa to the contralateral layer. The second suture and withdrawal were obliquely inserted into the serosa, muscularis, and submucosa without mucosa to the contralateral submucosa, muscularis, and serosa by taking a bite 1 mm apart from the cut end and forward from the first suture, as shown in Figure 1. Before tying the knots, each suture should be tightly approximated to avoid cutting through the fragile bowel tissues.
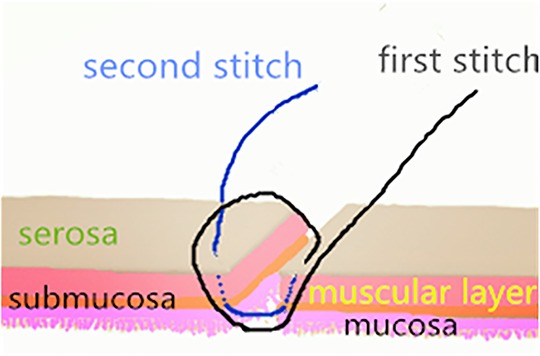
Figure 1. A schematic representation of the asymmetric figure-of-eight suture. The technique has been described in detail previously (17).
All patients were subjected to the same postoperative program, including antibiotics, administration of fluid, and placement of a nasogastric tube.
The follow-up was conducted via telephone or outpatient department interview for 3–6 months.
Ethical approval
All procedures performed in the present study were in accordance with the ethical standards of the 1964 Helsinki Declaration. Written informed consent was obtained from the parents and/or legal guardians of the patients.
Statistical analysis
The differences between the groups were analyzed using the SPSS Statistics version 26.0 software (IBM Corp., Armonk, NY, USA). Continuous data were analyzed using the Student's t-test. Fisher's exact test and the Pearson chi-square test were used to analyze categorical data. A P-value <0.05 was considered statistically significant.
Results
A total of 23 pediatric patients who underwent anastomosis were assessed for eligibility, including 13 (56.52%) who underwent anastomosis with a single-layer asymmetric figure-of-eight suture technique (Figure 2) and 10 (43.48%) who underwent anastomosis with a traditional single-layer interrupted suture technique (Figure 3). Demographic and clinical characteristics are listed in Table 1. The male-to-female ratio was 2.29:1. The BMI was significantly lower in group 1 than in group 2 (14.43 ± 3.23 vs. 19.38 ± 6.74; P = 0.036). The mean intestine anastomosis time in group 1 (18.83 ± 0.83 min) was significantly lower than in group 2 (22.70 ± 4.11 min; P = 0.005). The patients in group 1 had an earlier first postoperative bowel movement than those in group 2 (2.17 ± 0.72 vs. 2.80 ± 0.42; P = 0.023). The duration of nasogastric tube placement in group 1 was significantly shorter than that in group 2 (4.12 ± 1.42 vs. 5.60 ± 1.57; P = 0.043). No significant differences were found between the two groups with regard to sex distribution (P = 0.405), median age (P = 0.065), serum albumin level (P = 0.084), serum hemoglobin level (P = 0.054), C-reactive protein (P = 0.636), and white blood cell count (P = 0.230). Demographic and clinical characteristics are listed in Table 1.

Figure 2. A 5-month-old boy with generalized peritonitis and septic shock caused by a perforated terminal ileum due to a congenital fibrotic band compression. (A) Perforation (small Δ) and necrosis (arrow) at the terminal ileum; (B) completion of primary anastomosis (large Δ) using the asymmetric figure-of-eight suture technique.
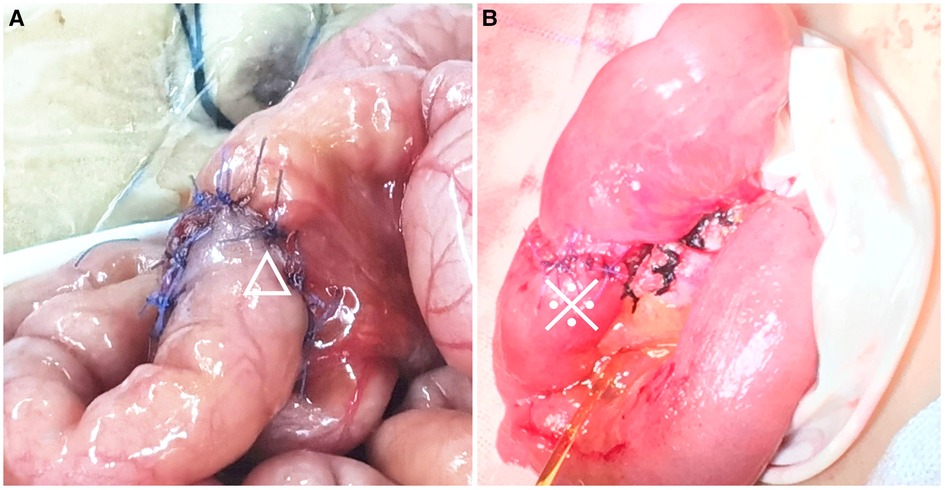
Figure 3. Comparison of the asymmetric figure-of-eight suture technique and traditional single-layer suture technique. (A) A 50-day-old boy with biliary atresia undergoing Kasai portoenterostomy, completion of end-to-side jejuno-jejunal anastomosis (△); (B) a six-year-old boy with Meckel's diverticulum complicated by strangulated internal hernia undergoing necrotic bowel resection and end-to-end ileoileal anastomosis (※).
Ileo-ileal, jejuno-jejunal, and portojejunal anastomoses were performed in groups 1 and 2. Major causes included gut malrotation, Meckel's diverticulum, perforation, gut or biliary atresia, duplication, small bowel obstruction, or neonatal necrotizing enterocolitis.
No major postoperative complications were found in any of the children.
Discussion
Intestinal anastomosis is an essential surgical technique to restore intestinal continuity (1). In the pediatric population, the primary intestinal anastomosis technique is usually chosen according to the patient's general status and intestinal condition. The disease spectrum includes neonatal necrotizing enterocolitis, Meckel's diverticulum with complications, intestinal atresia, and strangulated bowel obstruction. A feasible and easy-to-perform intestinal anastomotic technique is necessary to reduce major postoperative complications, especially for those who have severely altered intestinal morphology. A safe and efficacious bowel anastomosis includes the following technical aspects: meticulous technique; gentle tissue handling; accurate mucosa apposition; and avoidance of tension and ischemia at the anastomotic site (1, 9, 19, 22).
Although the use of the traditional double-layer intestinal anastomosis or single-layer suture technique for intestinal anastomosis is controversial (23), the single-layer extramucosal suture anastomosis technique is widely accepted by pediatric surgeons (14). In the present study, we created an easy-to-perform anastomotic technique with two stitches at different horizontal levels (17), which might minimize the tension and prevent the surgeon from cutting through the intestinal wall while tying the knot, especially in inflamed edematous intestinal walls (17, 24).
Several studies have reported that the early removal of the nasogastric tube and initiation of oral feeding can promote the recovery of intestinal function, shorten the length of the hospital stay, and prevent intestinal obstruction (25–28). In the present study, the mean duration of nasogastric tube placement was significantly shorter in group 1 than in group 2. The first day of postoperative bowel movement in group 1 was significantly shorter than that in group 2, suggesting a more rapid intestinal function recovery.
Our results showed that the mean anastomosis time was significantly shortened in the asymmetric figure-of-eight single-layer suture technique group than in the single-layer interrupted sutures group, partly owing to the requirement of fewer knots (17). Time-saving is also important for patients who experience severe complications pre- or intraoperatively, such as intestinal volvulus and shock. Postoperative complications, such as abscess or anastomotic leakage, were absent even in patients with high-risk primary anastomosis, such as necrotizing enterocolitis, and generalized peritonitis.
Limitations
This was a retrospective study. The sample size was relatively small, and the study was prone to the incomplete collection of individual data. Owing to the absence of a guideline to adhere to, the availability of intestinal anastomosis options may have been affected by the training or previous experience of pediatric surgeons. Furthermore, all patients were treated in a single medical center, and the results are not representative of other medical centers. Therefore, a multi-institutional trial with a large sample size determined using power analysis is required in the future.
Conclusion
The asymmetric figure-of-eight single-layer suture technique was feasible and efficacious. It was superior to the traditional single-layer technique for intestinal anastomosis. Further studies are needed to confirm the preliminary findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval were waived as this was a retrospective study. The patients' parents and/or legal guardians provided written informed consent to participate in the study.
Author contributions
TF, FC, and LG: conceptualization. ML, MZ, XR, CL, HY, X-LX, and G-JD: investigation. ML and TF: writing – original draft preparation. FC, TF, and LG: writing – review and editing. TF and LG: supervision. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the patients and their families and appreciate the cooperation of all staff. We thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen C. The art of bowel anastomosis. Scand J Surg. (2012) 101(4):238–40. doi: 10.1177/145749691210100403
2. Madani R, Day N, Kumar L, Tilney HS, Gudgeon AM. Hand-sewn versus stapled closure of loop ileostomy: a meta-analysis. Dig Surg. (2019) 36(3):183–94. doi: 10.1159/000487310
3. Naoi D, Horie H, Koinuma K, Kumagai Y, Ota G, Tojo M, et al. Intestinal mucosa staple line integrity and anastomotic leak pressure after healing in a porcine model. Surg Today. (2021) 51(10):1713–9. doi: 10.1007/s00595-021-02267-9
4. Sedano JVR, Castro BA, Alelu RM, Vázquez AG, Fraile AG, Novillo IC. Use of 5-mm staple in neonatal intestinal surgery. J Laparoendosc Adv Surg Tech A. (2021) 31(9):1092–5. doi: 10.1089/lap.2021.0181
5. Uppal A, Pigazzi A. New technologies to prevent anastomotic leak. Clin Colon Rectal Surg. (2021) 34(6):379–84. doi: 10.1055/s-0041-1735268
6. Giaccaglia V, Antonelli MS, Franceschilli L, Salvi PF, Gaspari AL, Sileri P. Different characteristics of circular staplers make the difference in anastomotic tensile strength. J Mech Behav Biomed Mater. (2016) 53:295–300. doi: 10.1016/j.jmbbm.2015.08.029
7. Saeidi H, Opfermann JD, Kam M, Wei S, Leonard S, Hsieh MH, et al. Autonomous robotic laparoscopic surgery for intestinal anastomosis. Sci Robot. (2022) 7(62):eabj2908. doi: 10.1126/scirobotics.abj2908
8. Fan C, Ma J, Zhang HK, Gao R, Li JH, Yu L, et al. Sutureless intestinal anastomosis with a novel device of magnetic compression anastomosis. Chin Med Sci J. (2011) 26(3):182–9. doi: 10.1016/s1001-9294(11)60046-1
9. Kar S, Mohapatra V, Singh S, Rath PK, Behera TR. Single layered versus double layered intestinal anastomosis: a randomized controlled trial. J Clin Diagn Res. (2017) 11(6):Pc01–pc4. doi: 10.7860/jcdr/2017/24817.9983
10. Singh R, Najmi HI, Chahal RK, Nikhil D. A comparative study of single-layered versus double-layered intestinal anastomosis. Cureus. (2022) 14(3):e23088. doi: 10.7759/cureus.23088
11. Sajid MS, Siddiqui MR, Baig MK. Single layer versus double layer suture anastomosis of the gastrointestinal tract. Cochrane Database Syst Rev. (2012) 1:Cd005477. doi: 10.1002/14651858.CD005477.pub4
12. Close K, Epstein KL, Sherlock CE. A retrospective study comparing the outcome of horses undergoing small intestinal resection and anastomosis with a single layer (Lembert) or double layer (simple continuous and cushing) technique. Vet Surg. (2014) 43(4):471–8. doi: 10.1111/j.1532-950X.2014.12143.x
13. Leslie A, Steele RJ. The interrupted serosubmucosal anastomosis-still the gold standard. Colorectal Dis. (2003) 5(4):362–6. doi: 10.1046/j.1463-1318.2003.00460.x
14. Ordorica-Flores RM, Bracho-Blanchet E, Nieto-Zermeño J, Reyes-Retana R, Tovilla-Mercado JM, Leon-Villanueva V, et al. Intestinal anastomosis in children: a comparative study between two different techniques. J Pediatr Surg. (1998) 33(12):1757–9. doi: 10.1016/s0022-3468(98)90279-2
15. Nieto JE, Dechant JE, Snyder JR. Comparison of one-layer (continuous Lembert) versus two-layer (simple continuous/cushing) hand-sewn end-to-end anastomosis in equine jejunum. Vet Surg. (2006) 35(7):669–73. doi: 10.1111/j.1532-950X.2006.00206.x
16. Höllwarth ME. Short bowel syndrome. In: Puri P, Höllwarth ME, editors. Pediatric surgery. Springer surgery atlas series. Berlin, Heidelberg: Springer (2006). p. 264–74.
17. Liu C, Wang Y, Zhao AR, Hu FA, Fan Q, Han G, et al. An alternative asymmetric figure-of-eight single-layer suture technique for bowel anastomosis in an in vitro porcine model. Front Surg. (2022) 9:896542. doi: 10.3389/fsurg.2022.896542
18. Burch JM, Franciose RJ, Moore EE, Biffl WL, Offner PJ. Single-layer continuous versus two-layer interrupted intestinal anastomosis: a prospective randomized trial. Ann Surg. (2000) 231(6):832–7. doi: 10.1097/00000658-200006000-00007
19. Aniruthan D, Pranavi AR, Sreenath GS, Kate V. Efficacy of single layered intestinal anastomosis over double layered intestinal anastomosis-an open labelled, randomized controlled trial. Int J Surg. (2020) 78:173–8. doi: 10.1016/j.ijsu.2020.04.066
20. Bailey HR, LaVoo JW, Max E, Smith KW, Butts DR, Hampton JM. Single-layer polypropylene colorectal anastomosis. Experience with 100 cases. Dis Colon Rectum. (1984) 27(1):19–23. doi: 10.1007/bf02554066
21. Slieker JC, Daams F, Mulder IM, Jeekel J, Lange JF. Systematic review of the technique of colorectal anastomosis. JAMA Surg. (2013) 148(2):190–201. doi: 10.1001/2013.jamasurg.33
22. Goulder F. Bowel anastomoses: the theory, the practice and the evidence base. World J Gastrointest Surg. (2012) 4(9):208–13. doi: 10.4240/wjgs.v4.i9.208
23. Milone M, Elmore U, Allaix ME, Bianchi PP, Biondi A, Boni L, et al. Fashioning enterotomy closure after totally laparoscopic ileocolic anastomosis for right colon cancer: a multicenter experience. Surg Endosc. (2020) 34(2):557–63. doi: 10.1007/s00464-019-06796-w
24. Awad S, El-Rahman AIA, Abbas A, Althobaiti W, Alfaran S, Alghamdi S, et al. The assessment of perioperative risk factors of anastomotic leakage after intestinal surgeries; a prospective study. BMC Surg. (2021) 21(1):29. doi: 10.1186/s12893-020-01044-8
25. Greer D, Karunaratne YG, Karpelowsky J, Adams S. Early enteral feeding after pediatric abdominal surgery: a systematic review of the literature. J Pediatr Surg. (2020) 55(7):1180–7. doi: 10.1016/j.jpedsurg.2019.08.055
26. Peng Y, Xiao D, Xiao S, Yang L, Shi H, He Q, et al. Early enteral feeding versus traditional feeding in neonatal congenital gastrointestinal malformation undergoing intestinal anastomosis: a randomized multicenter controlled trial of an enhanced recovery after surgery (ERAS) component. J Pediatr Surg. (2021) 56(9):1479–84. doi: 10.1016/j.jpedsurg.2021.02.067
27. Yadav PS, Choudhury SR, Grover JK, Gupta A, Chadha R, Sigalet DL. Early feeding in pediatric patients following stoma closure in a resource limited environment. J Pediatr Surg. (2013) 48(5):977–82. doi: 10.1016/j.jpedsurg.2013.02.013
Keywords: intestinal anastomosis, asymmetric figure-of-eight suture, postoperatively complications, pediatric, single layer suture
Citation: Liu M, Zhang M, Ren X, Liu C, Yu H, Xu X, Ding G, Fu T, Geng L and Cheng F (2023) Asymmetric figure-of-eight single-layer suture technique for intestinal anastomosis: A preliminary study. Front. Surg. 10:1109751. doi: 10.3389/fsurg.2023.1109751
Received: 28 November 2022; Accepted: 16 January 2023;
Published: 13 February 2023.
Edited by:
Andrea Balla, Hospital "San Paolo," ItalyReviewed by:
Riccardo Coletta, University of Florence, ItalyCarmelo Romeo, University of Messina, Italy
© 2023 Liu, Zhang, Ren, Liu, Yu, Xu, Ding, Fu, Geng and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Geng Y2ZjMDEyOEAxMjYuY29t Fengchun Cheng MzgxODExNDFAcXEuY29t
†ORCID Chen Liu orcid.org/0000-0002-0250-1875 Lei Geng orcid.org/0000-0003-0216-5602
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Mingzhu Liu1
Mingzhu Liu1 Guo-Jian Ding
Guo-Jian Ding Tingliang Fu
Tingliang Fu