
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 17 February 2023
Sec. Orthopedic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1096080
 Shanxi Wang1,†
Shanxi Wang1,† Xuan Fang1,†
Xuan Fang1,† Yunkun Qu1
Yunkun Qu1 Rui Lu1
Rui Lu1 Xiaojun Yu1
Xiaojun Yu1 Shaoze Jing2
Shaoze Jing2 Qing Ding1
Qing Ding1 Chaoxu Liu1
Chaoxu Liu1 Hua Wu1*
Hua Wu1* Yang Liu1*
Yang Liu1*
Background: To assess the clinical and radiographical outcomes of 3-level anterior cervical discectomy and fusion (ACDF) with a 3D-printed titanium cage in treating degenerative cervical spondylosis.
Methods: In this study, 25 patients with degenerative cervical spondylosis who underwent 3-level ACDF using a 3D-printed titanium cage from March 2019 to June 2021 were retrospectively enrolled. The patient-reported outcome measures (PROMs) were evaluated by visual analog scale (VAS) for the neck (VAS-neck) and arm pain (VAS-arm), Neck Disability Index (NDI) score, Japanese Orthopedic Association (JOA) score, SF-12 concise health survey, and the Odom criteria. The radiographical parameters, including C2-C7 lordosis, segmental angle, segmental height, and subsidence, were assessed. The mean duration of follow-up was 25.6 months.
Results: Bony fusion was achieved in all patients (100%). In three patients (12%) mild dysphagia was observed during the follow-up. The VAS-neck, VAS-arm, NDI score, JOA score, SF-12 score, C2-C7 lordosis, and segmental angle improved noticeably at the latest follow-up. Based on the Odom criteria, 22 patients (88%) reported satisfactory (excellent or good). The mean loss of C2-C7 lordosis and segmental angle between the immediate postoperative and the latest follow-up values were 1.6° ± 0.5° and 1.1° ± 0.5°, respectively. The mean subsidence was 0.9 ± 0.6 mm.
Conclusion: In patients with multi-level degenerative cervical spondylosis, 3-level ACDF using the 3D-printed titanium cage can effectively relieve the symptoms, stabilize the spine, and restore segmental height and cervical curvature. It is proven to be a reliable option for patients with 3-level degenerative cervical spondylosis. However, a future comparative study involving a larger population and longer follow-up time may be required to further evaluate the safety, efficacy and outcomes of our preliminary results.
Anterior cervical discectomy and fusion (ACDF) was first mentioned by Smith and Robinson in 1958. It is considered as a safe and effective method to relieve the symptoms of degenerative cervical spondylosis, a common progressive disease among the older population (1–4). With surgical treatment, the compression to nerve root and spinal cord can be relieved immediately, and the patient-reported outcome measures (PROMs) can be improved noticeably (2, 5, 6).
With the development of anesthetic and surgical techniques, there is an increase in the clinical application of ACDF. Furthermore, it has become a mature and prevalent surgical technique in treating degenerative cervical spondylosis. However, determining the type of fusion method that is the best for acquiring bony fusion remains controversial. Additionally, each fusion method has its proponents and inherent drawbacks (2, 7, 8).
In previous studies, a variety of implants were used to promote intervertebral fusion (9–14). Autograft iliac bone, the first implant used for interbody fusion, was replaced gradually due to its bone resorption, graft collapse, and donor-site complications (8, 9, 15). Although the allograft was designed to avoid donor-site complications, its low fusion rate restricted its application (8). Polyetheretherketone (PEEK) cage was the most commonly used biological substitute (15–17). Unfortunately, it may probably lead to a lack of osseointegration, implant subsidence, and even failure of fusion (18). The 3D-printed titanium cage is a new production. It is not only biocompatible but also resistant to corrosion and compression (11). Meanwhile, the porous structure also promotes bony ingrowth, contributing to bone incorporation (16). Several studies have demonstrated that applications of 3D-printed titanium cages in single-level and two-level ACDF can better facilitate interbody fusion and prevent subsidence without increased complications. Yet, based on the authors' knowledge, only a few studies have reported the applications of 3-level ACDF using 3D-printed titanium cages in treating degenerative cervical spondylosis (11, 15, 16, 19). In this study, the clinical and radiological outcomes of patients who underwent 3-level ACDF with a 3D-printed titanium cage were evaluated.
This study was authorized and approved by the Ethics Committee of our institution. From March 2019 to June 2021, 25 patients who underwent 3-level ACDF with a 3D-printed titanium cage for the treatment of degenerative cervical spondylosis were retrospectively enrolled. In this study, patients aged at least 18 years with symptomatic degenerative cervical spondylosis were included. All the included patients did not respond to conservative treatment before surgeries.
Of the 25 participants studied, 15 were female patients (60%). The mean age of the participants was 56.8 ± 6.1 years and their mean BMI was 23.2 ± 2.6 kg/m2. Four patients (16%) were smokers, and one patient (4%) had diabetes mellitus. Fourteen patients (56%) presented with radiculopathy symptoms, 10 patients (40%) with medullary symptoms, and one patient (4%) with combined symptoms. The most common operative segment was C4-C7 in 14 patients (56%), followed by C3-C6 in 11 patients (44%). Segmental instability was found in 18 patients (72%), and 28 segments (37%) out of 75 segments exist dynamic instability. The preoperative ASA classification was class I in four patients (16%), class II in 18 patients (72%), and class III in three patients (12%). The mean duration of follow-up was 25.6 ± 7.8 months (Table 1).
All the 3-level ACDF surgeries were operated on by the same senior spine surgeons. Following general anesthesia, the patients were given the supine position with their necks properly extended. The C-arm fluoroscope was used to confirm the location of the lesion segment and a standard right-side transverse incision was made. The skin, subcutaneous tissue, and platysma were dissected layer by layer until the front of the cervical vertebral was exposed. Then, the affected intervertebral disc was completely removed with the help of the distractor. After removing the osteophytes and the posterior longitudinal ligament thoroughly, adequate spinal cord and nerve root decompression could be achieved. The cartilaginous endplates were scraped off with a curette. Furthermore, a suitable empty 3D-printed titanium cage was implanted in the intervertebral space and then an anterior cervical plate was fixed. Finally, a drainage tube was retained before closing the incision.
Following surgery, the symptoms of all patients improved. Postoperative complications, such as dysphagia, hematoma, surgical-site infection, segmental instability, and pseudarthrosis, were recorded. Especially, the dysphagia status was described as none, mild, moderate, and severe (Table 2) (20). All patients were encouraged to wear the cervical collar for eight weeks and take rehabilitation measures early. After that, patients would return to the hospital for clinical and radiological assessments at 1, 2, 3, 6, 12 months, and annually thereafter.
All PROMs were recorded preoperatively, postoperatively, and at each follow-up. Some measurement scales in PROMs were used for evaluating the clinical outcomes. The visual analog scale (VAS) was applied to evaluate neck and arm pain levels before and after surgery, including VAS for the neck (VAS-neck) and VAS for the arm (VAS-arm) (21). The Neck Disability Index (NDI) score and Japanese Orthopedic Association (JOA) score were used for assessing the physical and neurological functions preoperatively and postoperatively (22, 23). The SF-12 concise health survey, which selected 12 items from the SF-36 questionnaire, was used to evaluate the general health status and the quality of life comprehensively (24). The minimum clinically important difference (MCID) was considered a threshold for clinical improvement (22). In this study, the MCID values for the above outcome measures were calculated at 2.6 points for VAS-neck pain, 4.1 for VAS-arm pain, 8 for NDI, 2.5 for JOA, 8.5 for SF-12 physical component summary (PCS), and 9.9 for SF-12 mental component summary (MCS) (22, 23, 25, 26). The Odom criteria were proven to be valid and reliable in assessing surgical outcomes and overall patient satisfaction. The following two ratings were used for determining patient satisfaction: satisfactory (excellent and good) or unsatisfactory (fair and poor) (21, 27).
Radiographs, including the anteroposterior, lateral plain, and flexion-extension radiographs, were collected before surgery, on the first day after surgery, and at each follow-up. The measured parameters included C2-C7 lordosis, segmental angle, and subsidence. The C2-C7 lordosis, also called cervical lordosis, was measured by using the Cobb angle between the lower endplates of C2 and C7. The segmental angle was only limited to fusion levels. Therefore, the measurement approach for this parameter was to use the Cobb angle between the upper endplate of the cephalad and the lower endplate of the caudal vertebrae (2). The subsidence was defined as a change of operative segmental height at the latest follow-up compared with the immediate postoperative height (11). The segmental height was defined as the distance between the midpoint of the superior border of the cephalad-affected vertebral body and the midpoint of the inferior border of the caudal-affected vertebral body. The angle of motion (ROM) ≤4° and translation ≤1.25 mm in the affected levels on flexion-extension images were considered a successful fusion (16).
The SPSS version 20.0 (IBM Corp, USA) was used for statistical analyses. Continuous variables were presented as the mean ± standard deviation (SD) or median (range). Categorical variables were recorded as numbers and percentages. The results of VAS-arm, VAS-neck, NDI, JOA, and SF-12 preoperatively and at the latest follow-up were compared using the Wilcon signed-rank test. The results of C2-C7 lordosis, segmental angle, and segmental height preoperatively and at the latest follow-up were compared using the Paired t-test. A P-value of <0.05 was considered to be statistically significant.
The mean operative time of 3-level ACDF was 136.5 ± 7.7 min. Primary healing of incision was achieved in all patients. The median (range) length of hospital stay was 10 days (8–13). The postoperative radiologic data showed that bony fusion was achieved in all patients. Three patients (12%) complained of mild dysphagia during the follow-up, all of which were recovered at latest follow-up. Complications, like hematoma, surgical-site infection, segmental instability, and pseudarthrosis were not noted in any patients after surgery. The typical case is shown in Figure 1.
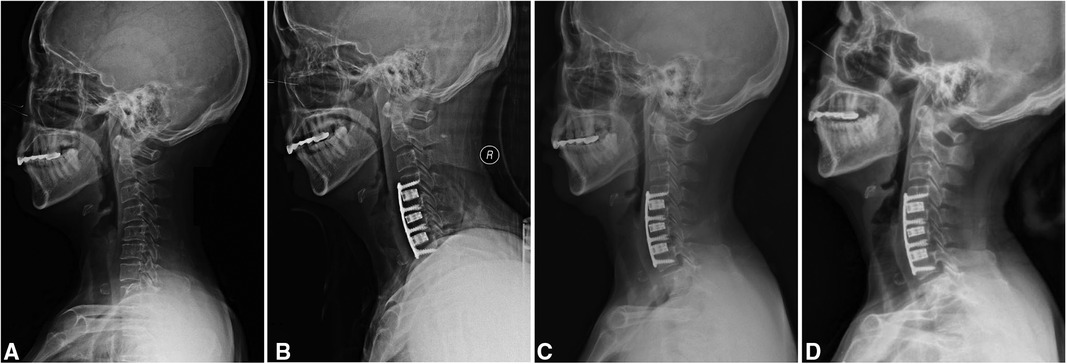
Figure 1. A 60-year-old woman with degenerative cervical spondylosis. (A) A preoperatively lateral radiograph in a neutral position. (B) This patient was treated with 3-level anterior cervical discectomy and fusion using a 3D-printed titanium cage (C4-7). (C) A radiograph at three months post-operatively shows that the implant is in a good position. (D) A radiograph at the latest follow-up shows a satisfactory outcome.
The median VAS-neck decreased from 6 points (4–8) preoperatively to 2 points (0–3) at the latest follow-up (P < 0.001), and the median VAS-arm decreased from 5 points (4–8) preoperatively to 0 point (0–3) at latest follow-up (P < 0.001) (Figure 2A). The median NDI decreased from 30 points (20–42) preoperatively to 8 points (3–17) at latest follow-up (P < 0.001) (Figure 2B). The median JOA improved from 13 points (7–15) preoperatively to 16 points (14–17) at latest follow-up (P < 0.001) (Figure 2C). The median PCS improved from 20 points (0 to 30) preoperatively to 70 points (40 to 95) at latest follow-up (P < 0.001), and the median MCS improved from 46 points (17–54) preoperatively to 75 points (42 to 92) at latest follow-up (P < 0.001) (Figure 2D). The median differences of VAS arm, VAS neck, NDI, JOA, PCS, and MCS before surgery and at latest follow-up were −4, −5, −22, 3, 50, and 29, respectively, which all reached MCIDs (Table 3). Patient satisfaction was satisfactory (excellent and good) in 22 patients (88%) and unsatisfactory (fair) in three patients (12%) (Figure 2E).
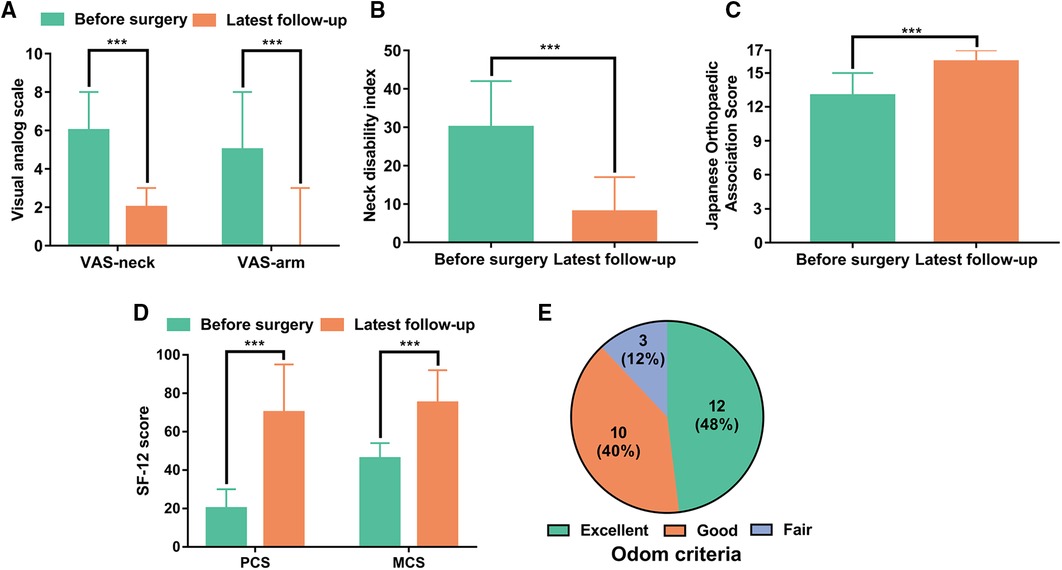
Figure 2. Patient-reported outcome measures before the operation and at the latest follow-up. (A) Visual analog scale for neck pain (VAS-neck) and arm pain (VAS-arm). (B) Neck Disability Index (NDI) score. (C) Japanese Orthopedic Association (JOA) score. (D) SF-12 physical component summary (PCS) and SF-12 mental component summary (MCS). (E) Patient satisfaction according to Odom criteria at the latest follow-up. ***P < 0.001.
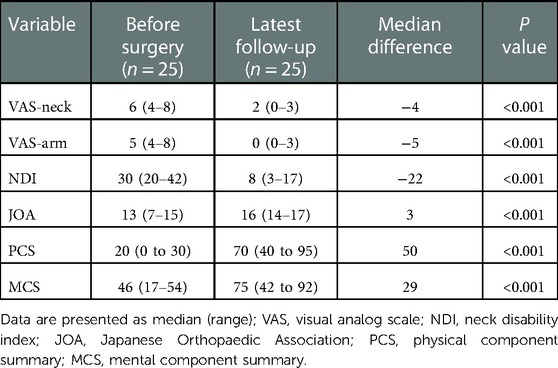
Table 3. Comparisons of patient-reported outcome measures between preoperatively and latest follow-up.
The mean preoperative, postoperative, and latest C2-C7 lordosis were 11.1° ± 4.0°, 21.6° ± 4.4°, and 20.0° ± 4.3°, respectively, and the mean preoperative, postoperative, and latest segmental angles were 5.8° ± 2.7°, 14.5° ± 3.3° and 13.4° ± 3.2°, respectively (Figure 3A). The mean preoperative, postoperative, and latest segmental heights were 69.3 ± 5.7 mm, 74.5 ± 6.2 mm and 73.6 ± 6.0 mm, respectively (Figure 3B). The mean C2-C7 lordosis, segmental angle, and segmental height at latest follow-up were significantly increased compared with preoperative data (P < 0.001, P < 0.001, P < 0.001). The mean loss of C2-C7 lordosis and segmental angle between immediate postoperative and the latest follow-up values were 1.6° ± 0.5° and 1.1° ± 0.5°, respectively. The mean subsidence was 0.9 ± 0.6 mm (Table 4).
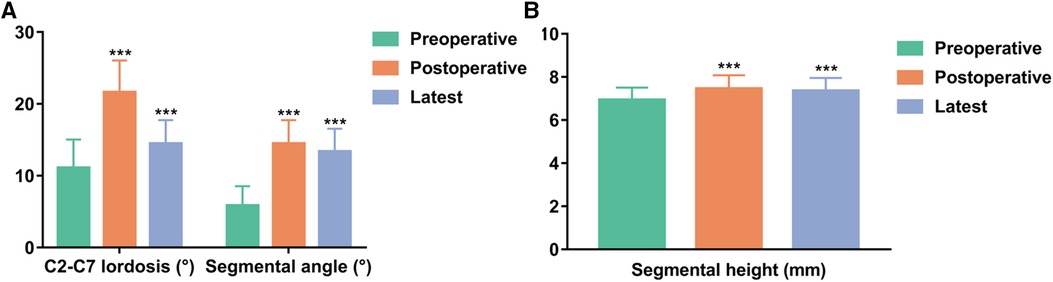
Figure 3. Comparisons of radiologic parameters. (A) C2-C7 lordosis and segmental angle. (B) Segmental height. ***P < 0.001 vs. preoperative data.
Degenerative cervical spondylosis has increased in the past decades, exerting a considerable impact on global health (28). Conservative treatments, like oral analgesics, cervical traction, and neck physical therapy can relieve pain and improve neurological function in most patients (4). For patients with surgical indications, ACDF is considered the standard surgery due to its safety and satisfactory clinical results (11). However, as the lesser common procedure, multilevel ACDF is complicated and remains controversial (21). With an increase in the number of fusion segments, the incidence of postoperative complications, like a higher rate of dysphagia, non-union, and subsidence-related complications, are experiencing a rise (5, 10, 29). Wewel et al. found that 3–4 level ACDF could result in pseudarthrosis in nearly half of the patients and had a higher revision rate (10). Another study also reported a higher non-union rate in the 3-level ACDF procedures (30). Hence, an effective fusion technique adopted by surgeons to acquire bony fusion and prevent subsidence-related complications is important.
In the past, autologous iliac bone was regarded as the gold standard for interbody fusion, however, Bolesta et al. reported that the non-union rate was up to 53% in 3-level ACDF using the iliac crest (18, 31). In another related research, the pseudarthrosis rate was 42% in 3-level ACDF using allograft materials (10). At present, the PEEK cage, characterized by cost-efficient and radiolucent, was commonly used for interbody fusion (15, 18). However, its material property was not suitable for bone ingrowth, which was seen as the primary reason for the postoperative non-union (15). A study revealed that the non-union rate in the 3-level ACDF group using PEEK cages for fusion was 14.3%, which was much higher than that of single- and two-level ACDF with PEEK cages (5, 32). The porous structure, promising mechanical properties, and rough surface of the 3D-printed titanium cage could allow bone cell ingrowth, making it easier for interbody fusion and improving the fusion rate (15, 16). In a previous study of 28 patients, the fusion rate in single- or two-level ACDF using a 3D-printed titanium cage was 100% (11). However, scanty information was available in the literature focusing on the fusion rate in 3-level ACDF with a 3D-printed titanium cage. In this study, a 3-level ACDF using a 3D-printed titanium cage in 25 patients with degenerative cervical spondylosis was performed. The mean follow-up time was 25.6 months, and all patients achieved bony fusion. Considering that the fusion rate can be affected by many factors, such as age and smoking, we reviewed the previous literature and found that the patient characteristics included in this study were similar to those in previous studies (5, 10, 33, 34). These data reveal that 3D-printed titanium cage is a feasible choice for interbody fusion.
The fundamental purpose of placing an intervertebral cage after discectomy was to maintain postoperative intervertebral height and cervical lordosis, as well as to prevent the development of subsidence-related complications (8, 35). Fujibayashi et al. presented two types of cage subsidence; transient subsidence, occurring in the early period after surgery, was about 1–3 mm and associated with cage stabilization, while progressive subsidence was associated with non-union (36). Another similar study found that slight subsidence could maintain cervical alignment and lordosis (37). Excessive subsidence could result in segmental kyphosis, adjacent segment degeneration, and failure of fusion (29). One innovative study also revealed that the mild subsidence (1–3 mm) had no effect on clinical outcomes, whereas the severe subsidence (>3 mm) was associated with poor neurological outcomes (38). However, the correlation between cage subsidence and long-term outcomes was still controversial (35, 39). Risk factors leading to cage subsidence were retrospectively studied in the early literature, including increased age, osteopenia, oversized cage, cervical alignment, and use of plate (29). Meanwhile, appropriate cervical curvature was a part of the successful treatment. Chen et al. performed 3-level ACDF using PEEK cage and plate fixation for 26 patients, the loss of cervical lordosis was 2° at 24 months after surgery, and the loss of disc height was about 1.4 mm (5). Louie et al. reported that for a 3-level ACDF using a PEEK cage, the mean subsidence was 1.7 mm after a mean 24.3-month follow-up (40). Achieving interbody fusion in a 3D-printed titanium cage is faster than in a PEEK cage, which can effectively prevent subsidence and loss of cervical lordosis (16). In our study, the loss of cervical lordosis at the latest follow-up was 1.6°, and the mean subsidence was 0.9 mm. Our study indicated that a 3D-printed titanium cage is an effective option for maintaining postoperative intervertebral height and cervical lordosis.
A large number of studies have shown that ACDF can significantly improve the PROMs of patients after surgery (9, 16, 21, 33). Lambrechts et al. reported 1024 patients who underwent ACDF, and all PROMs, including VAS neck and arm pain, NDI, JOA, and SF-12 scores, improved noticeably (41). Arts et al. retrospectively reviewed 49 patients who underwent single-level ACDF surgeries. The mean VAS arm and neck pain scores decreased from 56.1 points and 53.2 points preoperatively to 22.2 points and 23.8 points postoperatively at 12 months, respectively. The mean NDI decreased from 41.2 points preoperatively to 19.4 points postoperatively at 12 months (16). In this study, a significant reduction in neck and arm pain was observed. The disability, physical, and neurological functions of the patients showed a noticeable improvement at the latest follow-up compared with the preoperative data, as illustrated by the improvement in the SF-12, NDI, and JOA scoring systems. Moreover, the MCID was used to evaluate the improvement of clinical outcomes, and all PROMs achieved MCID. At the latest follow-up, 88% of patients responded with satisfactory outcomes (excellent or good) based on the Odom criteria.
The most common complication after ACDF is dysphagia (42, 43). Nanda et al. performed 3-level ACDF for 25 patients, four of whom had dysphagia in the postoperative period (44). Sun et al. compared the clinical outcomes of zero-profile spacer (ZP Group) and plate-cage (PC Group) for 3-level ACDF, 40.7% of patients in ZP Group and 47.1% of patients in PC Group experienced dysphagia at 48 h postoperatively, and 3.7% of patients in ZP Group and 23.5% of patients in PC Group still had dysphagia at 6 months after surgery (45). In another study about 3- or 4-level ACDF using allograft materials, 11% of patients had clinically significant dysphagia at discharge (10). In the present study, three patients (12%) complained of mild dysphagia during the follow-up, all of which were recovered at latest follow-up. Our data preliminarily support the 3D-printed titanium cage as an option for 3-level ACDF in treating degenerative cervical spondylosis with comparable incidence of complications to traditional approaches.
There are also some drawbacks in 3D-printed titanium cage utilization, the major concerns are the fatigue performance and mechanical strength. Although the design of porous structure can promote bone ingrowth and reduce elastic modulus, it may impair the fatigue performance and mechanical strength of implants(11). However, biomechanical assessment revealed better mechanical properties of 3D-printed titanium cage than those of conventional implants, which supports that 3D-printed titanium is a feasible implant for 3-level ACDF (46).
A few limitations were observed in the present study. First, a control group was lacking in this study. Future research should compare the outcomes of the 3D-printed titanium cage with other cages in 3-level ACDF. Second, the number of patients in this study is too small to perform an effective subgroup analysis including more relevant factors, such as osteoporosis and gender. Cervical spondylosis is more common in the elderly, often accompanied by osteoporosis and other diseases. Previous studies have shown that patients with osteoporosis have a lower fusion rate after ACDF (47, 48). Compared with PEEK cage, 3D-printed titanium cage can accelerate the achievement of interbody fusion (16). This may be a feasible option for people with osteoporosis. However, because of the few clinical application of 3D-printed titanium cage, there are no related studies about the application of ACDF using 3D-printed titanium cage in patients with osteoporosis have been reported, and further studies are needed to verify this in the future. At last, a longer duration of follow-up is needed to investigate long-term complications and cervical stabilization. Despite these limitations, the present study remains the first retrospective study evaluating on the efficacy of 3-level ACDF using the 3D-printed cage in treating cervical spondylosis.
In patients with multi-level degenerative cervical spondylosis, 3-level ACDF using the 3D-printed titanium cage can effectively relieve the symptoms, stabilize the spine, and restore segmental height and cervical curvature. The fusion rate of 100% can be reached. It is proven to be a reliable option for patients with multi-level degenerative cervical spondylosis. However, due to the lack of a corresponding control group and limited sample size, the safety, efficacy and outcomes of our preliminary results have not been fully confirmed. A future comparative study involving a larger population and longer follow-up time may be required to further evaluate the clinical outcomes between the 3D-printed titanium cage and traditional implants in 3-level ACDF. This will further help in supporting the findings of our study.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
HW and YL conceived and designed this study; XF and SXW wrote the manuscript; CXL and HW performed operations. RL, XJY, SZJ, and QD collected the data; YKQ performed the data analysis; HW and YL reviewed and revised this manuscript. All authors contributed to the article and approved the submitted version
This work was supported by the National Natural Science Foundation of China (51907078).
We express our gratitude to all the participants who generously agreed to be interviewed for this research. We would like to thank Bullet Edits Limited for proofreading the manuscript revision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lee JJ, Lee N, Oh SH, Shin DA, Yi S, Kim KN, et al. Clinical and radiological outcomes of multilevel cervical laminoplasty versus three-level anterior cervical discectomy and fusion in patients with cervical spondylotic myelopathy. Quant Imaging Med Surg. (2020) 10:2112–24. doi: 10.21037/qims-20-220
2. Canseco JA, Minetos PD, Karamian BA, Paziuk TM, Basques BA, DiMaria SL, et al. Comparison between three- and four-level anterior cervical discectomy and fusion: patient-reported and radiographic outcomes. World Neurosurg. (2021) 151:e507–16. doi: 10.1016/j.wneu.2021.04.073
3. Shedid D, Benzel EC. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery. (2007) 60:S7–13. doi: 10.1227/01.NEU.0000215430.86569.C4
4. Theodore N. Degenerative cervical spondylosis. N Engl J Med. (2020) 383:159–68. doi: 10.1056/NEJMra2003558
5. Chen Y, Lu G, Wang B, Li L, Kuang L. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking stand-alone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: a retrospective study with 2-year follow-up. Eur Spine J. (2016) 25:2255–62. doi: 10.1007/s00586-016-4391-x
6. Cha EDK, Lynch CP, Jadczak CN, Mohan S, Geoghegan CE, Singh K. Comorbidity influence on postoperative outcomes following anterior cervical discectomy and fusion. Neurospine. (2021) 18:271–80. doi: 10.14245/ns.2040646.323
7. Kim S, Alan N, Sansosti A, Agarwal N, Wecht DA. Complications after 3- and 4-level anterior cervical diskectomy and fusion. World Neurosurg. (2019) 130:e1105–10. doi: 10.1016/j.wneu.2019.07.099
8. Wen Z, Lu T, Wang Y, Liang H, Gao Z, He X. Anterior cervical corpectomy and fusion and anterior cervical discectomy and fusion using Titanium mesh cages for treatment of degenerative cervical pathologies: a literature review. Med Sci Monit. (2018) 24:6398–404. doi: 10.12659/MSM.910269
9. Yeung KKL, Cheung PWH, Cheung JPY. Anterior cervical discectomy and fusion for cervical myelopathy using stand-alone tricortical iliac crest autograft: predictive factors for neurological and fusion outcomes. J Orthop Surg. (2019) 27:2309499019869166. doi: 10.1177/2309499019869166
10. Wewel JT, Kasliwal MK, Adogwa O, Deutsch H, O'Toole JE, Traynelis VC. Fusion rate following three- and four-level ACDF using allograft and segmental instrumentation: a radiographic study. J Clin Neurosci. (2019) 62:142–6. doi: 10.1016/j.jocn.2018.11.040
11. Jin YZ, Zhao B, Lu XD, Zhao YB, Zhao XF, Wang XN, et al. Mid- and long-term follow-up efficacy analysis of 3D-printed interbody fusion cages for anterior cervical discectomy and fusion. Orthop Surg. (2021) 13:1969–78. doi: 10.1111/os.13005
12. Kim SH, Lee JK, Jang JW, Park HW, Hur H. Polyetheretherketone cage with demineralized bone matrix can replace iliac crest autografts for anterior cervical discectomy and fusion in subaxial cervical spine injuries. J Korean Neurosurg Soc. (2017) 60:211–9. doi: 10.3340/jkns.2015.0203.014
13. Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. (2008) 8:426–35. doi: 10.1016/j.spinee.2006.12.006
14. Ma F, Xu S, Liao Y, Tang Q, Tang C, Wang Q, et al. Using a mixture of local bone dust and morselized bone as graft materials in single- and double-level ACDF. BMC Musculoskelet Disord. (2021) 22:510. doi: 10.1186/s12891-021-04394-3
15. Mayer F, Heider F, Haasters F, Mehren C. Radiological and clinical outcomes after anterior cervical discectomy and fusion (ACDF) with an innovative 3D printed cellular Titanium cage filled with vertebral bone marrow. Biomed Res Int. (2022) 2022:6339910. doi: 10.1155/2022/6339910
16. Arts M, Torensma B, Wolfs J. Porous titanium cervical interbody fusion device in the treatment of degenerative cervical radiculopathy; 1-year results of a prospective controlled trial. Spine J. (2020) 20:1065–72. doi: 10.1016/j.spinee.2020.03.008
17. Buser Z, Brodke DS, Youssef JA, Meisel HJ, Myhre SL, Hashimoto R, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine. (2016) 25:509–16. doi: 10.3171/2016.1.SPINE151005
18. Zhou J, Xia Q, Dong J, Li X, Zhou X, Fang T, et al. Comparison of stand-alone polyetheretherketone cages and iliac crest autografts for the treatment of cervical degenerative disc diseases. Acta Neurochir. (2011) 153:115–22. doi: 10.1007/s00701-010-0821-4
19. Singh H, Kukowski NR, Lunati MP, Dawes A, Kim CH, Kim S, et al. Porous 3D printed Titanium cages in anterior cervical discectomy and fusion are associated with less subsidence, improved maintenance of segmental lordotic correction, and similar clinical outcomes as allograft. Global Spine J. (2022) 21925682221124527. doi: 10.1177/21925682221124527
20. Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine. (2002) 27:2453–8. doi: 10.1097/00007632-200211150-00007
21. Zhu D, Zhang D, Liu B, Li C, Zhu J. Can self-locking cages offer the same clinical outcomes as anterior cage-with-plate fixation for 3-level anterior cervical discectomy and fusion (ACDF) in mid-term follow-up? Med Sci Monit. (2019) 25:547–57. doi: 10.12659/MSM.911234
22. Carreon LY, Glassman SD, Campbell MJ, Anderson PA. Neck disability Index, short form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J. (2010) 10:469–74. doi: 10.1016/j.spinee.2010.02.007
23. Kato S, Oshima Y, Matsubayashi Y, Taniguchi Y, Tanaka S, Takeshita K. Minimum clinically important difference and patient acceptable symptom state of Japanese orthopaedic association score in degenerative cervical myelopathy patients. Spine. (2019) 44:691–7. doi: 10.1097/BRS.0000000000002928
24. Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. J Clin Epidemiol. (1998) 51:1171–8. doi: 10.1016/s0895-4356(98)00109-7
25. Fong DYT, Chan BKY, Li S, Wan CH, Kazis LE. Average and individual differences between the 12-item MOS short-form health survey version 2 (SF-12 V.2) and the veterans RAND 12-item health survey (VR-12) in the Chinese population. Health Qual Life Outcomes. (2022) 20:102. doi: 10.1186/s12955-022-02010-z
26. Lee JS, Hobden E, Stiell IG, Wells GA. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med. (2003) 10:1128–30. doi: 10.1111/j.1553-2712.2003.tb00586.x
27. Broekema AEH, Molenberg R, Kuijlen JMA, Groen RJM, Reneman MF, Soer R. The odom criteria: validated at last: a clinimetric evaluation in cervical spine surgery. J Bone Joint Surg Am. (2019) 101:1301–8. doi: 10.2106/JBJS.18.00370
28. Hurwitz EL, Randhawa K, Yu H, Cote P, Haldeman S. The global spine care initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J. (2018) 27:796–801. doi: 10.1007/s00586-017-5432-9
29. Lee YS, Kim YB, Park SW. Risk factors for postoperative subsidence of single-level anterior cervical discectomy and fusion: the significance of the preoperative cervical alignment. Spine. (2014) 39:1280–7. doi: 10.1097/BRS.0000000000000400
30. Wang JC, McDonough PW, Kanim LE, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine. (2001) 26:643–6. discussion 646-647. doi: 10.1097/00007632-200103150-00015
31. Bolesta MJ, Rechtine GR 2nd, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation: a prospective study. Spine. (2000) 25:2040–4. discussion 2045-2046. doi: doi: 10.1097/00007632-200008150-00007
32. Zapolska G, Kwiatkowski M, Turek G, Mariak Z, Hermanowicz A. Biomechanical evaluation of single- and multi-level anterior cervical discectomy and fusion with polyetheretherketone cages: radiological and clinical outcomes. Neurol Neurochir Pol. (2019) 53:358–62. doi: 10.5603/PJNNS.a2019.0040
33. Xiao B, Wu B, Rong T, Cui W, Sang D, Liu B. Clinical impact of 3-level anterior cervical decompression and fusion (ACDF) on the occipito-atlantoaxial complex: a retrospective study of patients who received a zero-profile anchored spacer versus cage-plate construct. Eur Spine J. (2021) 30:3656–65. doi: 10.1007/s00586-021-06974-2
34. Zhou J, Li X, Dong J, Zhou X, Fang T, Lin H, et al. Three-level anterior cervical discectomy and fusion with self-locking stand-alone polyetheretherketone cages. J Clin Neurosci. (2011) 18:1505–9. doi: 10.1016/j.jocn.2011.02.045
35. Noordhoek I, Koning MT, Jacobs WCH, Vleggeert-Lankamp CLA. Incidence and clinical relevance of cage subsidence in anterior cervical discectomy and fusion: a systematic review. Acta Neurochir. (2018) 160:873–80. doi: 10.1007/s00701-018-3490-3
36. Fujibayashi S, Neo M, Nakamura T. Stand-alone interbody cage versus anterior cervical plate for treatment of cervical disc herniation: sequential changes in cage subsidence. J Clin Neurosci. (2008) 15:1017–22. doi: 10.1016/j.jocn.2007.05.011
37. Jang JW, Lee JK, Lee JH, Hur H, Kim TW, Kim SH. Effect of posterior subsidence on cervical alignment after anterior cervical corpectomy and reconstruction using titanium mesh cages in degenerative cervical disease. J Clin Neurosci. (2014) 21:1779–85. doi: 10.1016/j.jocn.2014.02.016
38. Chen Y, Chen D, Guo Y, Wang X, Lu X, He Z, et al. Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech. (2008) 21:489–92. doi: 10.1097/BSD.0b013e318158de22
39. Wu WJ, Jiang LS, Liang Y, Dai LY. Cage subsidence does not, but cervical lordosis improvement does affect the long-term results of anterior cervical fusion with stand-alone cage for degenerative cervical disc disease: a retrospective study. Eur Spine J. (2012) 21:1374–82. doi: 10.1007/s00586-011-2131-9
40. Louie PK, Sexton AC, Bohl DD, Tabaraee E, Presciutti SM, Mayo BC, et al. Rigid-Plating and cortico-cancellous allograft are effective for 3-level anterior cervical discectomy and fusion: radiographic and clinical outcomes. Neurospine. (2020) 17:146–55. doi: 10.14245/ns.1836052.026
41. Lambrechts MJ, Toci GR, Issa TZ, Siegel NS, O'Connor P, Siniakowicz C, et al. Patients from socioeconomically distressed communities experience similar clinical improvements following anterior cervical discectomy and fusion. Spine. (2022) 47:1701–9. doi: 10.1097/brs.0000000000004455
42. Fountas KN, Kapsalaki EZ, Nikolakakos LG, Smisson HF, Johnston KW, Grigorian AA, et al. Anterior cervical discectomy and fusion associated complications. Spine. (2007) 32:2310–7. doi: 10.1097/BRS.0b013e318154c57e
43. Okano I, Salzmann SN, Ortiz Miller C, Hoshino Y, Oezel L, Shue J, et al. Risk factors for postoperative dysphagia and dysphonia following anterior cervical spine surgery: a comprehensive study utilizing the hospital for special surgery dysphagia and dysphonia inventory (HSS-DDI). Spine J. (2021) 21:1080–8. doi: 10.1016/j.spinee.2021.02.011
44. Nanda A, Sharma M, Sonig A, Ambekar S, Bollam P. Surgical complications of anterior cervical diskectomy and fusion for cervical degenerative disk disease: a single surgeon's experience of 1,576 patients. World Neurosurg. (2014) 82:1380–7. doi: 10.1016/j.wneu.2013.09.022
45. Sun B, Shi C, Wu H, Xu Z, Lin W, Shen X, et al. Application of zero-profile spacer in the treatment of three-level cervical spondylotic myelopathy: 5-year follow-up results. Spine. (2020) 45:504–11. doi: 10.1097/brs.0000000000003312
46. Yin X, Jiang L, Yang J, Cao L, Dong J. Application of biodegradable 3D-printed cage for cervical diseases via anterior cervical discectomy and fusion (ACDF): an in vitro biomechanical study. Biotechnol Lett. (2017) 39:1433–9. doi: 10.1007/s10529-017-2367-5
47. Zhang L, Wang J, Feng X, Tao Y, Yang J, Wang Y, et al. Outcome evaluation of zero-profile device used for single-level anterior cervical discectomy and fusion with osteoporosis compared without osteoporosis: a Minimum three-year follow-up study. World Neurosurg. (2018) 124:E1–E9. doi: 10.1016/j.wneu.2018.10.024
Keywords: 3D-printed titanium cage, anterior cervical discectomy and fusion, degenerative cervical spondylosis, 3-level ACDF, subsidence
Citation: Wang S, Fang X, Qu Y, Lu R, Yu X, Jing S, Ding Q, Liu C, Wu H and Liu Y (2023) Is 3D-printed Titanium cage a reliable option for 3-level anterior cervical discectomy and fusion in treating degenerative cervical spondylosis?. Front. Surg. 10:1096080. doi: 10.3389/fsurg.2023.1096080
Received: 11 November 2022; Accepted: 11 January 2023;
Published: 17 February 2023.
Edited by:
John K. Yue, University of California, United StatesReviewed by:
Hansen Deng, University of Pittsburgh Medical Center, United States© 2023 Wang, Fang, Qu, Lu, Yu, Jing, Ding, Liu, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Wu d3VodWFAaHVzdC5lZHUuY24= Yang Liu eWFuZ2xpdUB0amgudGptdS5lZHUuY24=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.