- 1Department of Hepatopancreatobiliary Surgery, Affiliated Hospital of Qinghai University, Qinghai, China
- 2Qinghai Province Research Key Laboratory for Echinococcosis, Qinghai, China
- 3Department of Gastroenterology, Xining Second People's Hospital, Xining, Qinghai, China
Objective: To summarize the single-centre experience of Ex vivo Liver Resection and Autotransplantation (ELRA) to treat end-stage hepatic alveolar echinococcosis (HAE).
Methods: Retrospective analysis of clinical data and follow-up data of 13 patients admitted to the Affiliated Hospital of Qinghai University from January 2015 to December 1, 2020, with the Ex vivo Liver Resection and Autotransplantation for hepatic alveolar echinococcosis.
Result: 13 patients underwent successful total/ semi-ex-vivo liver resection combined with Ex vivo Liver Resection and Autotransplantation with no intra-operative deaths. the median standard liver volume was 1,118 ml (1,085–1,206.5 ml); the median residual liver volume was 634 ml (526.5–1,338 ml); The median weight of the autograft was 845.8 g (619.5–1,020.5 g), the median operation time was 14.5 h (11.5–16.15 h); the median anhepatic period time was 290 min (257–312.5 min). The median intraoperative blood loss was 1,900 ml (1,300–3,500 ml); the median number of erythrocyte suspensions entered was 7.5 u (6–9u). The median length of hospital stay was 32 days (24–40 days). Postoperative complications occurred in 9 patients during hospitalization,with 7 patients graded at grade III or higher by Clavien-Dindo; 4 patients died postoperatively. 1 patient had recurrent abdominal distension with massive thoracoabdominal fluid and coagulation dysfunction 8 months after surgery and was considered to have small liver syndrome. 1 patient developed HAE recurrence during the follow-up, which was considered intraoperative incisional implantation.
Conclusion: ELRA is one of the most valuable therapeutic measures for the treatment of end-stage complicated hepatic alveolar echinococcosis. Precise preoperative assessment of liver function, individualized intraoperative duct reconstruction, and precise management of the postoperative disease can achieve better treatment results.
Introduction
Hepatic Alveolar echinococcosis (HAE) is a zoonotic disease caused by echinococcosis infection. Since the disease has no specific symptoms in the early stages, most of them are already in the middle and late stages when they go to the hospital, and the lesions usually involve the first and second hepatic hilum and even erode the posterior inferior vena cava of the liver (1, 2); The multiple intrahepatic lesions with more severe destruction of intrahepatic structures have led to a relatively small number of patients, only 35%, undergoing radical hepatectomy (3, 4), Liver transplantation was recognized as the best treatment for patients with advanced hepatic alveolar echinococcosis because of the destruction of the liver and surrounding organs and the reduced prognosis for patients with such advanced disease (5). However, allogeneic liver transplantation can be associated with a shortage of donor's livers during treatment, and lifelong immunosuppressive medication, which leads to a significantly higher risk of recurrence of encapsulated worms and affects the use of liver transplantation in HAE (5, 6). Pichlmayr's team (7) first completed the resection of hepatic malignancies in 1988 using ELRA as a radical approach for tumours that conventional surgery could not remove. To overcome the dilemma faced by liver transplantation in HAE, in 2011, Professor Wen Hao's team (8) first reported the application of the isolated liver resection technique combined with ELRA to the treatment of HAE patients, which several centres subsequently adopted in China for the treatment of HAE with good results. The clinical data and follow-up data of 13 ELRA patients admitted to the Affiliated Hospital of Qinghai University from January 2015 to December 1, 2020, are retrospectively analyzed and reported.
Patients and methods
Data of patients undergoing surgery: The data of 13 patients admitted to the Affiliated Hospital of Qinghai University from January 2015 to January 2020 who underwent ELRA for advanced hepatic vesicular encrustation disease were retrospectively analyzed, and their main characteristics were as follows: (1) the target lesion was challenging to resect in vivo, the reserved hepatic involved vasculature was difficult to resect and reconstruct, and bleeding from the liver and blood vessels close to the liver was difficult to control; (2) the prepared left hepatic vein-inferior vena cava venous confluence site invasion, pre-existing hepatic portal vein tertiary and higher branch invasion; (3) patient in the good physical condition and normal liver and renal function before surgery. There were 3 cases (23.1%) of males and 10 (76.9%) of females, aged 18–59 years, with a mean age of 38.3 years. Patients presented with symptoms of jaundice before surgery in 8 cases; recurrent upper abdominal discomfort in 5 cases; Indocyanine green retention rate at 15 min <10% in 6 cases and >10% in 7 cases; Child-Pugh A in 8 cases and grade B in 5 cases; all patients underwent preoperative PHI staging (HX-PHI Staging system) (9), The severity of vascular erosion was classified into 3 grades and different types according to the preoperative imaging assessment of the operated patients; the specific typing and grading levels are shown in Table 1. this study complied with the Declaration of Helsinki. All patients had postoperative pathology confirmed as hepatic echinococcosis; the patients and their families signed an informed consent form. The patient's preoperative clinical data and preoperative assessment results are shown in Table 2.
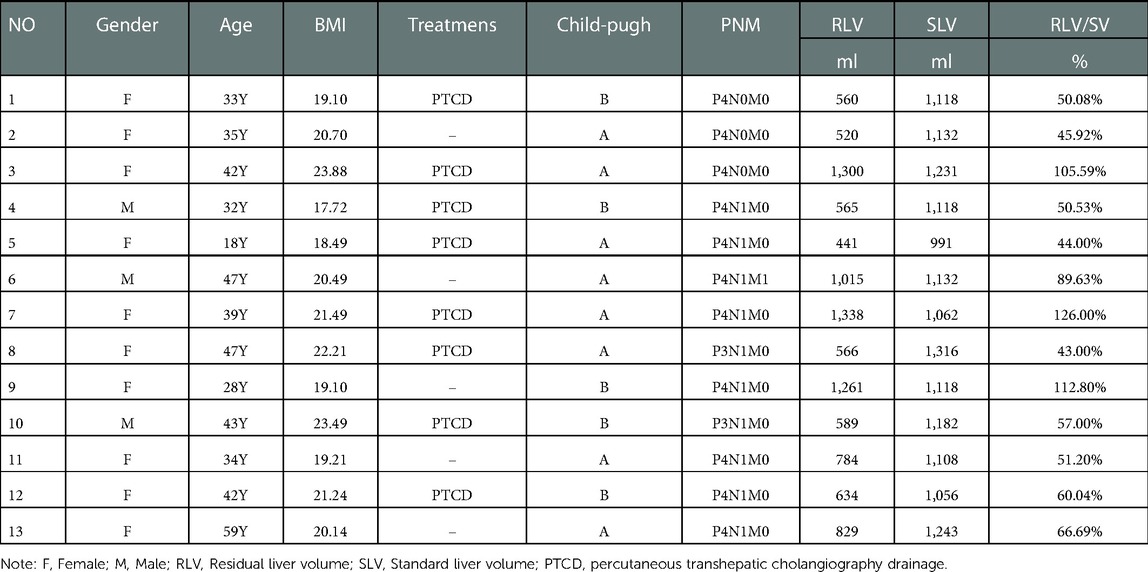
Table 2. Clinical data and preoperative evaluation of 13 patients with advanced hepatic alveolar echinococcosis.
Preoperative surgical feasibility assessment
Imaging was performed to understand the size, extent of infiltration,vascular and biliary involvement of the liver lesion (10, 11) (Figure 1A); for patients with extensive invasion and/or compression of the posterior hepatic inferior vena cava, an inferior vena cava angiogram was required; the degree of inferior vena cava stenosis was assessed and the presence or absence of collateral circulation established (Figure 1B); all patients were treated with three-dimensional reconstruction of the liver and a liver model was created (Figure 1C) to visualize the size of the lesion, calculate the actual liver volume, liver lesion volume, remaining liver volume, and standard liver volume to assess the surgical outcome to ensure the safety of the procedure (12, 13); the ICGR 15-minute retention rate was performed 1 week before surgery in all patients to assess the reserve function of the patient's liver to predict the occurrence of liver failure after surgery; all patients were treated preoperatively according to semi-ex Vivo All patients were prepared preoperatively for hepatectomy or hepatectomy combined with autologous transplantation.
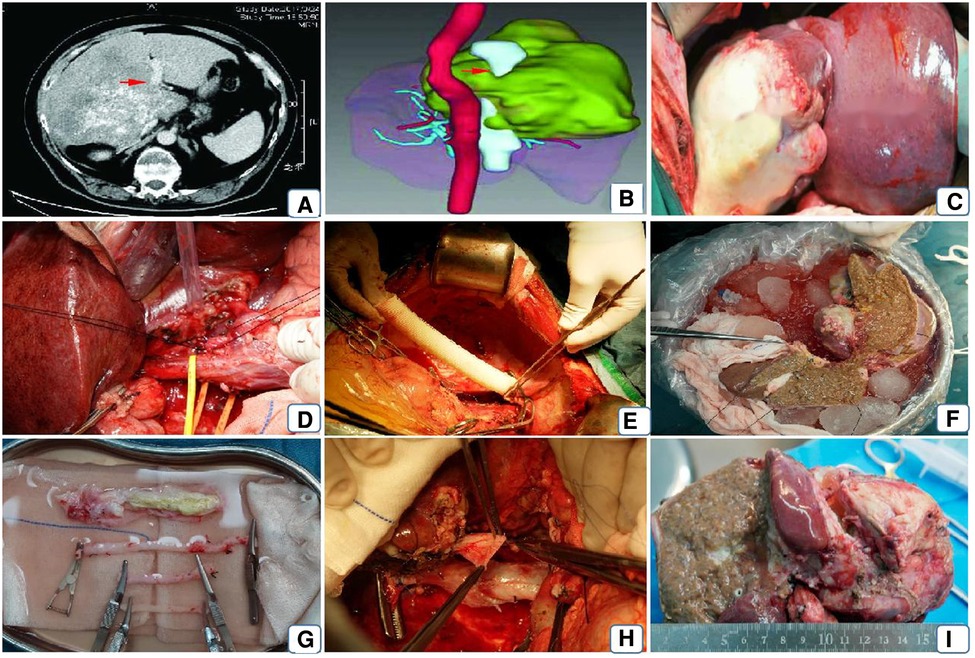
Figure 1. Patient's preoperative imaging assessment and surgical procedure. Note: (A) preoperative vesicular encapsulated lesion approximately 17*17 cm in diameter, invading the right branch of the portal vein (red arrow); (B) preoperative 3D reconstruction showing the lesion invading the encapsulated inferior vena cava and the left and middle hepatic veins; (C) opening the abdominal cavity to expose the normal liver and the lesion tissue; (D) dissecting the first hepatic portal, blocking the portal vein, the innominate hepatic artery and the common hepatic duct respectively and sharply dissecting them; (E) using artificial vessels connect the suprahepatic and intrahepatic inferior vena cava and end-lateral anastomosis of the portal vein to the artificial vessel; (F) ex vivo resection of the lesion and repair of the plastic inflow and outflow tract; (G) reconstruction of the inflow, outflow tract or inferior vena cava of the liver using autologous or allogeneic vessels; (H) replacement of the artificial inferior vena cava by the isolated free and repaired autologous inferior vena cava; (I) resected lesion.
Surgical procedures
After general anaesthesia, an incision is made through a herringbone incision, layer by layer, until the abdominal cavity is entered. Once the lesion is seen, intraoperative ultrasonography is used to demonstrate the intrahepatic invasion of the lesion further, to make a preliminary determination of the status of the lesion to the hilar, and to examine other extrahepatic organs for the presence of extrahepatic metastases. Adequate freeing of the liver, with the the third porta hepatis (at the lower part of the vena cava sulcus, at the outlet of the right posterior inferior hepatic vein and caudate vein) as utterly free as possible or, if the the third porta hepatis is found to be invaded by a lesion, in vitro separation after adequate dissection; sequential blocking and dissection of the CHD (common hepatic duct), PHA (Proper hepatic artery), SHIVC (suprahepatic inferior vena cava), IHIVC (Inferhepatic inferior vena cava) RHIVC (Retrohepatic inferior vena cava) from the body (Figure 1D). The inferior vena cava was reconstructed by end-to-end anastomosis of the artificial vessel-superior and inferior vena cava using a 6-0 prolene wire (Figure 1E). A temporary portal vein shunt was established by end-to-end anastomosis of the PV to the artificial IVC. These two routine steps maintain hemodynamic stability during the procedure. Blood gas analysis, including pH, LAC, and SaPO2, was performed at 1-hour intervals, and anaesthetic adjustments were made accordingly. After the liver is removed from the body, the liver is continuously lavaged with 0-4° lavage solution via the portal vein, followed by sequential lavage of the hepatic artery and the intra- and extrahepatic bile ducts. The ultrasonic knife and bipolar electrocoagulation forceps were used to separate the liver parenchyma along the hepatic sickle ligament and carefully isolate the liver lesion, followed by complete resection of the diseased liver (Figure 1F), the healthy side intact. After the reserved liver has been repaired ex vivo (Figure 1I), the reconstructed hepatic vein is lateralized to the artificial inferior vena cava or, if the patient's inferior vena cava can be preserved, to the vena cava with an end-to-side anastomosis. If the inferior vena cava is reconstructed outside the body, the artificial IVC is removed from the body at the time of liver transplantation, the autologous reconstructed vessel is connected to the superior and inferior hepatic vena cava, and the transplanted hepatic vein is then anastomosed end-to-end with the IVC (Figure 1G,H). Successive anastomoses are performed on the portal vein and the hepatic artery. If the patient's common bile duct can be preserved, an interrupted pair of common bile duct anastomoses are performed. If it is not possible to preserve, a choledochojejunostomy is performed.
Postoperative management and follow-up
Postoperative complications were assessed according to Clavien-Dindo classification; all patients were routinely given albendazole for 1 year postoperatively and were followed up by outpatient review and telephone call after discharge, which included the patient's abdominal ultrasound, total abdominal CT, liver function, patient survival and the presence of recurrence, up to December 2020.1.5.
Statistical analysis
The data were statistically analyzed using SPSS 23.0, with measures expressed as mean, standard deviation (X ± S) or median, and counts expressed as a percentile.
Results
The age range of the 13 patients who underwent ELRA was 18–59 years, with a median age was 38.3 years. Preoperative PTBD was performed in 6 patients, and TBIL concentrations were reduced to ≤60 umol/ L. ICG retention at 15 min was <10% in 6 patients (46.1%) and ICG R 15 was >10% in 7 patients (53.9%). PHI class I (P3I1-3H1-2) in 4 cases (30.8%), class II (P1H2I3, P2H2I3) in 3 cases (23.07%), and class III (P2H1I2, P2H2I2) in 6 cases (46.1%) (Table 1); There were 4 of HAE lesions invaded the diaphragm (30.8%), 3 of HAE lesions invaded the adrenal gland (23.07%), 2 of HAE lesions invaded the kidney (15.4%) and 4 HAE lesions combined with peripheral multi-organ invasion(30.8%); According to the standard liver volume calculation formula of West China Hospital, we calculated the standard liver volume of all patients (14). The median standard liver volume was 1118 ml (1,085–1,206.5 ml), the median residual liver volume was 634 ml (range 526.5–1,338 ml) and calculated GLV/SLV 43-112%, with a mean of 69.42% (Table 1).
The median weight of the autograft was 845.8 g (619.5–1,020.5 g). To maintain hemodynamic stability during the hepatoless period, 12 patients underwent temporary inferior vena cava reconstruction and portal bypass. 1 patient underwent intra-operative extracorporeal vein-venous diversion, of which 7 patients underwent direct reconstruction of the inferior vena cava using autologous vessels and 4 patients underwent temporary replacement of the inferior vena cava using artificial vessels. In two patients, a PV-subhepatic IV end-lateral anastomosis was used for TPS. In one patient, the inferior vena cava was not reconstructed because of the intraoperative formation of retroperitoneal collateral circulation. No postoperative renal insufficiency or gastrointestinal symptoms were observed. The specific reconstruction modalities of the first and second hepatic hilum after transcatheter repair and plastic surgery are shown in Table 1; the median operation time was 14.5 h (11.5–16.15 h), the median anhepatic phase time was 290 min (257–312.5 min). The median intraoperative blood loss was 1,900 ml (1,300–3,500 ml), the median number of erythrocyte suspensions entered was 7.5 u (6–9u). There were 4 patients had no significant complications during hospitalization, and 9 had complications, including 2 cases of biliary leakage, 4 cases of thoracoabdominal effusion, one case of pulmonary infection, one case of vascular anastomotic stenosis, and 1case of recurrence. 4 patients had complications of Clavien-Dindo classification grade III or higher (Table 1). 4 patients died, with a mortality rate of 30.8%. Ultrasound, CT, and blood indices were examined every 3 months to study liver function and the long-term prognosis of ERAT. All patients were regularly treated with albendazole for at least one year after surgery. The median follow-up was 43 months (2–63 months), and one case developed persistent ascites and hypoproteinemia at 8 months postoperatively, which was considered a small liver syndrome (15). One patient developed a chest wall mass at 36 months postoperatively, considered a recurrence of incisional implantation with a high probability of recurrence (16), and was discharged with surgical excision and improvement.
Discussion
Hepatic alveolar echinoccosis is a disease that can infect both humans and livestock and is also known as “worm cancer” because of its tumour-like growth pattern (infiltrative growth), for which radical resection is the treatment of choice (17, 18). On the other hand, the lack of medical resources in areas with a high prevalence of worms has deprived these patients of the best opportunity for radical resection in clinical practice. The advent of ELRA has given these patients, unable to undergo radical resection, the opportunity for reoperation (19, 20). At the same time, ELRA, as a challenging liver surgery, poses a severe challenge to the multidisciplinary cooperation and operator skills in each treatment centre due to the complexity of the operation, the high degree of difficulty, the long operation time, and the high trauma.
Preoperative evaluation
Among the indications for ELRA proposed by scholars at home and abroad in the past (21, 22, 23), one of the more important points is the relationship between lesions and blood vessels. We evaluated the number, size, relationship with surrounding tissues, and liver quality of lesions to ensure safety and effectiveness through preoperative 3D reconstruction techniques (24). This technique is more intuitive and more straightforward than conventional two-dimensional planar CT and allows the liver to be viewed from different angles with the blood vessels (25, 26); thus facilitating the surgical planning of ELRA; in addition, some patients with end-stage HAE have a combination of biliary infiltration leading to obstructive jaundice. Obstructive jaundice or elevated bilirubin impairs the regenerative capacity of the residual liver (26), requiring routine preoperative biliary drainage by PTCD or Endoscopic nasalbileduct drainage (ENBD) to relieve hyperbilirubinemia and biliary obstruction. The procedure's safety is effectively ensured by careful assessment of the surgical operation, liver function, and residual liver volume, combined with delicate surgical techniques.
Management of anhepatic phase
The management of the anhepatic phase is another critical issue that determines whether the procedure can be performed successfully (25). In previously reported ELRAs (27, 28), veno-venous bypass (VVB) was usually routinely used to control hemodynamic stability during the hepatoless phase; however, in another study (29), complications such as postoperative pulmonary thromboembolism and post-reperfusion syndrome were seen in up to 30% of patients. In our medical centre, 11 patients underwent temporary inferior vena cava reconstruction, one did not, and one patient who underwent extracorporeal vein-venous diversion died postoperatively due to pulmonary embolism; in the patient who did not undergo reconstruction, we found abundant retroperitoneal collateral circulation on preoperative inferior vena cava angiography and no significant hemodynamic fluctuations after intraoperative clamping of the vena cava. Therefore we recommend that all patients undergo routine temporary IVC reconstruction combined with portal shunts for hemodynamic stability and to reduce the incidence of postoperative infections caused by bacterial translocation (30, 31). In addition, hypothermic perfusion is also a core technique for liver protection (29), facilitating the maintenance of vascular patency, flushing of red blood cells and anaerobic metabolites from the transplanted liver, and reducing the extent of mitochondrial and nuclear damage; 12 of the 13 patients at our medical centre were perfused intraoperatively with University of Wisconsin solution (UW fluid) and one with HTK fluid, and the median anhepatic phaseliver-free period time in all cases was 290(257–312.5) minutes, with the longest time being 365 min, with no abnormal blood flow abnormal changes after hepatic blood flow was restored. Therefore, our centre believes that the use of cold perfusion of the liver and temporary inferior vena cava reconstruction combined with portal shunt during the hepatoless period can effectively reduce the hemodynamic instability associated with hepatocellular destruction, gastrointestinal stasis and ischaemic reperfusion injury after liver restoration.
Reconstruction methods of blood vessels and bile ducts
The reconstruction of ELRA graft vessels and bile ducts is complex; All are individualized protocols (32, 33). Our centre described the characteristics of vascular infiltration in 13 surgical patients concerning the vascular infiltration typing of hepatic vesicular peritoneal PHI proposed by Prof. Wang's team (13) (Table 1). A preliminary reconstruction plan had been made preoperatively according to the degree of vascular infiltration. The reconstruction method was finally determined according to the intraoperative findings. Hepatic artery reconstruction was performed with end-to-end anastomosis of the intrinsic hepatic artery; portal vein reconstruction was performed with lateral graft liver.The biliary tract is reconstructed by Hepatic duct (HD) and common bile duct (CBD) anastomosis,Roux-en-Y hepaticojejunostomy, or a combination of these two approaches,depending on the intraoperative situation. As the inferior vena cava is the most severely invaded, the decision to reconstruct the vena cava is based on the presence or absence of retroperitoneal collateral circulation. Intraoperative ultrasound should be performed at the end of all ductal grafts to detect any stenosis or occlusion (33, 34).
Management of postoperative complications
Management of postoperative complications: Despite the surgeons' increasing knowledge of ELRA, sophisticated intraoperative operations, and improved preoperative assessment, the incidence of postoperative complications is still high, with biliary leakage being the most common, which can be as high as 46.7% (35, 36, 37), and is thought to be related to longer ischaemic time, infection, and biliary anastomotic stenosis; whereas in our current study cohort, biliary leakage occurred in two cases (15.4%), which is significantly lower than the incidence previously reported. There were 2 cases (15.4%), which was significantly lower than the previously reported incidence, and this was closely related to the thorough preoperative planning and precise intraoperative operations at our medical centre. Four patients (30.7%) with complications of pleural effusion and 342 ascites were considered to be due to ischemia-reperfusion injury 343 to the liver and their symptoms improved after active symptomatic treatment; in one patient, a spherical mass in the chest wall was found 36 months after surgery, and a right subxiphoid-rib arch and a mixed density shadow in the right cardio-diaphragmatic angle were seen on CT plain scan of the chest and abdomen; the author compared the location of the recurrent lesion with the preoperative images and preferred intraoperative incisional implantation metastasis (38).
In summary, for patients with advanced HAE, Ex vivo Liver Resection and Autotransplantation have a broad application prospect. It does not require waiting for a liver source does not require long-term postoperative immunosuppressive drugs. Its economic burden is less than that of allogeneic liver transplantation. However, liver transplantation is a complex, challenging and prolonged procedure with more complications, and the patient's general condition is poor, which can easily lead to various postoperative complications. Therefore, accurate preoperative evaluation of the liver, individualized intraoperative pipeline reconstruction, and delicate surgical operations are vital steps to successful surgery for patients eligible for this surgical modality. At the same time, multidisciplinary consultations should be strengthened when some rare complications arise. Early diagnosis and treatment should be provided to improve the success rate and patient survival after liver transplantation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The study followed the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of Scientific Research and Clinical Trial of the Affiliated Hospital of Qinghai University (Approval Identifier: P-SL-2019054). All patients provided written informed consent for the collection and publication of their medical information at the first visit to our center, which was filed in their medical records, and the ethics committees approved this consent procedure.
Author contributions
JY, XC and LH selected the topic and wrote and revised the article; HW, YZ, MP, and CY provided critical revision and reviewed the literature under the paper's supervision; ZW and HF were responsible for the final draft. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by grants from 2022 Science and Technology Plan Project of Qinghai Science and Technology Department (Qinghai Provincial Key Laboratory of Hydatid Disease Research).
Acknowledgments
We thank all the patients and their families for participating in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang X. Expert consensus on diagnosing and treating complicated hepatic vesicular encystment (2020 edition). Chin J Gen Surg Basic Clin Sci. (2020) 27:18–23. doi: 10.7507/1007-9424.201911096
2. McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. (2003) 362(9392):1295–304. doi: 10.1016/S0140-6736(03)14573-4
3. Wen H. New advances in the diagnosis and surgical treatment of hepatic encystment disease. Chin J Gastrointest Surg. (2011) 10:290–2. doi: 10.3760/cma.j.issn.1007-8118.2011.05.027
4. Akbulut S, Sahin TT. Comment on surgical approaches for definitive treatment of hepatic alveolar echinococcosis: results of a survey in 178 patients. Parasitology. (2020) 147(13):1408–10. doi: 10.1017/S0031182020001390
5. Du C, Liu Z, Yang X, Yan L, Li B, Wen T, et al. Hepatectomy for patients with alveolar echinococcosis: long-term follow-up observations of 144 cases. Int J Surg. (2016) 35:147–52. doi: 10.1016/j.ijsu.2016.09.094
6. Buttenschoen K, Carli Buttenschoen D, Gruener B. Long-term experience on surgical treatment of alveolar echinococcosis. Langenbecks Arch Surg. (2009) 394(4):689–98. doi: 10.1007/s00423-008-0392-5
7. Pichlmayr R, Grosse H, Hauss J, Gubernatis G, Lamesch P, Bretschneider HJ. Technique and preliminary results of extracorporeal liver surgery (bench procedure) and surgery on the in situ perfused liver. Br J Surg. (1990) 77(1):21–26. doi: 10.1002/bjs.1800770107
8. Wen H, Dong JH, Zhang JF, Zhao JM, Shao YM, Duan WD, et al. Extracorporeal hepatectomy combined with autologous liver transplantation for treatment of hepatic alveolar cysticercosis. Chinese J Gastroenterol Surg. (2011) 10:148–9. doi: 10.3760/cma.j.issn.1673-9752.2011.02.024
9. Qiu Y, Yang X, Shen S, Huang B, Wang W. Vascular infiltration-based surgical planning in treating end-stage hepatic alveolar echinococcosis with ex vivo liver resection and autotransplantation. Surgery. (2019) 165(5):889–96. doi: 10.1016/j.surg.2018.11.007
10. Xiao Q, Ye QF, Wang W, Xia ZP, Ming YZ, Wang YF, et al. Advances in preoperative evaluation of autologous liver transplant patients. Chin J Hepatobiliary Surg. (2016) 22:141–4. doi: 10.16139/j.1007-9610.2016.03.002
11. Yang C, Yang HJ, Deng SP, Zhang Y. Current status of ex-vivo liver resection and autologous liver transplantation for end-stage hepatic alveolar echinococcosis. Ann Palliat Med. (2020) 9(4):2271–8. doi: 10.21037/apm-20-184
12. Tien-Tian X, Yan-Qiu S, Qiang Z, Xiumin H, Yong-Hai Z, Chang-Chun Q, et al. The value of three-dimensional visualization technology in the preoperative assessment of autologous liver transplantation for end-stage hepatic multifocal echinococcosis. Chin J Schistosomiasis Control. (2018) 30:646–51. doi: 10.16250/j.32.1374.2018098
13. Yanqiu S, Yonghai Z, Mei Y, Jingjing W, Qingxin Z. Application of CT and MRI in autologous liver transplantation for isolated liver resection in advanced hepatic alveolar echinococcosis. Chin J Med Imaging. (2015) 23:610–3. doi: 10.3969/j.issn.1005-5185.2015.08.012
14. Zheng-Rong S, Lu-Nan Y, Bo L, Tian-Fu W. Evaluation of standard liver volume formulae for Chinese adults. World J Gastroenterol. (2009) 15(32):4062–66. CNKI:SUN: ZXXY.0.2009-32-01619705504
15. Ye HW, Zhou Y, Lu Q, Zhang LQ, Deng Y, Xu XL, et al. A case of postoperative microhepatic syndrome after extracorporeal hepatectomy combined with residual liver autotransplantation for hepatic alveolar peritonitis. J Clin Hepatobiliary Dis. (2019) 35:858–60. CNKI:SUN:GDWZ.0.2015-04-003
16. Wang ZX, Li Y, Wang JY, Li WX, Ren L, Wang HJ, et al. Report of a case of recurrence after autologous liver transplantation for hepatic alveolar encapsulation disease. J Clin Hepatobiliary Dis. (2020) 36:1832–4. doi: 10.3969/j.issn.1001-5256.2020.08.033
17. Azoulay D, Lim C, Salloum C, Andreani P, Fesuy F. Complex liver resection using standard total vascular exclusion. Venovenous bypass, and in situ hypothermic portal perfusion: an audit of 77 consecutive cases. Ann Surg. (2015) 262(1):93–104. doi: 10.1097/SLA.0000000000000787
18. Azoulay D, Salloum C, Eshkenazi R, Schwartz C, Lahat E, Lim C. Access to the portal system via the mesentery for establishing venous bypass in liver transplantation. Liver Transpl. (2019) 25(5):807–10. doi: 10.1002/lt.25452
19. Kato T, Regina L, Peter W, Joshua G, Adam S, Benjamin H, Karim M, et al. Ex vivo resection and autotransplantation for conventionally unresectable tumors - an 11-year single center experience. Ann Surg. (2020) 272(5):766–72. doi: 10.1097/SLA.0000000000004270
20. Wang H, Liu Q, Wang Z, Zhang F, Li X, Wang X. Clinical outcomes of Ex Vivo liver resection and liver autotransplantation for hepatic alveolar echinococcosis. Journal of Huazhong University of Science and Technology [Medical Sciences]. (2012) 32(4):598–600. doi: 10.1007/s11596-012-1003-9
21. Wen H, Dong JH, Zhang JH, Zhao JM, Li T. Ex vivo liver resection and autotransplantation for End-stage alveolar echinococcosis: a case series. Am J Transplant. (2016) 16(2):615–24. doi: 10.1111/ajt.13465
22. Yang X, Qiu Y, Huang B, Wang W, Shen S, Feng X, et al. Novel techniques and preliminary results of ex vivo liver resection and autotransplantation for end-stage hepatic alveolar echinococcosis: a study of 31 cases. Am J Transplant. (2018) 18(7):1668–79. doi: 10.1111/ajt.14621
23. Tuerganaili A, Jia-Hong D, Ying-Mei S, Jin-Ming Z, Tao L, Tuerhongjiang T, et al. Ex vivo liver resection, and autotransplantation as an alternative to allotransplantation for end-stage hepatic alveolar echinococcosis. J Hepatol. (2018) 69(5):1037–46. doi: 10.1016/j.jhep.2018.07.006
24. Yang C, Yang HJ, Deng S, Zhang Y. Advances in the surgical treatment of advanced hepatic vesicular encapsulated worms with insufficient remaining liver volume. Chin J General Surg. (2021) 30(01):98–104. doi: 10.13437/j.cnki.jcr.2021.09.018
25. Ma H-L, Fan X-T, Shi X-J, He F-P. Clinical study of autologous liver transplantation by isolated hepatectomy for end-stage hepatic alveolar encapsulation disease. Chin J Transplant (Electronic Version). (2017) 11:1–4. doi: 10.3877/cma.j.issn.1674-3903.2017.01.001
26. Turan A, Shao YM, Zhao JM, Li T, Ran B, Jiang TM, et al. Clinical practice of enhancing the “quantity and quality” of the functional liver in autologous liver transplantation for hepatic vesicular disease: a clinical case study of 12 cases. Chin J Med. (2017) 97:270–5. doi: 10.11847/zgggws2016-32-07-00
27. Wang W, Ye QF, Fan XL, Xiong Y, Fu Z, Wang YF. Intraoperative venous diversion technique for autologous liver transplantation.
28. Ye QF. Basic and clinical research on autologous liver transplantation technology. Hubei Province: Wuhan University (2015-03-30).
29. Hoffmann K, Weigand MA, Hillebrand N, Büchler MW, Schemmer P. Is veno-venous bypass still needed during liver transplantation? A review of the literature. Clin Transplant. (2009) 23(1):1–8. doi: 10.1111/j.1399-0012.2008.00897.x
30. Zawistowski M, Nowaczyk J, Jakubczyk M, Domagala P. Outcomes of ex vivo liver resection and autotransplantation: a systematic review and meta-analysis. Surgery. (2020) 168(4):631–42. doi: 10.1016/j.surg.2020.05.036
31. Yu ZA, ECHL C, Chong YA, HY A, JL B, ZB Guo, et al. In situ reconstruction of vascular inflow/outflow to left lateral liver section, ex-vivo liver resection and autologous liver transplantation of remaining liver remnant for hepatic alveolar echinococcosis. Int J Surg Case Rep. (2020) 69:39–43. doi: 10.1016/j.ijscr.2020.03.023
32. Jianyong L, Jingcheng H, Wentao W, Lunan Y, Jichun Z, Bing H, et al. Ex vivo liver resection followed by autotransplantation to a patient with advanced alveolar echinococcosis with a replacement of the retrohepatic inferior vena cava using autogenous vein grafting: a case report and literature review. Medicine (Baltimore). (2015) 94(7):e514. doi: 10.1097/MD.0000000000000514
33. Shu JJ, Gong Y, Ou X, Dai HS, Zhang CC, Liu W, et al. Clinical efficacy of isolated hepatectomy combined with autologous liver transplantation for complex occupying lesions of the liver. Chin J Gastrointest Surg. (2020) 19:869–75. doi: 10.3760/cma.j.cn115610-20200522-00377
34. Zhang Y, Yang C, Wang L, Yang HJ, Liu J, Xian D, et al. Isolated hepatectomy and autologous liver transplantation combined with complex hepatic vein reconstruction for end-stage hepatic alveolar encapsulated disease. Chin J General Surg Basic Clini. (2018)25:1236–41. CNKI:SUN:ZPWL.0.2019-08-016
35. Cheng Z, Ye QF. Techniques and complications associated with autologous liver transplantation. Chin J Hepatobiliary Surg. (2017) 23:563–5. doi: 10.3760/cma.j.issn.1007-8118.2017.08.020
36. Ye QF, Fan XL, Ming YZ, Ke C, Wang YF, Peng GZ, et al. Intraoperative and postoperative complications of autologous liver transplantation and their prevention and treatment. Chin J Hepatobiliary Surg. (2013) 19:564–7. doi: 10.3760/cma.j.issn.1007-8118.2013.08.002
37. Chao G, Chengjie Y, Yamin G, Gang W. Analysis of autologous liver transplantation postoperative complications for highland hepatic vesicular echinococcosis. Organ Transplant. (2016) 7:449–53. doi: 10.3969/j.issn.1674-7445.2016.06.007
Keywords: ex vivo liver resection and autotransplantation echinococcosis, prognosis, complications, echinococcoses, China
Citation: Yuan J, Chen X, Hou L, Wang H, Zhou Y, Pang M, YangDan C, Wang Z and Fan H (2023) Single-center experience of Ex vivo liver resection and autotransplantation for complex hepatic alveolar echinoccosis. Front. Surg. 10:1089788. doi: 10.3389/fsurg.2023.1089788
Received: 4 November 2022; Accepted: 18 January 2023;
Published: 15 February 2023.
Edited by:
Jeroen Van Vugt, Erasmus Medical Center, NetherlandsReviewed by:
Michael Kueht, University of Texas Medical Branch at Galveston, United StatesTevfiktolga Sahin, İnönü University, Türkiye
© 2023 Yuan, Chen, Hou, Wang, Zhou, Pang, YangDan, Wang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhixin Wang TmljZV9ndXlAeWVhaC5uZXQ= Haining Fan ZmFuaGFpbmluZ0BtZWRtYWlsLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Jiaqi Yuan
Jiaqi Yuan Xiaobin Chen
Xiaobin Chen Lizhao Hou1,2,†
Lizhao Hou1,2,†