
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 17 March 2023
Sec. Genitourinary Surgery and Interventions
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1095545
Objective: Inguinal lymph node metastasis (ILNM) is significantly associated with poor prognosis in patients with squamous cell carcinoma of the penis (SCCP). Patient prognosis could be improved if the probability of ILNM incidence could be accurately predicted at an early stage. We developed a predictive model based on machine learning combined with big data to achieve this.
Methods: Data of patients diagnosed with SCCP were obtained from the Surveillance, Epidemiology, and End Results Program Research Data. By combing variables that represented the patients' clinical characteristics, we applied five machine learning algorithms to create predictive models based on logistic regression, eXtreme Gradient Boosting, Random Forest, Support Vector Machine, and k-Nearest Neighbor. Model performance was evaluated by ten-fold cross-validation receiver operating characteristic curves, which were used to calculate the area under the curve of the five models for predictive accuracy. Decision curve analysis was conducted to estimate the clinical utility of the models. An external validation cohort of 74 SCCP patients was selected from the Affiliated Hospital of Xuzhou Medical University (February 2008 to March 2021).
Results: A total of 1,056 patients with SCCP from the SEER database were enrolled as the training cohort, of which 164 (15.5%) developed early-stage ILNM. In the external validation cohort, 16.2% of patients developed early-stage ILNM. Multivariate logistic regression showed that tumor grade, inguinal lymph node dissection, radiotherapy, and chemotherapy were independent predictors of early-stage ILNM risk. The model based on the eXtreme Gradient Boosting algorithm showed stable and efficient prediction performance in both the training and external validation groups.
Conclusion: The ML model based on the XGB algorithm has high predictive effectiveness and may be used to predict early-stage ILNM risk in SCCP patients. Therefore, it may show promise in clinical decision-making.
Penile carcinoma (PC) is a rare malignant tumor of the genitourinary system that exhibits a predilection for the glans and inner prepuce (1). The overall incidence of PC in men in developed countries such as Europe and the United States is less than 1 in 100,000, and it accounts for approximately 0.24% of all new cancer cases in men (2, 3). In contrast, PC accounts for 2% of malignancies in men in less developed countries or regions such as Africa and South America, and in some places like Uganda, the incidence is even higher than 8/100,000 (4, 5). This phenomenon could be linked to risk factors such as poor economic conditions, a high prevalence of HPV infection, and phimosis (6, 7). Squamous cell carcinoma accounts for more than 95% of penile cancers and is the most common histologic type (8). The risk of developing squamous cell carcinoma of the penis (SCCP) increases with age, with the peak age range being 50 to 70 years (9). For the surgical treatment of SCCP, the aim of primary tumor treatment is to completely remove the tumor and preserve as many organs as possible without compromising control of the tumor. However, for advanced invasive tumors, partial or radical penectomy is unavoidable (10).
Lymph node invasion of SCCP conforms to the anatomy, and it most commonly metastasizes to the inguinal lymph nodes, followed by the pelvic lymph nodes. Superficial and deep inguinal lymph nodes are the first regional lymph nodes to be invaded, most commonly in the superior medial region, either unilaterally or bilaterally (11). Previous studies have shown that the occurrence of lymph node metastases in SCCP patients is significantly associated with poor prognosis (12). The survival rate of patients with SCCP decreases dramatically with increase in lymph node invasion. The five-year survival rate of patients with inguinal lymph node metastasis (ILNM) is only 50%–80%, but the five-year survival rate of patients who develop pelvic lymph node and peripheral lymph node invasion is less than 33% (13, 14). Due to limitations in imaging techniques, up to 25% of cases in patients with clinically negative lymph nodes cannot be detected despite the presence of micrometastases (15). Therefore, identifying independent risk factors for the development of ILNM at an early stage and establishing corresponding risk prediction models for identifying patients at high risk of ILNM can improve the survival prognosis of this group of SCCP patients through more frequent imaging and more comprehensive clinical treatment in the early stage of diagnosis.
Early studies had shown that stage and grade of the tumor as well as lymphatic and vascular embolization were associated with the risk of lymph node metastasis (16, 17). Ficarra et al. showed that pT stage, histologic grade, venous embolization, and lymphatic embolization were independent predictors of lymph node metastasis in SCCP (18). Peak et al. developed a predictive model based on the clinicopathological characteristics of patients recorded in the National Cancer Database (NCDB), which showed better efficacy in their internal validation (19). In previous studies, several prediction nomograms were established based on logistic regression. The traditional logistic regression algorithm has some limitations in terms of nonlinear complex computation, with area under the curve (AUC) both less than 0.8, so this leaves room for further optimization (20, 21). Machine learning (ML) is an advanced algorithmic model that automatically learns and improves performance by identifying complex nonlinear relationships in different patterns, and is considered superior to traditional algorithms (22). As one of the components of artificial intelligence, ML has been widely used in clinical practice, such as for epidemic prediction (23, 24), and survival analysis (25, 26). ML models have also been proposed for the prediction of lymph node metastasis in a variety of malignancies (27, 28). Based on these promising clinical applications of ML models, the present study aimed to develop and validate a novel ML-based model for predicting the risk of early-stage ILNM in patients with SCCP.
A retrospective cohort design was adopted. Data were obtained from the SEER research database, which covers roughly 27.8% of the US population. The SEER database is a comprehensive data source, which is public and identifiably accessible that data analysis is treated as non-human subjects. Therefore no institutional review board approval and informed consent were required. We used the ICD-O-3 site codes C60.0 to C60.8 and histological codes 8,051–8,052 and 8,070–8,075 to identify SCCP patients. To develop the ideal ML model, several variables were obtained, including survival data, age at diagnosis, race, marital status at diagnosis, primary site, tumor size, tumor grade, surgical procedure, inguinal lymph node dissection (ILND), lymph-vascular invasion (LVI), TNM stage, radiotherapy data, and chemotherapy data. We grouped ethnicity into white, black and other; and marital status into married and other. An external validation set was constructed by collecting data on the same variables from our center based on the same criteria. A flow chart for patient selection from the SEER database is shown in Supplementary Figure S1. Early-stage ILNM was defined as N1 and N2 stage, i.e., unilateral or bilateral untreated ILNM.
For continuous variables, Student's t-test was used for normally distributed data and the Mann Whitney U-test was used for non-normally distributed data. The chi-square test was used to analyze categorical data. Categorical variables were one-hot-encoded before incorporated into the ML algorithms.The odds ratio (OR) with 95% confidence intervals (CI) was calculated using univariate and multivariate logistic regression analysis. Only two-sided p-values <0.05 were considered to indicate statistical significance. We used five different ML algorithms to analyze our data: LR, XGB, RF, SVM, and kNN. The model with the highest average AUC was considered as the best algorithm. Furthermore, the ML-based model was tuned to avoid overfitting, and the accuracy of the algorithm was tested using the ten-fold cross-validation method. Detailed packages used in the development of our ML models including XGB 1.2.1, lightGBM 3.2.1, and sklearn 0.22.1. In the RF algorithm, the number of decision trees was set to 100, the maximum tree depth was set to 10, and the maximum number of leaf nodes was set to 50. In the kNN algorithm, the number of leaves was set to 30, and the number of nearest neighbors was set to 5. R 4.1.2 (https://www.r-project.org/), Python 3.10 (https://www.python.org/), and SEER*Stat (https://seer.cancer.gov/seers tat/) were used in this study.
Baseline data for the training cohort and external validation cohort are provided in Table 1. In the training cohort, the variables with p values <0.05 were age, tumor size, marital status, tumor grade, T-stage, ILND, LVI, radiotherapy, and chemotherapy. Patients who developed early-stage ILNM were younger compared to those with stage N0 ILNM; had undergone ILND; and had larger tumor size, poorer grade, and higher T-stage. Further, a higher proportion of patients with early-stage ILNM had LVI and were receiving radiotherapy/chemotherapy. No statistically significant differences were found in race or primary site. The correlations between the variables chosen as predictors were analyzed and visualized by a heatmap using Spearman's rank correlation coefficient (Figure 1).
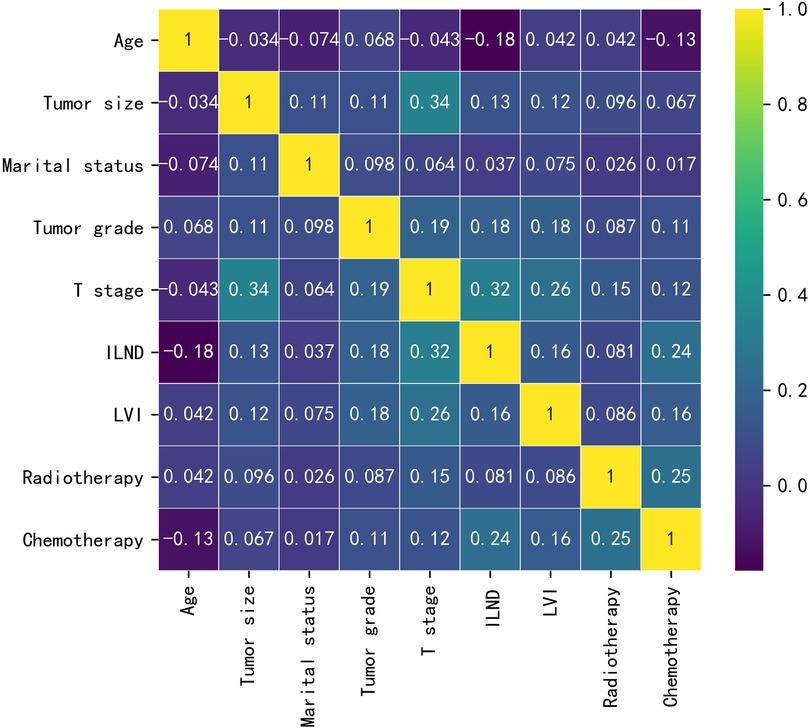
Figure 1. The results of Spearman correlation analysis between all the variables. The heat map shows the correlation between SCCP patients’ clinical and pathological features.
We retrieved patients' survival data from the SEER database. Cancer-specific survival (CSS) was considered as the endpoint, and Kaplan-Meier survival analysis was used to estimate the survival. As shown in Figure 2, patients with early-stage ILNM had significantly worse CSS (p < 0.0001).
As illustrated in Table 2, univariate logistic regression analysis showed that age, marital status, tumor grade, T-stage, ILND, LVI, radiotherapy, and chemotherapy were all significantly associated with the occurrence of early-stage ILNM in the overall population (p < 0.05). In multivariable logistic regression analysis, the factors with statistical significance included tumor grade (p = 0.001), T stage (p = 0.05), ILND (OR = 11.044, 95% CI = 6.984–17.846, p < 0.001), radiotherapy (OR = 4.64, 95% CI = 2.361–9.145, p < 0.001), and chemotherapy (OR = 9.612, 95% CI = 4.971–19.138, p < 0.001).
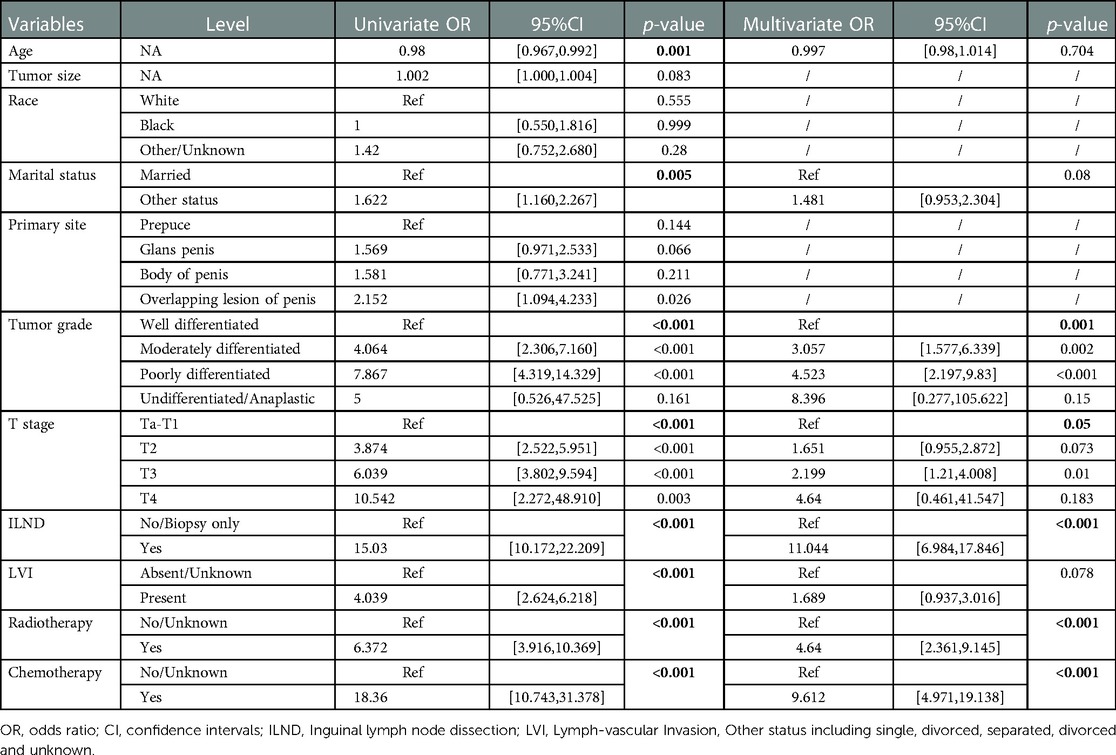
Table 2. Univariable and multivariate logistic regression analysis of the training cohort for predicting ILNM risk.
To compare the predictive efficiency of the five ML algorithm models, ten-fold cross validation was applied in this study (Figure 3). Both the RF model (AUC = 0.924, 95% CI = 0.902–0.946) and the XGB model (AUC = 0.922, 95% CI = 0.900–0.945) performed well in the prediction of early-stage ILNM risk. The decision curve analysis of models was also subsequently constructed. The confusion matrix for ten-fold cross validation of training cohort is shown in Table 3. The learning curves of models in the training cohort are shown in Supplementary Figure S2. For the external validation cohort, as shown in Figure 4, the XGB model showed the best performance among the five algorithms according to receiver operating characteristic curve analysis (AUC = 0.853, 95% CI = 0.743–0.964). Since the XGB model was found to be stable and efficient in both the training and validation groups, we finally chose the XGB model (accuracy = 0.848, sensitivity = 0.887, specificity = 0.837 in the training cohort) as the final prediction model. Our ML algorithm model can be embedded in a web calculator or applet to allow clinicians to assess the risk of ILNM in patients with SCCP.
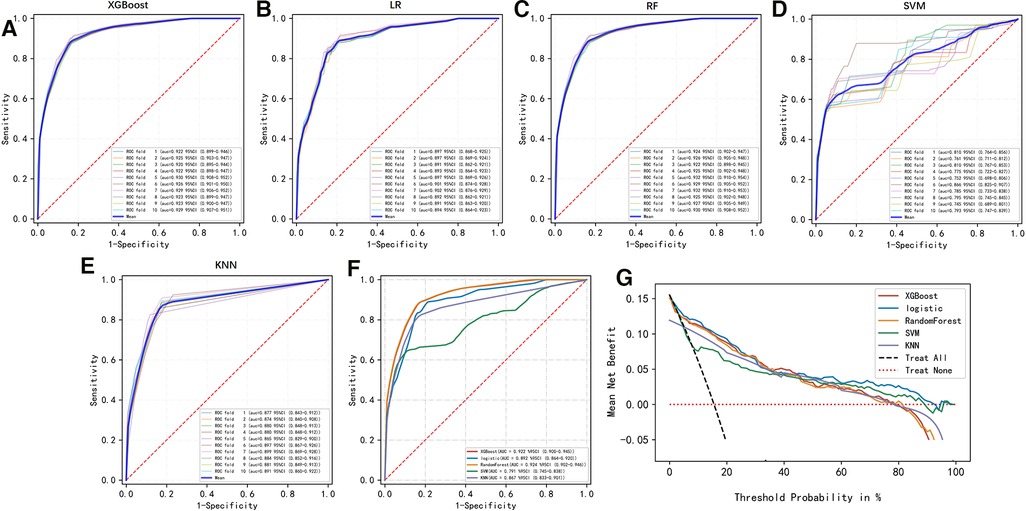
Figure 3. (A–F) Ten-fold cross ROC curves of five ML models in the training cohort. LR, logistic regression; XGB, eXtreme gradient boosting; RF, random forest; SVM, support vector machine; KNN, k-nearest neighbor. (G) Decision curve analysis graph showing the net benefit against threshold probabilities based on decisions from model outputs. The curves referred to as “All” represent the prediction that all the patients would progress to ILNM, and the curves referred to as “None” represent the prediction that no patients were ILNM.
The importance of patient clinical features in the XGB model is shown in Figure 5, and is listed here in descending order of importance: ILND, chemotherapy, radiotherapy, tumor grade, and T stage.
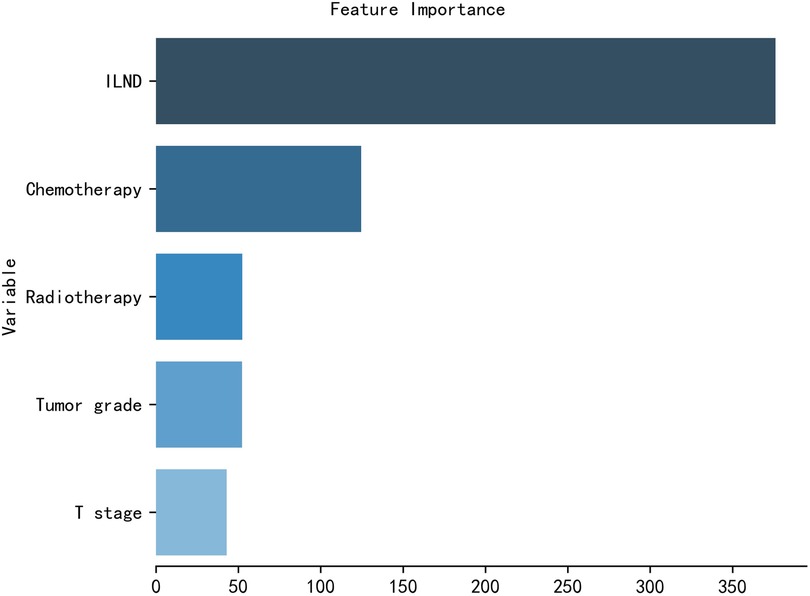
Figure 5. The XGB model was used to calculate the importance of each feature. The bar chart depicts the relative significance of the variables.
The occurrence of ILNM in SCCP is associated with poor patient prognosis. ILND is the most important procedure for the prevention and treatment of ILNM (29). However, surgeons must consider the balance between the survival benefit to the patient and the high incidence of postoperative complications (30). The use of dynamic anterior node biopsy has been advocated to avoid unnecessary ILND, but the risk of false-negative results remains unavoidable (31), thus threatening the postoperative quality of patient survival. Therefore, early and accurate prediction of LNM is imminent. Previous studies on the SEER database suggest that age at diagnosis, tumor grade, tumor size, T stage, primary site, and LVI may be predictors of ILNM. However, these studies did not take into account the postoperative adjuvant therapy received by the patients. The models based on these variables had AUC of 0.776 and 0.795, respectively (20, 21). The advent of ML algorithms has made it possible to improve model performance.
In this study, we included SCCP patients no distant metastases and non-N3 stage, and developed an accurate predictive model for predicting the risk of early-stage ILNM based on multiple clinical and pathological indicators. Our aim is to screen the SCCP population with a high risk of ILNM for more careful perioperative management of this group of patients, with the ultimate goal of improving patient prognosis. To the best of our knowledge, this is the first model based on a variety of ML algorithms to predict early-stage ILNM with big data and performing external validation. This study included 1,056 cases of SCCP from the SEER database for model establishment and 74 cases from an independent institution in China for external validation. We adopted five ML methods: LR, XGB, RF, SVM, and KNN. By integrating the findings related to the effectiveness and stability of the models in the training and external validation groups, we finally identified XGB as the best prediction model algorithm for early-stage ILNM risk prediction. In the training cohort, the XGB algorithm model (AUC = 0.922, 95% CI = 0.900–0.945) showed superior performance and also performed well in the external validation. The model can be embedded as a web calculator or applied by way of a mobile terminal application. By entering the values or classification results of each indicator, the individual risk of progression can be calculated and, for patients at high risk, more aggressive physical examination, imaging, adjuvant therapy or even surgery should be performed postoperatively.
In both the baseline analysis and the univariate regression, a lower proportion of married than unmarried individuals had early-stage ILNM. This might partly be attributable to the fact that married people tend to have better economic conditions and personal hygiene. In addition, having physical intimacy with a partner increases the likelihood of detecting masses in the groin area and visiting a hospital at an earlier stage of the disease. In our study, the site of the primary tumor did not show a significant association with ILNM risk, but this is not consistent with the findings of previous studies (21). Because delayed intervention can adversely affect the survival of patients with PC diagnosed with lymph node involvement, treatment guidelines recommend lymph node dissection. Therefore, management of regional lymph nodes is very important for patient survival (32). Our findings indicate the importance of ILND for the detection of early-stage ILNM. LVI is defined as infiltration of tumor cells in the lymphatic or hematologic system. Previous studies have shown that LVI is significantly associated with lymph node metastasis and distant metastasis (33). Recently, there is evidence that the presence of LVI is an important risk factor for occult micrometastases in patients with penile cancer and affects the overall survival of patients (34). The correlation of risk with T stage and nuclear grade observed in the present study is in line with previous literature (21, 34). Meanwhile, patients with indications for radiation and chemotherapy, have a higher risk of developing early ILNM, based on clinician judgment.
Our study has certain limitations. First, the number of patients used for external validation at our center is small, and we hope to further improve it at a later stage through multi-center collaboration. Second, ML algorithms are inconvenient for use in clinical practice, and we hope to develop an online calculator app of our XGB model for clinicians in the future. In addition, there are also some limitations to the data in the SEER database. The lack of data on immunohistochemistry, genomics, patient physical indicators, underlying diseases, and hematological indicators reduces the possibility of improving the accuracy of the model, and we hope to remedy this through the establishment of a multicenter database. In addition, the practical value of the model was determined in a predominantly Caucasian database, so its applicability in other regions (including China) is unclear due to the inevitable differences in ethnicity and treatment levels in different countries/regions. Finally, the indications for ILND or adjuvant therapy vary from one medical center to another, so there may be some errors in its practical application. Nevertheless, our study yielded encouraging results that ML algorithms appear to have greater efficacy potential for early ILNM risk prediction in patients with SCCP compared to traditional logistic regression analysis.
We have built a precise, big data-based ML model for predicting early-stage ILNM in patients with SCCP. External validation proved that our novel model has excellent predictive accuracy and clinical utility. Therefore, in the future, it may guide clinicians' decisions and improve the long-term prognosis of patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LD,CZ,KW contributed to the idea and design. CZ, KW, YZ collected and analyzed the data. LD, CW, WX, SL drew the figures and tables. LD and CZ wrote the draft. LD, CZ, KW, YZ, CW, WX, SL, WL and JW contributed to manuscript writing and revision. All authors contributed to the article and approved the submitted version.
This work was supported by The second round of Xuzhou Medical Leading Talents Training Project (XWRCHT20210027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1095545/full#supplementary-material.
1. O'Sullivan B, Brierley J, Byrd D, Bosman F, Kehoe S, Kossary C, et al. The tnm classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. (2017) 18:849–51. doi: 10.1016/S1470-2045(17)30438-2
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Ca Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
3. Bray F, Klint A, Gislum M, Hakulinen T, Engholm G, Tryggvadottir L, et al. Trends in survival of patients diagnosed with male genital cancers in the nordic countries 1964-2003 followed up until the end of 2006. Acta Oncol. (2010) 49:644–54. doi: 10.3109/02841860903575315
4. Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. (2009) 20:449–57. doi: 10.1007/s10552-008-9276-9
5. Coelho R, Pinho JD, Moreno JS, Garbis D, Do NA, Larges JS, et al. Penile cancer in maranhao, northeast Brazil: the highest incidence globally? Bmc Urol. (2018) 18:50. doi: 10.1186/s12894-018-0365-0
6. Maden C, Sherman KJ, Beckmann AM, Hislop TG, Teh CZ, Ashley RL, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. (1993) 85:19–24. doi: 10.1093/jnci/85.1.19
7. Koifman L, Vides AJ, Koifman N, Carvalho JP, Ornellas AA. Epidemiological aspects of penile cancer in rio de janeiro: evaluation of 230 cases. Int Braz J Urol. (2011) 37:231–40, 240-3. doi: 10.1590/s1677-55382011000200010
8. Erbersdobler A. Pathologic evaluation and reporting of carcinoma of the penis. Clin Genitourin Cancer. (2017) 15:192–5. doi: 10.1016/j.clgc.2016.08.003
9. Christodoulidou M, Sahdev V, Houssein S, Muneer A. Epidemiology of penile cancer. Curr Probl Cancer. (2015) 39:126–36. doi: 10.1016/j.currproblcancer.2015.03.010
10. Audenet F, Sfakianos JP. Psychosocial impact of penile carcinoma. Transl Androl Urol. (2017) 6:874–8. doi: 10.21037/tau.2017.07.24
11. Leijte JA, Kirrander P, Antonini N, Windahl T, Horenblas S. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur Urol. (2008) 54:161–8. doi: 10.1016/j.eururo.2008.04.016
12. Wen S, Ren W, Xue B, Fan Y, Jiang Y, Zeng C, et al. Prognostic factors in patients with penile cancer after surgical management. World J Urol. (2018) 36:435–40. doi: 10.1007/s00345-017-2167-5
13. Hakenberg OW, Comperat EM, Minhas S, Necchi A, Protzel C, Watkin N. Eau guidelines on penile cancer: 2014 update. Eur Urol. (2015) 67:142–50. doi: 10.1016/j.eururo.2014.10.017
14. Srinivas V, Morse MJ, Herr HW, Sogani PC, Whitmore WJ. Penile cancer: relation of extent of nodal metastasis to survival. J Urol. (1987) 137:880–2. doi: 10.1016/s0022-5347(17)44281-9
15. Bandieramonte G, Colecchia M, Mariani L, Lo VS, Pizzocaro G, Piva L, et al. Peniscopically controlled co2 laser excision for conservative treatment of in situ and t1 penile carcinoma: report on 224 patients. Eur Urol. (2008) 54:875–82. doi: 10.1016/j.eururo.2008.01.019
16. Lopes A, Hidalgo GS, Kowalski LP, Torloni H, Rossi BM, Fonseca FP. Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol. (1996) 156:1637–42. doi: 10.1016/s0022-5347(01)65471-5
17. Slaton JW, Morgenstern N, Levy DA, Santos MJ, Tamboli P, Ro JY, et al. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol. (2001) 165:1138–42. doi: 10.1016/S0022-5347(05)66450-6
18. Ficarra V, Zattoni F, Cunico SC, Galetti TP, Luciani L, Fandella A, et al. Lymphatic and vascular embolizations are independent predictive variables of inguinal lymph node involvement in patients with squamous cell carcinoma of the penis: gruppo uro-oncologico del nord est (northeast uro-oncological group) penile cancer data base data. Cancer. (2005) 103:2507–16. doi: 10.1002/cncr.21076
19. Peak TC, Russell GB, Dutta R, Rothberg MB, Chapple AG, Hemal AK. A national cancer database-based nomogram to predict lymph node metastasis in penile cancer. Bju Int. (2019) 123:1005–10. doi: 10.1111/bju.14652
20. Shao Y, Tu X, Liu Y, Bao Y, Ren S, Yang Z, et al. Predict lymph node metastasis in penile cancer using clinicopathological factors and nomograms. Cancer Manag Res. (2021) 13:7429–37. doi: 10.2147/CMAR.S329925
21. Zhang W, Gao P, Gao J, Wu X, Liu G, Zhang X. A clinical nomogram for predicting lymph node metastasis in penile cancer: a seer-based study. Front Oncol. (2021) 11:640036. doi: 10.3389/fonc.2021.640036
22. Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. (2015) 13:8–17. doi: 10.1016/j.csbj.2014.11.005
23. Ngabo D, Dong W, Ibeke E, Iwendi C, Masabo E. Tackling pandemics in smart cities using machine learning architecture. Math Biosci Eng. (2021) 18:8444–61. doi: 10.3934/mbe.2021418
24. Iwendi C, Huescas CGY, Chakraborty C, Mohan S. COVID-19 health analysis and prediction using machine learning algorithms for Mexico and Brazil patients. J Exp Theor Artif Intell. (2022) 35(12):1226–37.e7. doi: 10.1080/0952813X.2022.2058097
25. Deo RC. Machine learning in medicine. Circulation. (2015) 132:1920–30. doi: 10.1161/CIRCULATIONAHA.115.001593
26. Vougas K, Sakellaropoulos T, Kotsinas A, Foukas GP, Ntargaras A, Koinis F, et al. Machine learning and data mining frameworks for predicting drug response in cancer: an overview and a novel in silico screening process based on association rule mining. Pharmacol Ther. (2019) 203:107395. doi: 10.1016/j.pharmthera.2019.107395
27. Wei L, Huang Y, Chen Z, Lei H, Qin X, Cui L, et al. Artificial intelligence combined with big data to predict lymph node involvement in prostate cancer: a population-based study. Front Oncol. (2021) 11:763381. doi: 10.3389/fonc.2021.763381
28. Li W, Zhou Q, Liu W, Xu C, Tang ZR, Dong S, et al. A machine learning-based predictive model for predicting lymph node metastasis in patients with ewing's sarcoma. Front Med (Lausanne). (2022) 9:832108. doi: 10.3389/fmed.2022.832108
29. Alkatout I, Naumann CM, Hedderich J, Hegele A, Bolenz C, Junemann KP, et al. Squamous cell carcinoma of the penis: predicting nodal metastases by histologic grade, pattern of invasion and clinical examination. Urol Oncol. (2011) 29:774–81. doi: 10.1016/j.urolonc.2009.10.014
30. Pettaway CA, Pagliaro L, Theodore C, Haas G. Treatment of visceral, unresectable, or bulky/unresectable regional metastases of penile cancer. Urology. (2010) 76:S58–65. doi: 10.1016/j.urology.2010.03.082
31. Lam W, Alnajjar HM, La-Touche S, Perry M, Sharma D, Corbishley C, et al. Dynamic sentinel lymph node biopsy in patients with invasive squamous cell carcinoma of the penis: a prospective study of the long-term outcome of 500 inguinal basins assessed at a single institution. Eur Urol. (2013) 63:657–63. doi: 10.1016/j.eururo.2012.10.035
32. Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. (2005) 173:816–9. doi: 10.1097/01.ju.0000154565.37397.4d
33. Hamy AS, Lam GT, Laas E, Darrigues L, Balezeau T, Guerin J, et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res Treat. (2018) 169:295–304. doi: 10.1007/s10549-017-4610-0
Keywords: machine learning algorithms, prediction model, penis cancer, squamous cell carcinoma, inguinal lymph node metastases, real-world research
Citation: Ding L, Zhang C, Wang K, Zhang Y, Wu C, Xia W, Li S, Li W and Wang J (2023) A machine learning-based model for predicting the risk of early-stage inguinal lymph node metastases in patients with squamous cell carcinoma of the penis. Front. Surg. 10:1095545. doi: 10.3389/fsurg.2023.1095545
Received: 11 November 2022; Accepted: 7 February 2023;
Published: 17 March 2023.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Andrey O Morozov, I.M. Sechenov First Moscow State Medical University, Russia© 2023 Ding, Zhang, Wang, Zhang, Wu, Xia, Li, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Li bGl6aGl4aW44OG1tQDE2My5jb20= Junqi Wang d2pxNjhAc2luYS5jbg==
†These authors share first authorship
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.