
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg., 16 February 2023
Sec. Surgical Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1092128
This article is part of the Research TopicArtificial Intelligence in Colorectal CancersView all 7 articles
 Ju Houqiong1,2,†
Ju Houqiong1,2,† Wan Ziwen2,3,†
Wan Ziwen2,3,† Zhong Chonghan1,2
Zhong Chonghan1,2 He Penghui1,2
He Penghui1,2 Yu Hongxin1,2
Yu Hongxin1,2 Lu Weijie1,2
Lu Weijie1,2 Liu Dongning1,2*
Liu Dongning1,2* Li Taiyuan1,2*
Li Taiyuan1,2*
Background: Natural orifice specimen extraction surgery (NOSES), as a new star of minimally invasive techniques, has been increasingly favored and promoted in the field of surgery around the world. Most previous studies were comparative studies of laparoscopic NOSES and conventional laparoscopic surgery. However, there is little research on comparing robotic colorectal cancer NOSES with conventional robotic-assisted colorectal cancer resection surgery.
Participant and methods: This study is a retrospective study of propensity score matching (PSM). This study included Ninety-one propensity score-matched pairs of the participant who had undergone robotic colorectal cancer resection surgery at our center between January 2017 and December 2020. The covariates used in the propensity score included gender, age, BMI, ASA score, maximum tumor diameter, the tumor's height from the anal verge, histological differentiation, AJCC stage, T stage, N stage, and history of previous abdominal surgery. The outcome measurement criteria included postoperative complications, inflammatory response, pelvic floor function, anal function, cosmetic outcome, quality of life, disease-free survival (DFS), and overall survival (OS).
Results: The robotic NOSES group had faster recovery time from gastrointestinal function (P = 0.014), shorter abdominal incision length (P < 0.001), less pain (P < 0.001), less additional analgesia required (P < 0.001), and lower postoperative indicators of white blood cell count (P < 0.001) and C-reactive protein content compared to the robotic-assisted resection surgery (RARS) group (P = 0.035). Additionally, the robotic NOSES group had significantly better body imagery (P < 0.001), cosmetic scores (P < 0.001), somatic function (P = 0.003), role function (P = 0.039), emotional function (P = 0.001), social function (P = 0.004), and overall function (P < 0.001) than the RARS group. The two groups demonstrated no significant difference between DFS and OS.
Conclusion: Robotic colorectal cancer NOSES is a safe and feasible minimally invasive procedure and offers shorter abdominal incisions, less pain, less surgical stress response, and better postoperative quality of life. Therefore, this technique can be further promoted for colorectal cancer patients eligible for NOSES.
Colorectal cancer (CRC) is the third-leading malignant tumor and the second-highest contributor to cancer deaths globally (1). The current treatment protocol for CRC is still a multidisciplinary and comprehensive diagnosis and treatment model based on radical surgery (2). Since 1991, the world's first laparoscopic rectal cancer surgery, which is representative of minimally invasive surgery because of its minor trauma, rapid recovery, and long-term treatment of tumors, is not different from open surgery, with rapid promotion and application (3–5).
The unique advantages of the surgical robot make colorectal surgery operations more precise and intelligent, providing more options for minimizing invasion of the colorectum. A multicenter, large-sample, randomized controlled study in China (6) and several meta-analyses (7–9) revealed that robotic surgery for colorectal cancer has better lumpectomy quality and demonstrates an oncology outcome similar to conventional laparoscopic surgery for the long term.
However, conventional robotic or laparoscopic surgery requires an additional incision to complete specimen removal. The abdominal wall incision is the most direct and compelling evidence of the minimally invasive outcome of the procedure. Additionally, adjuvant abdominal wall-assisted incisions are associated with an increased potential for postoperative incision-related complications, composed of wound infections, incisional hernias, and even incisional implant metastases (10, 11). The minimally invasive natural orifice specimen extraction surgery (NOSES) has tackled this complication. Moreover, NOSES can provide good near-term outcomes while satisfying the need for radical tumor treatment (12–14). The combination of surgical robots and NOSES can bring incredible benefits to colorectal cancer patients.
The proximity between the sigmoid column and rectum to the anal location provides a favorable predisposition for transanal specimen retrieval without significantly increasing the difficulty of the surgical operation. Moreover, few studies compared robotic colorectal cancer NOSES with conventional robotic-assisted colorectal cancer resection surgery (RARS). Hence, participants who underwent robotic sigmoid or upper segment of rectum cancer surgery at our center were selected for this study. The safety and feasibility of robotic colorectal cancer NOSES surgery were further confirmed by comparing and analyzing the short- and long-term outcomes of robotic colorectal cancer NOSES with those of conventional robotic colorectal cancer surgery. This study aimed to provide more reliable and accurate proof of clinical evidence for robotic colorectal cancer NOSES.
The study was a retrospective single-center team-based study conducted at First Affiliated Hospital of Nanchang University. All procedures were per the moral criteria of the Center and the Declaration of Helsinki. The study included all recipients of respectable sigmoid and upper segment rectal cancers without distant metastases diagnosed by imaging and histology in the Department of Gastrointestinal Surgery at our center and undergoing radical robotic resection in the period beginning January 2017 through December 2020.
Inclusion criteria: (1) postoperative pathologically confirmed colorectal adenocarcinoma; (2) tumor located in the sigmoid colon, recto-b junction, and upper rectum (lower edge of tumor located above the peritoneal reflex) according to imaging, colonoscopy, intraoperative findings, and postoperative pathology; (3) patient's body mass index (BMI) <35 kg/m2; (4) no distant metastasis according to preoperative examination and intraoperative findings.
Exclusion criteria: (1) concurrent combination of other malignancies; (2) emergency surgery for bleeding, obstruction, or perforation; (3) patients receiving preoperative radiotherapy; (4) a lack of follow-up data or incomplete data; (5) stoma prophylaxis patients or patients with stoma caused by other reasons.
The inclusion and exclusion criteria for both groups were referred to in the criterion mentioned above. Ultimately, 282 patients were included. Among them, 138 patients undergoing robotic natural orifice specimen extraction surgery were in the NOSES group, and 144 who underwent conventional robotic-assisted resection surgery were in the RARS group.
In the NOSES and RARS groups, successful anesthesia was followed by intraoperative confirmation of no distant metastases. Colon cancer surgery and rectal cancer surgery were performed according to complete mesocolic excision (CME) and total mesorectal excision (TME), respectively. Surgery was performed by the same surgeon who had received standardized training in robotic surgery and completed over 200 colorectal cancer procedures.
In the RARS group, the inferior mesenteric vessels were carefully dissected, the lymphatic adipose tissue was cleared, and the rectum or colonic mesentery was freed, with at least 2 cm from the below edge of the tumor, the mesentery naked, and the closed rectum cut. The adjacent organs and pelvic autonomic nerves must be protected during the operation. A minor incision was taken to remove the specimen from the lower abdomen, and a reconstruction of the digestive tract was excised and completed. Both sigmoid and rectal cancer resections were performed with a circular stapler for end-to-end anastomosis.
In the NOSES group, there was no difference from the conventional robot in freeing the intra-abdominal tract, lymph node dissection, and tumor resection. However, differences were observed in the route of specimen removal and gastrointestinal tract reconstruction. Transrectal specimen extraction for sigmoid and upper rectal cancer was NOSES IV. The procedure consisted of sufficient mild dilation and transanal flushing of the rectum cavity by iodine saline. The rectum was dissected by a linear stapler at 4 cm away from the lower margin of the tumor, while a sterile protected package was placed through the assistant hole and dragged out of the anus through the rectum. The staple holder was held with a toothed clamp and delivered to the abdominal cavity through the sterile-protected package. At 3 cm below the sigmoid nakedness, the intestinal canal was incised with an ultrasonic knife. The staple holder was disposed into the intestinal cavity of the sigmoid colon through the incision and pushed into the proximal colon until the connecting rod crossed the sigmoid nakedness. After gently straightening the wire, the sigmoid colon was disconnected with a linear stapler at the sigmoid colon tube nudity. The specimen was dragged out of the body via the anus with toothed forceps placed in the protective sleeve. The rectal stump was closed with a linear stapler. Dissection was performed at the sigmoid colon to find the broken end of the silk thread. Then, the staple holder was dragged out distally by tugging on the silk thread, and the outline of the staple holder rod was seen. Afterward, the staple holder rod was pulled out through the silk thread, and the pelvic cavity was rinsed with iodine saline. Active bleeding was checked. Subsequently, gastrointestinal reconstruction was performed to end-to-end anastomose the descending colon and rectum with a circular stapler. The end-to-end anastomosis of the descending colon and rectum was completed by placing a loop anastomosis clutch through the anus after the absence of sigmoid mesenteric torsion was determined. The gas injection test checks whether there is leakage in the anastomosis, and the dangerous triangle of the anastomosis is stitched with the assistance of the robot. The critical operation for transrectal specimen retrieval is illuminated in Figure 1.
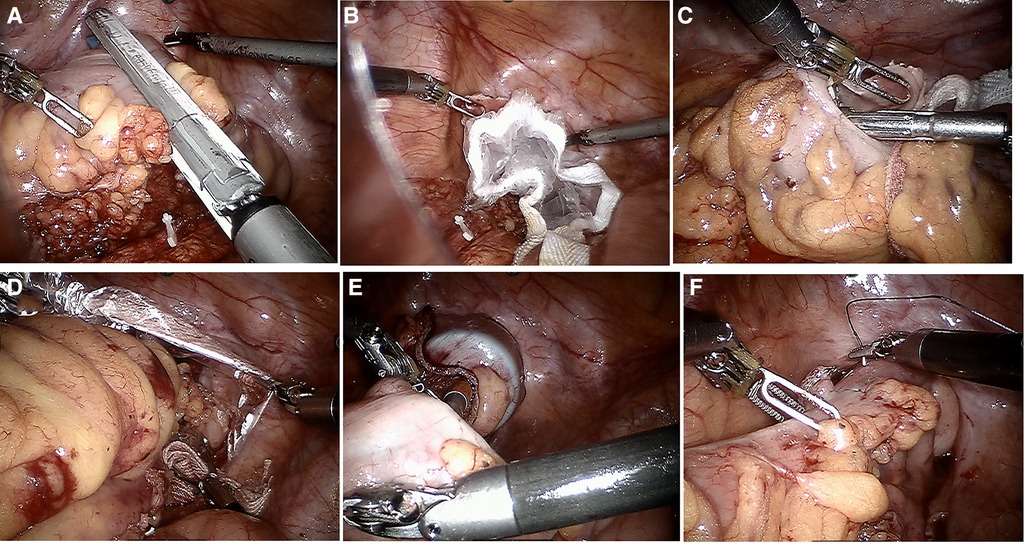
Figure 1. Key surgical steps of natural orifice specimen extraction surgery (A–F). (A) Excisional occluder to dissociate tumor specimens; (B) sterile protective sleeve is placed into the rectum to establish sterile access; (C) staple holder placed into the proximal rectum; (D) transanal removal of the specimen; (E) end-to-end sigmoid-rectal anastomosis; (F) intraluminal reinforced anastomosis.
Data collection. Each case was requested to report demographic information, preoperative diagnosis, operational state, postoperative pathology, postoperative complications, recovery information, and long-term follow-up information. Overall survival (OS) was defined as the time from surgery to death from any cause. Disease-free survival (DFS) was defined as the time from surgery to disease recurrence or death from any cause. (1) Patients were asked about their attitudes toward their physical appearance and how satisfied they were with the scar's appearance 1 month after surgery. The Body Imagery Questionnaire (BIQ) was used in cholecystectomy (15) and nephrectomy patients (16). (2) A PFDI-20 score was employed to assess patients’ symptoms 6 months after surgery, including urinary tract symptoms, gastrointestinal symptoms, and pelvic organ prolapse (17). (3) Patient Scar Assessment Questionnaire and Scoring System (PSAQ) was adopted to assess the cosmetic outcome of patients at 3 months postoperatively, as well as the outcome of any linear scar surgical treatment (18). (4) EORTC QLQ—C30 scale was utilized to assesses patients’ quality of life at 3 months postoperatively (19). (5) After 6 months after surgery, patients were assessed for incontinence using the Wexner incontinence score (20).
Follow-up. Routine follow-up was scheduled 1 month after surgery by National Comprehensive Cancer Network guidelines, every 3 months for 2 years, and every 6 months for 5 years until patient death or study termination. The information was available by e-mail or telephone if the participant did not return for observation. All patients were followed until death or September 31, 2022.
Since the propensity score matching method (PSM) can minimize selectivity bias, it was used to counterbalance the baseline information among the two groups. PSM was matched at a ratio of 1:1 for propensity scores against the above baseline information. A logistic regression model was applied to assign these variables to the baseline information among 282 patients with a caliper value of 0.02. The measures conformed to a normal distribution and were presented as mean ± SD. The measures that were not normally distributed were performed using independent samples t-test or Mann-Whitney U test, indicated as median and quartiles, respectively. Count data were presented as frequencies and percentages using the χ2 test or Fisher's exact probability method. DFS and OS were calculated using the Kaplan–Meier method and compared by the Log-rank test. P < 0.05 indicated statistical significance. IBM SPSS Statistics (version 26.0, SPSS Inc, Chicago, IL) was used for all statistical analyses.
All procedures were performed successfully. In the present study, PSM was performed for gender, age, BMI, ASA score, maximum tumor diameter, the height of tumor from the anal verge, histological differentiation, AJCC stage, T stage, N stage, and previous abdominal surgery, and 91 pairs of patients were successfully matched. Afterward, confounding bias was eliminated, and all 91 pairs of participants had comparable baseline information (P > 0.05) (Table 1).
As suggested by comparing the perioperative figures, the operative time was essentially the same between both groups (147.1 ± 25.7 min in the RARS group vs. 149.8 ± 31.7 min in the NOSES group, P = 0.535). The intraoperative blood loss was similar (54.3 ± 28.0 ml in the RARS group vs. 54.0 ± 30.3 ml in the NOSES group, P = 0.949). Gastrointestinal recovery function was better in the NOSES group than that in the RARS group (60.8 ± 16.6 h in the RARS group vs. 54.4 ± 18.1 h in the NOSES group, P = 0.014). The abdominal incision length was significantly shorter than that in the RARS group (11.1 ± 0.6 cm in the RARS group vs. 4.8 ± 0.3 cm in the NOSES group, P < 0.001). Regarding postoperative recovery indicators, the number of postoperative hospital days was the same in the RARS and NOSES groups (P = 0.934) (Table 2). However, compared to the RARS group, the NOSES group reported better pain score results (P < 0.001) and had significantly fewer patients requiring additional analgesics (P < 0.001). Concerning surgical stress, the white blood cell counts and C-reactive protein levels were compared between the two groups at 1, 3, and 5 days postoperatively. Our results demonstrated that compared to the RARS group, the NOSES group had lower indicators of inflammation (P < 0.001, P = 0.035) (Figure 2, Table 3). With respect to postoperative complications, nine were observed in the NOSES group, while 13 were in the RARS group. Notably, the NOSES group had fewer wound complications.
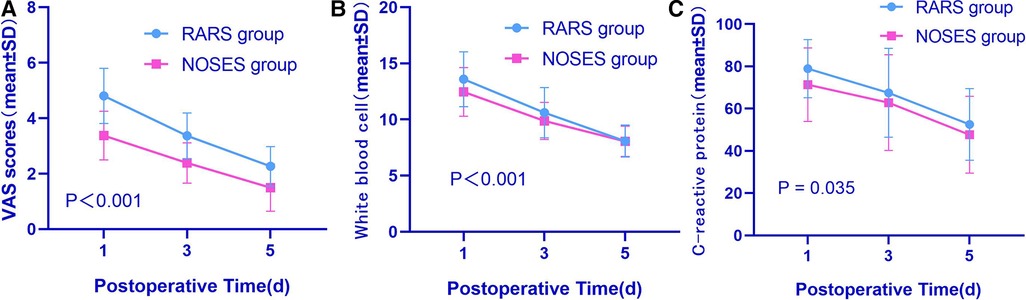
Figure 2. Comparative perioperative indexes in the two groups of patients after PSM (A–C). (A) VAS scores between two groups; (B) white blood cell scores between two groups; (C) C-reactive protein scores between two groups. The P-value was calculated by repeated measures statistical analysis. VAS, visual analogue scale; RARS, robotic-assisted colorectal cancer resection surgery; NOSES, natural orifice specimen extraction surgery.
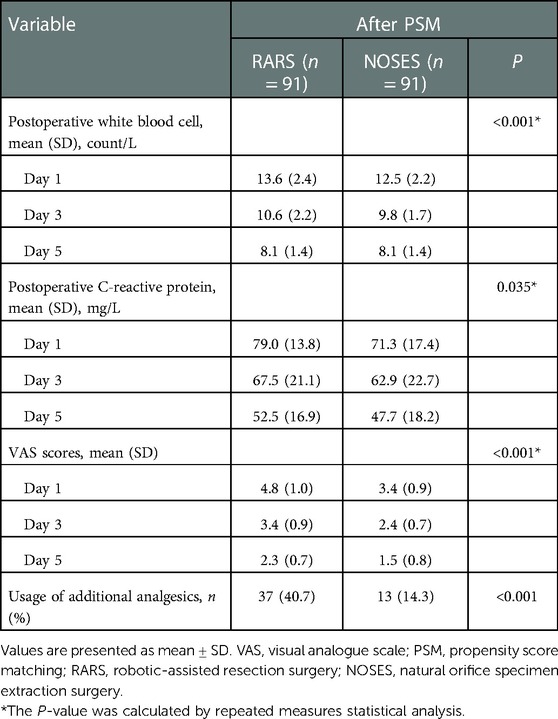
Table 3. Comparison of postoperative stress response and pain condition of patients between the NOSES group and RARS group.
At 1 month postoperatively, the NOSES group had significantly better body imagery scores (P < 0.001) and cosmetic outcomes (P < 0.001) than the RARS group (Figure 3). The PFDI-20 scores of the NOSES and RARS groups were not significantly different 3 months after surgery. Besides, somatic function (P = 0.003), role function (P = 0.039), emotional function (P = 0.001), social function (P = 0.004), and overall function (P < 0.001) in the NOSES group were significantly better than those in the RARS group (Figure 4). The NOSES group had less fatigue (P = 0.024), pain (P = 0.004), and diarrhea (P = 0.044) at 3 months postoperatively. Additionally, the PASQ Total Subscale Score and Global Subscale Score at 3 months postoperatively in the NOSES group were significantly lower than those in The RARS group (Table 4). Nonetheless, the RARS and NOSES groups maintained an identical standard of anal function at the 6-month postoperative evaluation of anal functional capacity (Table 5).
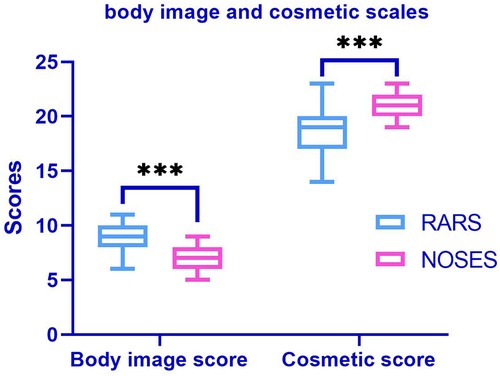
Figure 3. Comparative body imagery questionnaire (BIQ) in the two groups of patients after PSM. The body image score (among 5 and 20, a lower score means better body image). Cosmetic score (among 3 and 24, a higher score means better cosmetic results). ***P < 0.001. RARS, robotic-assisted colorectal cancer resection surgery; NOSES, natural orifice specimen extraction surgery.
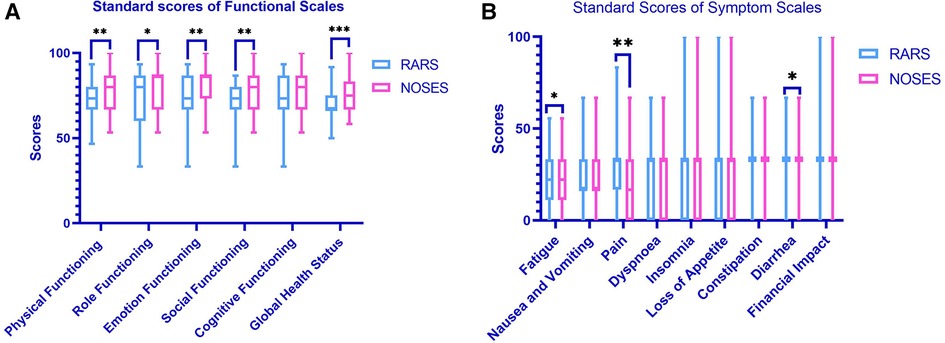
Figure 4. Comparative EORCT quality of life questionnaire-core 30 results in the two groups of patients after PSM (A,B). (A) Functional scales between two groups (a higher score means better functional results); (B) symptom scales between two groups (a lower score means better symptom results). *P < 0.05, **P < 0.01, ***P < 0.001. RARS, robotic-assisted colorectal cancer resection surgery; NOSES, natural orifice specimen extraction surgery.
At the last follow-up as of September 31, 2022, the median follow-up was 51 months (17–67) and 38 months (16–68) for the RARS group and the NOSES group, respectively. The 2-year overall survival rate was 94.5% and 96.7% in the RARS group and the NOSES group, respectively. The Log-rank test revealed no statistically insignificant difference in overall survival between the two groups (P = 0.234). Furthermore, both groups were not significantly different in 2-year disease-free survival (P = 0.757) (Figure 5).
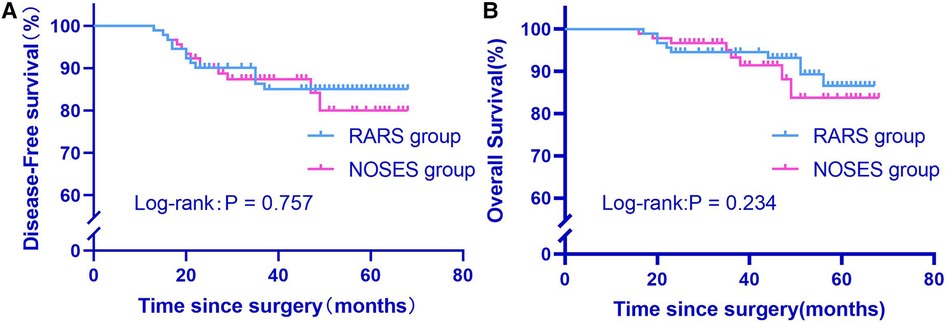
Figure 5. Comparison of overall survival and disease-free survival between two groups after propensity score matching (A,B). RARS, robotic-assisted colorectal cancer resection surgery; NOSES, natural orifice specimen extraction surgery.
Given the continuous advancement of minimally invasive technology, the surgery for radical colorectal cancer has changed from traditional open-to-laparoscopic surgery to present-day robotic surgery with the coexistence of all three (21). In 2000, the FDA approved the use of robotic surgery systems in clinical practice, leading to yearly increasing reports of robotic NOSES for colorectal cancer (22–24). Currently, most of the studies are retrospective, and there are confounding factors influencing the authenticity of some of the findings (25). This study applied propensity score matching to effectively weaken the group's confounding effect and balance the differences. The results were more objective, realistic, and comparable.
Surgical safety is the primary prerequisite for performing robotic colorectal cancer NOSES. Efetov et al.'s (26) findings concluded that NOSES has the benefit of reduced intra-abdominal infection potential risk. Our study strictly adhered to the principles of aseptic surgery during the operation, removed the specimen using a sterile-protected package, disinfected it promptly, and flushed the abdominal cavity with plenty of salines. Regarding postoperative complications, the intra-abdominal infections in the NOSES and the RARS groups did not differ significantly. Meanwhile, the mean operative time and bleeding volume were similar in the two groups, consistent with Liu et al. (27). Concerning the tumor-free principle, no difference between groups was observed in the number of lymph nodes cleared and the percentage of positive tumor margins. This was attributed to the broader field of view and flexible robotic arm of the robotic surgical system. These findings further confirmed that robotic NOSES for colorectal cancer has surgical efficacy that is not inferior to robotic-assisted colorectal cancer resection.
The short-term outcome of surgery is a crucial indicator to evaluate the quality of robotic colorectal cancer NOSES. Since the length of the abdominal wall incision is noticeably shorter with robotic NOSES, patients also experience considerably less postoperative pain. They require less additional analgesia, and patients can get up and move around early. Therefore, the restoration of gastrointestinal function was earlier for the NOSES group than for the RARS group. Similarly, the leukocyte indicators and C-reactive protein levels of the two groups were compared at 1, 3, and 5 days postoperatively. Particularly, surgical stress may promote the growth of preexisting micrometastases or trigger tumor growth (28, 29). Inflammatory indexes in the NOSES group were lower than those in the RASR group, suggesting that the NOSES group had less organismal disturbance and a more pronounced minimally invasive advantage for the patients.
Most current retrospective studies often overlooked the postoperative quality of life assessment. The cosmetic results of NOSES are also one of the reasons for patients choosing this procedure, especially young female patients. When surgical incision complications or scarring were expected to heal fully, the patients’ body imagery and cosmetic outcomes were explored, starting with their feelings. It was demonstrated that body imagery and cosmetic outcomes in the NOSES group were significantly better than those in the RARS group. The findings were consistent with the PSAQ scale. The EORTC QLQ—C30 scale, divided into a functional scale and a symptom scale, has been used in breast cancer (30) and prostate cancer (31). The EORTC QLQ—C30 scale was divided into functional and symptom scales, validated in breast and prostate cancer. The results implied that the NOSES group had better results than the RARS group. This could be explained that the more prominent the postoperative surgical scar, the more negative the patient's psychological cues about cancer treatment, and the greater the irritability and panic, the worse the results provided by the EORTC QLQ—C30 scale. Some researchers believe that injury to anal function may occur during transanal removal of the specimen and anastomosis of the intestinal canal (32, 33). In the indications for NOSES (34), the patient's BMI and tumor size were strictly controlled. The anal sphincter function was not damaged during the procedure. Thus, the anal function was kept at the same level in both groups regarding the Wexner score. Moreover, no significantly different OS and DFS for the two groups were observed concerning long-term oncologic outcomes.
This study also has some limitations. First, there will be a certain amount of selection bias as this is a retrospectively studying. Hence, propensity score matching was selected to reduce variance. Secondly, the sample size was insufficient due to the slight size limitation of a single-center study. It is ensured in this study that the same surgeon-led specialty team performs all procedures, so as to curtail variability from background or surgical skills among surgeons. For this reason, our center is conducting a multicenter, prospective randomized controlled study of robotic NOSES (35) to lay a solid foundation of robotic NOSES for colorectal cancer to guide the surgical treatment of colorectal cancer better.
Robotic colorectal cancer NOSES is a safe and feasible minimally invasive technique and offers shorter abdominal incisions, less pain, less surgical stress response, and better postoperative quality of life. Therefore, this technique can be further promoted for colorectal cancer patients eligible for NOSES.
JH and WZ wrote the main manuscript text. ZC, HP, YH and LW prepared the tables and figures. LD and LT revised and approved the manuscript text. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Jiangxi Key Research and Development Plan (20202BBG73032).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
3. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MHGM, de Lange-de Klerk ESM, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. (2015) 372:1324–32. doi: 10.1056/NEJMoa1414882
4. Jiang W-Z, Xu J-M, Xing J-D, Qiu H-Z, Wang Z-Q, Kang L, et al. Short-term outcomes of laparoscopy-assisted vs open surgery for patients with low rectal cancer: the LASRE randomized clinical trial. JAMA Oncol. (2022) 8(11):1607–15. doi: 10.1001/jamaoncol.2022.4079
5. Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WCJ, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. (2009) 10:44–52. doi: 10.1016/S1470-2045(08)70310-3
6. Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. (2022) 7(11):991–1004. doi: 10.1016/S2468-1253(22)00248-5
7. Fleming CA, Cullinane C, Lynch N, Killeen S, Coffey JC, Peirce CB. Urogenital function following robotic and laparoscopic rectal cancer surgery: meta-analysis. Br J Surg. (2021) 108:128–37. doi: 10.1093/bjs/znaa067
8. Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, et al. Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. (2018) 267:1034–46. doi: 10.1097/SLA.0000000000002523
9. Safiejko K, Tarkowski R, Koselak M, Juchimiuk M, Tarasik A, Pruc M, et al. Robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection: a systematic review and meta-analysis of 19,731 patients. Cancers. (2021) 14:180. doi: 10.3390/cancers14010180
10. Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparoscopic vs open colectomy. Surg Endosc. (2002) 16:1420–5. doi: 10.1007/s00464-002-8837-3
11. Skipworth JRA, Khan Y, Motson RW, Arulampalam TH, Engledow AH. Incisional hernia rates following laparoscopic colorectal resection. Int J Surg. (2010) 8:470–3. doi: 10.1016/j.ijsu.2010.06.008
12. Guan X. Short-term and oncological outcomes of natural orifice specimen extraction surgery (NOSES) for colorectal cancer in China: a national database study of 5055 patients. Science Bulletin. (2022) 67(13):1331–4. doi: 10.1016/j.scib.2022.05.014
13. Thakkar S, Pancholi A, Carleton N. Natural orifice specimen extraction for colorectal cancer removal: the best of both worlds. Gastrointest Endosc. (2021) 94:651–2. doi: 10.1016/j.gie.2021.05.028
14. Wolthuis AM, Meuleman C, Tomassetti C, D’Hooghe T, Fieuws S, de Buck van Overstraeten A, et al. How do patients score cosmesis after laparoscopic natural orifice specimen extraction colectomy? Colorectal Dis. (2015) 17:536–41. doi: 10.1111/codi.12885
15. Lurje G, Amygdalos I, Kambakamba P, Petrowsky H, Lesurtel M, Zehnder A, et al. Cosmesis and body image in patients undergoing single-port versus conventional laparoscopic cholecystectomy: a multicenter double-blinded randomized controlled trial (SPOCC-trial). Ann Surg. (2015) 262:8. doi: 10.1097/SLA.0000000000001474
16. Park SK, Olweny EO, Best SL, Tracy CR, Mir SA, Cadeddu JA. Patient-reported body image and cosmesis outcomes following kidney surgery: comparison of laparoendoscopic single-site, laparoscopic, and open surgery. Eur Urol. (2011) 60:1097–104. doi: 10.1016/j.eururo.2011.08.007
17. Barber MD, Walters MD, Cundiff GW, PESSRI Trial Group. Responsiveness of the pelvic floor distress inventory (PFDI) and pelvic floor impact questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. Am J Obstet Gynecol. (2006) 194:1492–8. doi: 10.1016/j.ajog.2006.01.076
18. Mundy LR, Miller HC, Klassen AF, Cano SJ, Pusic AL. Patient-reported outcome instruments for surgical and traumatic scars: a systematic review of their development, content, and psychometric validation. Aesthetic Plast Surg. (2016) 40:792–800. doi: 10.1007/s00266-016-0642-9
19. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
20. Brown HW, Dyer KY, Rogers RG. Management of fecal incontinence. Obstet Gynecol. (2020) 136:811–22. doi: 10.1097/AOG.0000000000004054
21. Cheng CL, Rezac C. The role of robotics in colorectal surgery. Br Med J. (2018) 360(j5304). doi: 10.1136/bmj.j5304
22. Yao H, Li T, Chen W, Lei S, Liu K, Jin X, et al. Safety and feasibility of robotic natural orifice specimen extraction surgery in colorectal neoplasms during the initial learning curve. Front Oncol. (2020) 10:1355. doi: 10.3389/fonc.2020.01355
23. Zhou JJ, Li TG, Lei SL, Chen WD, Liu KJ, Liu B, et al. Analysis of robotic natural orifice specimen extraction surgery on 162 cases with rectal neoplasms. Zhonghua Wei Chang Wai Ke Za Zhi. (2020) 23:384–9. doi: 10.3760/cma.j.cn.441530-20191017-00453
24. Yao H, Li T, Chen W, Lei S, Liu K, Liu B, et al. Role of robotic natural orifice specimen extraction surgery in colorectal neoplasms. Sci Rep. (2021) 11:9818. doi: 10.1038/s41598-021-89323-z
25. Aslaner A, Çakır T, Eyvaz K, Kazım Kazan M, Can Çakır R, Doğan U, et al. Comparison of robotic-assisted resection alone and with natural orifice specimen extraction for rectal cancer by using Da vinci Xi. Eur Rev Med Pharmacol Sci. (2022) 26:6665–70. doi: 10.26355/eurrev_202209_29767
26. Efetov SK, Tulina IA, Kim VD, Kitsenko Y, Picciariello A, Tsarkov PV. Natural orifice specimen extraction (NOSE) surgery with rectal eversion and total extra-abdominal resection. Tech Coloproctol. (2019) 23(9):899–902. doi: 10.1007/s10151-019-02058-y
27. Liu D, Luo R, Wan Z, Zhu W, He P, Ye S, et al. Clinical outcomes and prognostic factors of robotic assisted rectal cancer resection alone versus robotic rectal cancer resection with natural orifice extraction: a matched analysis. Sci Rep. (2020) 10:12848. doi: 10.1038/s41598-020-69830-1
28. Behrenbruch C, Shembrey C, Paquet-Fifield S, Mølck C, Cho H-J, Michael M, et al. Surgical stress response and promotion of metastasis in colorectal cancer: a complex and heterogeneous process. Clin Exp Metastasis. (2018) 35:333–45. doi: 10.1007/s10585-018-9873-2
29. Tai L-H, de Souza CT, Bélanger S, Ly L, Alkayyal AA, Zhang J, et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. (2013) 73:97–107. doi: 10.1158/0008-5472.CAN-12-1993
30. Mierzynska J, Taye M, Pe M, Coens C, Martinelli F, Pogoda K, et al. Reference values for the EORTC QLQ-C30 in early and metastatic breast cancer. Eur J Cancer. (2020) 125:69–82. doi: 10.1016/j.ejca.2019.10.031
31. Gamper EM, Musoro JZ, Coens C, Stelmes J-J, Falato C, Groenvold M, et al. Minimally important differences for the EORTC QLQ-C30 in prostate cancer clinical trials. BMC Cancer. (2021) 21:1083. doi: 10.1186/s12885-021-08609-7
32. Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. Laparoscopic natural orifice specimen extraction-colectomy: a systematic review. World J Gastroenterol. (2014) 20:12981–92. doi: 10.3748/wjg.v20.i36.12981
33. Costantino FA, Diana M, Wall J, Leroy J, Mutter D, Marescaux J. Prospective evaluation of peritoneal fluid contamination following transabdominal vs. transanal specimen extraction in laparoscopic left-sided colorectal resections. Surg Endosc. (2012) 26:1495–500. doi: 10.1007/s00464-011-2066-6
34. Guan X, Liu Z, Longo A, Cai J-C, Chen WT-L, Chen L-C, et al. International consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep. (2019) 7(1):24–31. doi: 10.1093/gastro/goy055
Keywords: robotic surgery, natural orifice specimen extraction, quality of life, colorectal cancer, survival
Citation: Houqiong J, Ziwen W, Chonghan Z, Penghui H, Hongxin Y, Weijie L, Dongning L and Taiyuan L (2023) Comparison of transabdominal wall specimen retrieval and natural orifice specimen extraction robotic surgery in the outcome of colorectal cancer treatment. Front. Surg. 10:1092128. doi: 10.3389/fsurg.2023.1092128
Received: 7 November 2022; Accepted: 16 January 2023;
Published: 16 February 2023.
Edited by:
Nicola Tartaglia, University of Foggia, ItalyReviewed by:
Simone Guadagni, University of Pisa, Italy© 2023 Houqiong, Ziwen, Chonghan, Penghui, Hongxin, Weijie, Dongning and Taiyuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Taiyuan anlsaXRhaXl1YW5Ac2luYS5jb20= Liu Dongning bGl1ZG9uZ25pbmcxOTgyQHNpbmEuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.