- 1Department of Cardiovascular Surgery, Qilu Hospital of Shandong University, Jinan, China
- 2Department of Endocrinology, Qilu Hospital of Shandong University, Jinan, China
- 3Shandong Institute of Medical Device and Pharmaceutical Packaging Inspection, Jinan, China
Objectives: This observational study aims to explore the predictive role of postoperative arterial lactate in off-pump coronary artery bypass grafting (CABG)-associated acute kidney injury (AKI).
Materials and methods: A total of 500 consecutive patients who underwent off-pump CABG from August 2020 to August 2021 at the Department of Cardiovascular Surgery, Qilu Hospital of Shandong University, were included. Logistic regression analysis was used to confirm the independent risk factors of off-pump CABG-associated AKI. Receiver operating characteristic (ROC) curve was performed to evaluate the discrimination ability and Hosmer–Lemeshow goodness of fit test was performed to evaluate the calibration ability.
Results: The incidence of off-pump CABG-associated AKI was 20.6%. Female gender, preoperative albumin, baseline serum creatinine, 12 h postoperative arterial lactate and duration of mechanical ventilation were independent risk factors. The area under the ROC curve (AUC) of 12 h postoperative arterial lactate for predicting off-pump CABG-associated AKI was 0.756 and the cutoff value was 1.85. The prediction model that incorporated independent risk factors showed reliable predictive ability (AUC = 0.846). Total hospital stay, intensive care unit stay, occurrence of other postoperative complications, and 28-day mortality were all significantly higher in AKI group compared to non-AKI group.
Conclusion: 12 h postoperative arterial lactate was a validated predictive biomarker for off-pump CABG-associated AKI. We constructed a predictive model that facilitates the early recognition and management of off-pump CABG-associated AKI.
1. Introduction
Acute kidney injury (AKI) is one of the most common postoperative complications in adult cardiac surgery. According to the new Kidney Disease: Improving Global Outcome (KDIGO) consensus criteria (1), the incidence of cardiac surgery-associated acute kidney injury (CSA-AKI) in adults can reach up to 42% (2). Notably, minimal elevations of serum creatinine (SCr) are closely associated with short-term and long-term mortality in patients after cardiac surgery, even for those whose renal function has returned to normal (3–7). Therefore, the early prediction and prevention of CSA-AKI have attracted great attention.
Cardiopulmonary bypass (CPB) is an important independent risk factor for CSA-AKI (8). Renal hypoperfusion, inflammation, and oxidative stress during CPB all increase the risk of CSA-AKI (9–11). Most studies on the risk factors of CSA-AKI have included all types of cardiac surgery without specifically discussing off-pump coronary artery bypass grafting (CABG). Off-pump CABG was developed to reduce the risk of perioperative complications caused by CPB and improve the long-term prognosis of patients (12). However, there is no strong evidence that off-pump CABG is effective in preserving renal function.
Several large-scale studies have found that compared with on-pump CABG, off-pump CABG could indeed reduce the incidence of postoperative AKI, but it did not show better effects on postoperative renal replacement therapy (RRT) and mortality (13–16). This suggests that off-pump CABG may not have significant advantage in improving outcomes in patients with AKI. Considering the wide application of off-pump CABG in cardiac surgery, early recognition and management of AKI in patients undergoing off-pump CABG deserves attention.
Arterial lactate is considered as a biomarker of tissue hypoxia and hypoperfusion. Hyperlactatemia in critically ill patients is closely associated with increased mortality and poor prognosis (17–22). Several studies have found that high lactate level may be linked to the development of CSA-AKI (5, 23–25). However, studies on the relationship between off-pump CABG-associated AKI and lactate are lacking.
The present study aims to explore the predictive role of postoperative arterial lactate in AKI associated with off-pump CABG and construct a predictive model that facilitates the early recognition and management of AKI through a single center retrospective study.
2. Materials and methods
2.1. Study population
We retrospectively analyzed the patients who underwent off-pump CABG surgery in the Department of Cardiovascular Surgery, Qilu Hospital of Shandong University, from August 2020 to August 2021. Exclusion criteria were patients undergoing on-pump CABG (including intraoperative conversion to CPB), patients undergoing RRT before operation and patients lacking postoperative SCr data. The study has been approved by the Medical Ethics Committee of Qilu Hospital of Shandong University, and patient information was kept anonymously to maintain confidentiality.
2.2. Surgical procedures
All off-pump CABG surgeries were performed by experienced cardiac surgeons. Median sternal incision was performed routinely. Left internal mammary artery (LIMA), great saphenous vein, and sometimes radial artery were harvested as vascular grafts. After heparinization, the LIMA was anastomosed to the left anterior descending (LAD) artery, and then anastomosis of aorta to saphenous vein grafts to other stenosed coronary arteries was performed. Anastomotic modes were determined based on the anastomotic site. During the anastomosis, suction stabilizers were used to stabilize the beating heart, and deep pericardial sutures and intra-coronary shunts were routinely used.
2.3. Study variables and data analysis
A number of variables were included in this study. Preoperative variables included: age, gender, body mass index, smoking, hypertension, diabetes mellitus (DM), hyperlipemia, history of cerebral disease including cerebral hemorrhage and infarction, chronic obstructive pulmonary disease, previous heart failure, arrhythmia including atrial fibrillation and frequent premature ventricular complexes, percutaneous coronary intervention, New York Heart Association (NYHA) functional classification, recent contrast media exposure (within 7 days before operation), left ventricular ejection fraction (LVEF), laboratory reports, three-vessel coronary heart disease, and left main coronary artery disease. Intraoperative variables included: emergency surgery, operation time, erythrocyte transfusion, urine volume, and fluid replacement. Postoperative variables included: central venous pressure and mean arterial pressure at admission, intra-aortic balloon pump (IABP), low cardiac output syndrome (LCOS), RRT, vasoactive medicine application including dopamine and norepinephrine, duration of mechanical ventilation, erythrocyte transfusion, laboratory reports, complications, length of stay, length of ICU stay, and death. All data were checked twice.
2.4. Definition of renal function
We used the 2021 Chronic Kidney Disease Epidemiology Collaboration equation (26) for calculating the estimated glomerular filtration rate (eGFR). AKI was diagnosed and staged according to the KDIGO guidelines published in 2012 (1): increase in SCr ≥ 0.3 mg/dl (≥26.5 μmol/L) within 48 h or increase in SCr to ≥ 1.5 times baseline levels in 7 days or urine volume < 0.5 ml/kg/h for 6 h. Any of the above is diagnosed as AKI. The latest SCr level within 7 days before operation was defined as the baseline, and AKI from ICU admission to discharge were defined as postoperative AKI.
Severity classification AKI stage I: increase in SCr ≥ 0.3 mg/dl (≥26.5 μmol/L) within 48 h or increase in SCr to 1.5–1.9 times baseline levels or urine volume < 0.5 ml/kg/h for 6–12 h. AKI stage II: increase in SCr to 2.0–2.9 times baseline levels or urine volume < 0.5 ml/kg/h for ≥12 h. AKI stage III: increase in SCr to ≥4.0 mg/dl (≥353 μmol/L) or increase in SCr to ≥3 times baseline levels or urine volume < 0.3 ml/kg/h for ≥24 h or anuria for 12 h or initiation of RRT.
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS 25.0 and R project software. Continuous variables conforming to normal distribution are expressed as mean ± SD and compared by Student's t-test. Non-normally distributed continuous variables are expressed as median and 25th to 75th percentiles and were compared by Mann–Whitney U-test. Rank variables were also compared using Mann–Whitney U-test. Categorical variables are expressed as frequency and percentage and were analyzed using χ2 test or Fisher's exact test. P < 0.05 (two-side) was considered significant. Logistic regression analysis was performed on the significant variables in univariate analysis to identify independent risk factors. The results of multivariate analysis are presented as odds ratio (OR) and 95% confidence interval (CI). A receiver operating characteristic (ROC) curve was performed to exhibit the predictive ability. The cut-off value was determined when the Youden index was maximum. Hosmer–Lemeshow goodness of fit test was performed to evaluate the calibration of the prediction model. P > 0.05 indicated a good calibration ability. Internal validation was performed using the bootstraping method with 1,000 replicates.
3. Results
3.1. Clinical characteristics of the study population
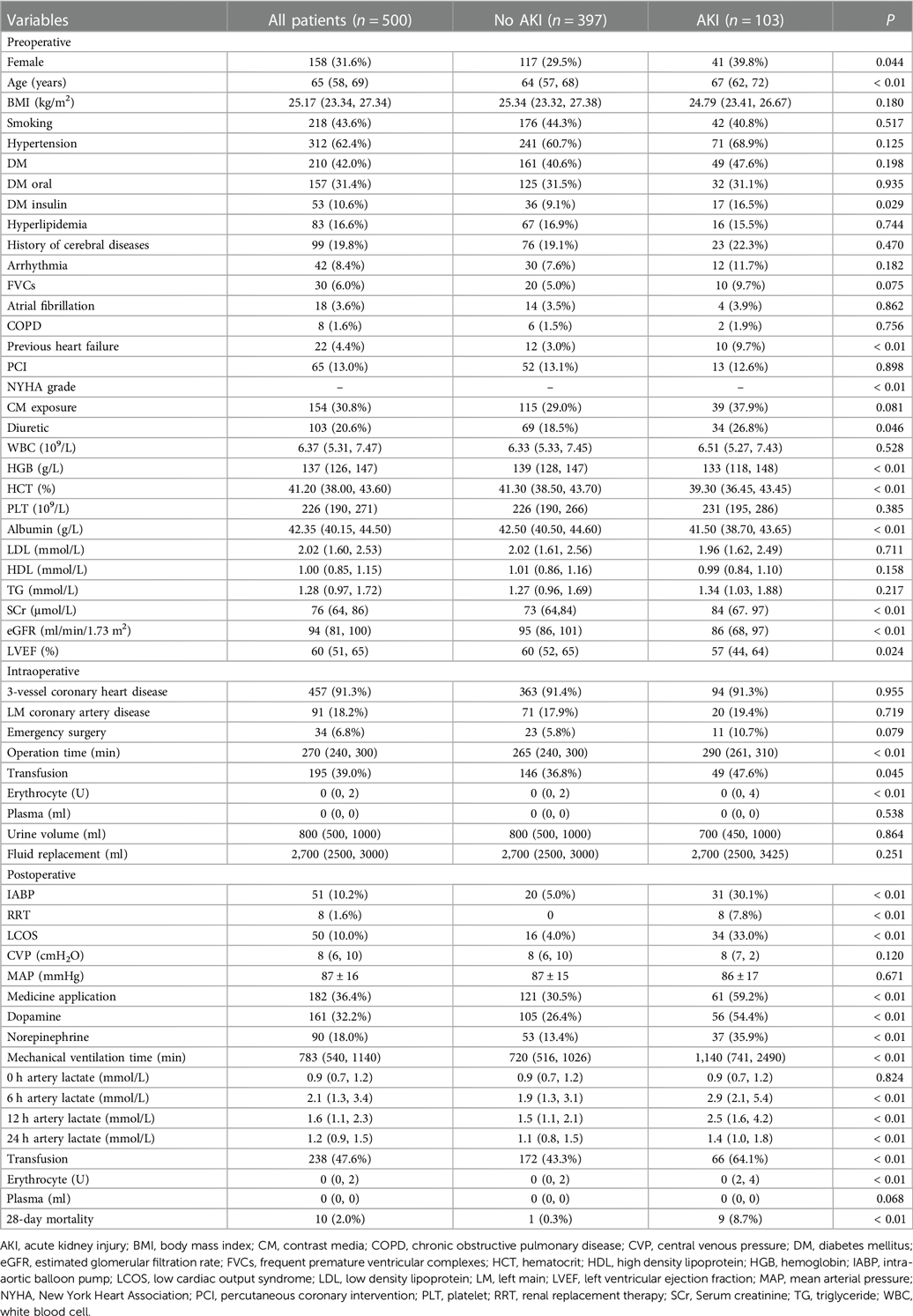
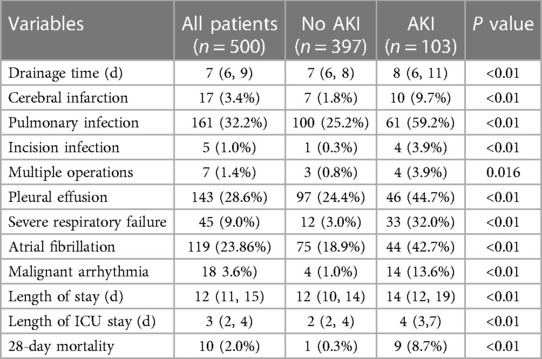
The study population consisted of 500 patients, including 342 males (68.4%) and 158 females (31.6%). The mean age of the patients was 64 years, and the mean hospital stay was 13 days. The incidence of off-pump CABG-associated AKI was 20.6% (103 patients), including 76.7% (79 patients) with AKI stage I, 7.77% (8 patients) with AKI stage II and 15.5% (16 patients) with AKI stage III. The incidence of postoperative RRT in patients diagnosed with AKI was 7.8%, and 28-day mortality of off-pump CABG was 2.0% (Table 1).
3.2. Independent risk factors of off-pump CABG-associated AKI
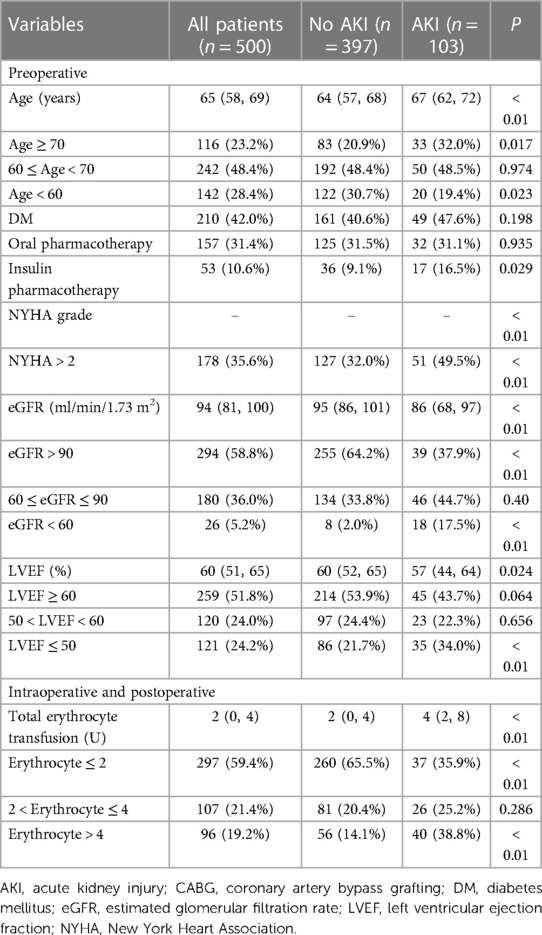
Hypothesis testing revealed that there were statistical differences between AKI and non-AKI patient groups including age, gender, DM, previous heart failure, NYHA functional classification, preoperative diuretic use, preoperative hemoglobin, preoperative hematocrit, preoperative albumin, baseline SCr, preoperative eGFR, preoperative LVEF, erythrocyte transfusion, IABP application, LCOS, vasoactive medicine application, duration of mechanical ventilation, arterial lactate level after operation (Table 1). In order to more precisely identify risk factors, we categorized the following variables: age, DM, NYHA functional classification, eGFR, LVEF and total erythrocyte transfusion (Table 2).
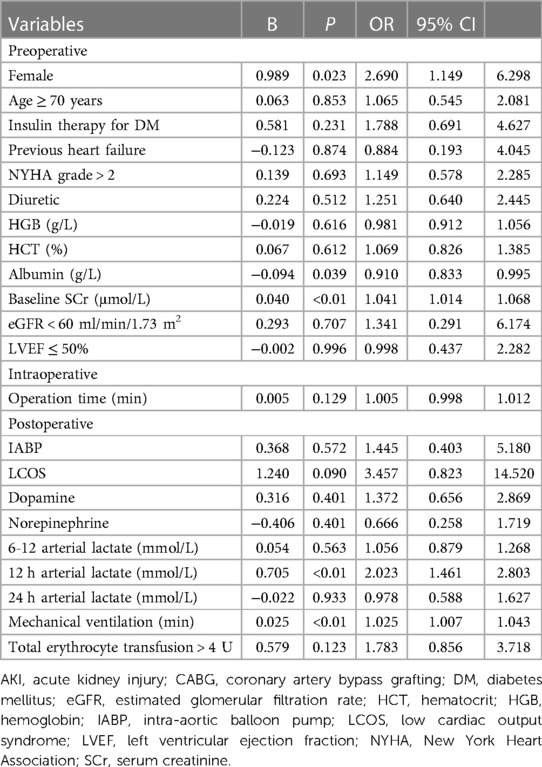
We included the variables that are statistically significant in univariate analysis for logistic regression analysis. The results showed that female gender, baseline SCr level, preoperative albumin level, 12 h postoperative arterial lactate level and duration of mechanical ventilation were independent risk factors of off-pump CABG-associated AKI (Table 3).
3.3. Arterial lactate level for predicting off-pump CABG-associated AKI
The level of arterial lactate peaked at 6 h after operation. We found that patients in the AKI group had significantly higher arterial lactate levels than patients in the non-AKI group at each postoperative time point, and the difference was the most obvious at 12 h after operation (OR = 2.023, 95% CI = 1.461–2.803). We performed ROC curve analysis to evaluate the ability of 12 h arterial lactate level for predicting off-pump CABG-associated AKI. The area under the ROC curve (AUC) of 12 h arterial lactate level was 0.756 (Figure 1A). The cutoff value was derived to be 1.85 according to the maximum value of the Youden index. The sensitivity was 71.8% and the specificity was 67.8%. The above results confirmed that 12 h arterial lactate after operation is a reliable biomarker for predicting off-pump CABG-associated AKI.
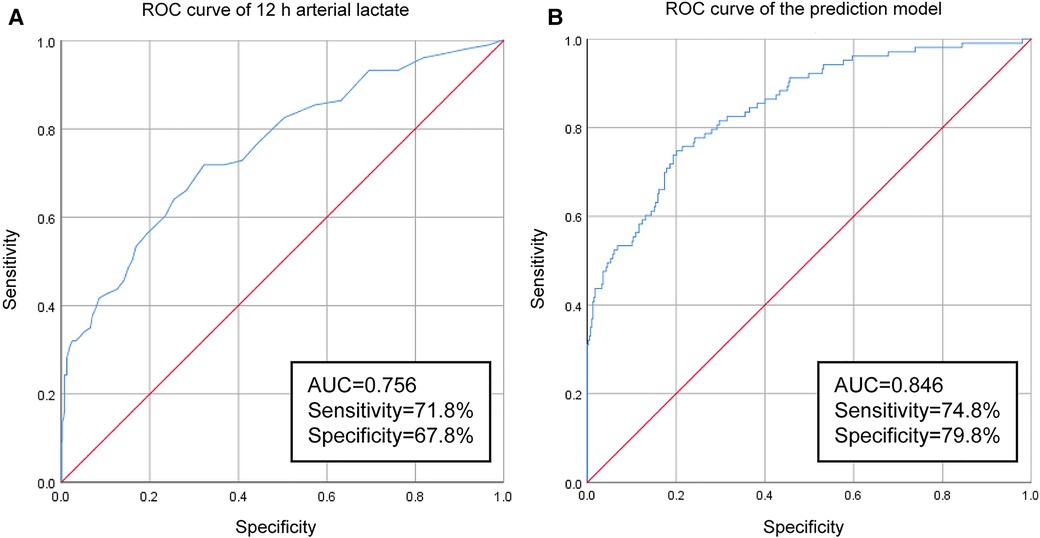
Figure 1. Results of ROC-curve analysis: (A) ROC curve of 12 h arterial lactate for predicting off-pump CABG-associated AKI. (B) ROC curve of the prediction model.
3.4. Prediction model of off-pump CABG-associated AKI
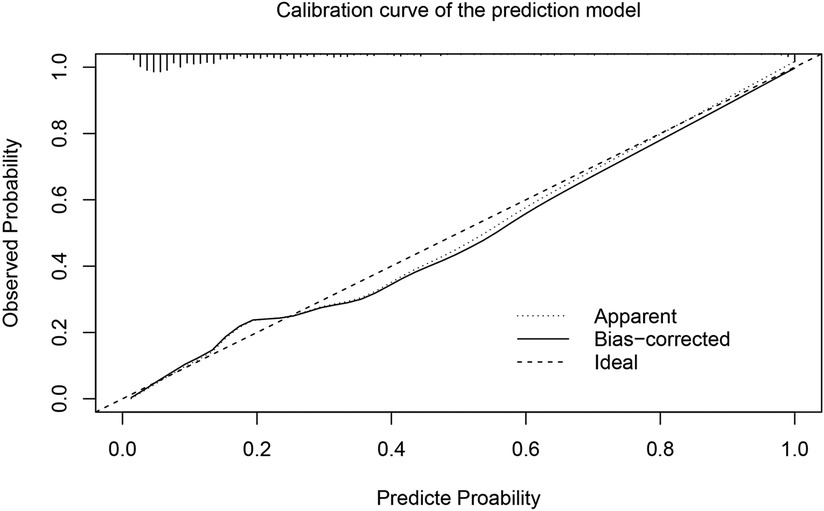
In order to more accurately identify high-risk patients with AKI at an early stage, we incorporated the five independent risk factors into a prediction model. The AUC of the new prediction model was 0.846, which exhibited great predictive performance (Figure 1B). The sensitivity was 74.8% and the specificity was 79.8%. The calibrated c-index after internal validation with the bootstrapping method was 0.839. In addition, the result of Hosmer–Lemeshow goodness of fit test confirmed the good calibration ability of the prediction model (P = 0.642) and the calibration curve also showed good concordance between predicted and actual probabilities (Figure 2).
3.5. Postoperative complications and short-term prognosis
We performed statistical analysis of postoperative complications and 28-day mortality between the AKI group and the non-AKI group. Postoperative LCOS, cerebral infarction, pulmonary infection, incision infection, secondary surgery, pleural effusion, severe respiratory failure, atrial fibrillation and malignant arrhythmia were more frequent in patients with AKI (Table 4). Moreover, patients in the AKI group had longer total hospital and ICU stays, and significantly higher 28-day mortality (Table 4). The above statistical results indicated that patients with off-pump CABG-associated AKI had a worse short-term prognosis.
4. Discussion
The development of AKI during hospitalization significantly increases the risk of subsequent chronic kidney diseases and death (27). Second only to sepsis, patients undergoing cardiac surgery are more likely to develop AKI because of hemodynamic instability, CPB, and increased inflammatory response (28). Therefore, research on risk factors of CSA-AKI has attracted much attention and many predictive models have been developed for this purpose.
Due to the lack of consensus on the definition of AKI, the initial predictive models, such as the Cleveland Clinic score, the Mehta score, and the Simplified Renal Index, were mainly targeted at severe AKI requiring RTT (29–31). Later on, according to the definition of AKI established by the KDIGO guidelines, many researchers have developed predictive models for CSA-AKI at all stages (32, 33). However, the mechanisms underlying AKI after off-pump CABG differ from on-pump cardiac surgery. During CPB, hypothermia, hypoperfusion, non pulsatile blood flow, systemic inflammatory response, vasoactive agent, acidosis, intravascular haemolysis, etc., all lead to renal vasoconstriction and renal hypoperfusion (34–36). Ischemia reperfusion injury following CPB further contributes to renal impairment (2). Off-pump CABG may avoid risk factors associated with CPB that cause renal injury. To the best of our knowledge, there are few published studies addressing the risk factors of off-pump CABG-associated AKI (37–44). Our study revealed the predictive role of 12-h postoperative arterial lactate in off-pump CABG-associated AKI and provided a new prediction model for the early recognition and management.
Lactate is an important biomarker to evaluate and monitor tissue perfusion in critically ill patients. High arterial lactate level can reflect the imbalance of tissue oxygen metabolism which contribute to the development of AKI. In the study by Lopez-Delgado and coworkers, 24-h postoperative arterial lactate was an independent risk factor of CSA-AKI (5). Zhang and colleagues found that normalized lactate load was independently associated with CSA-AKI (23). A prospective trial involving 100 low-risk patients showed that postoperative lactate was a reliable predictor of CSA-AKI (45). The above studies have demonstrated a correlation between postoperative lactate and CSA-AKI. However, they only included cardiac surgery with CPB but not involving off-pump CABG. Our study found that the arterial lactate levels at 6 h, 12 h and 24 h after off-pump CABG in the AKI group were significantly higher than that in the non-AKI group. And we confirmed that 12 h arterial lactate was a potent predictor of off-pump CABG-associated AKI, which has not been reported in other studies.
Arterial lactate is a rapidly available indicator. Both circulating hypovolemia and microcirculatory derangement may lead to tissue hypoperfusion, which causes tissue hypoxia and increases lactate levels. Persistent high lactate levels are closely associated with organ failure (46, 47). In addition, elevated lactate levels may be a consequence of AKI. Legouis and colleagues proposed that changes in renal gluconeogenesis during AKI may increase mortality by affecting systemic metabolism (48). Their study found that the level of blood lactate in mice exposed to renal ischemia-reperfusion injury increased significantly and the clearance rate of lactate decreased significantly. Patients with AKI are more likely to suffer from impaired metabolism (low to normal glucose/high lactate), which is closely related to increased mortality. And in their study, thiamine supplementation can reduce mortality in patients with AKI by increasing lactate clearance and glucose production.
Our study focused more on the predictive value of lactate levels at early time points. Preoperative and intraoperative predictors were very timely, but not accurate enough. Predictors during late postoperative period are accurate, but not timely. The 12 h postoperative arterial lactate is both timely and reliable in predicting off-pump CABG-associated AKI. And we constructed a prediction model with good discrimination and calibration by combining the other four independent risk factors. There are several well-known risk factors for AKI, such as hypertension, DM, erythrocyte transfusion, LCOS, etc., which were significantly different between AKI and non-AKI group. However, they were not independent risk factors in the multivariate analysis, which may be due to dilution by other risk factors.
Preoperative albumin was included in the prediction model as an independent risk factor (OR = 0.910, 95% CI = 0.833–0.995). The association of albumin and AKI has been discussed mainly in patients with liver dysfunction. Albumin infusion can effectively improve circulating volume and decrease inflammatory response (49, 50). The combination of albumin and vasoconstrictors is the most important treatment for hepatorenal syndrome and can effectively improve the renal function (51, 52). In the study by Lee and colleagues, preoperative serum albumin levels <4.0 g/L was an independent risk factor for off-pump CABG-associated AKI (OR = 1.83, 95% CI = 1.27–2.64), and administration of 20% exogenous albumin before operation was able to reduce the risk of AKI (40, 53). Our study also confirmed the association. However, more studies are needed on whether albumin supplementation can prevent CSA-AKI.
Statistical analysis of short-time outcome after off-pump CABG indicated a higher incidence of postoperative complications and prolonged hospital stay among patients in the AKI group, which convinced us that the impact of off-pump CABG-associated AKI cannot be overlooked.
There are two limitations in our study. First, as a single center retrospective study, the findings were not validated in an external study population. Second, our study addressed only in-hospital complications and short-term survival but did not perform follow-up on long-term prognosis of patients. In the future, we would like to strengthen the management of patients based on the risk factors, such as albumin expanding volume therapy or thiamine improving glucose metabolism, to find out which strategy is able to prevent off-pump CABG-associated AKI.
5. Conclusion
Our study demonstrated the predictive value of 12 h postoperative arterial lactate in off-pump CABG-associated AKI and constructed a reliable prediction model which contributes to the early recognition and management of off-pump CABG-associated AKI.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Qilu Hospital of Shandong University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RY performed data analysis, statistics, and draft writing. TL performed data collection. LL performed language processing. XM and YB designed the study and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the corresponding author, Xiangbin Meng, whom did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors would like to thank Xirui Zhu, Wangding Pan, Jianshu Zhang, Kai Jin, and Shouqing Han for their assistance with the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Khwaja A. Kdigo clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120(4):c179–84. doi: 10.1159/000339789
2. Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. (2017) 13(11):697–711. doi: 10.1038/nrneph.2017.119
3. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. (2009) 119(18):2444–53. doi: 10.1161/circulationaha.108.800011
4. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of Serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. (2004) 15(6):1597–605. doi: 10.1097/01.asn.0000130340.93930.dd
5. Lopez-Delgado JC, Esteve F, Torrado H, Rodríguez-Castro D, Carrio ML, Farrero E, et al. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified rifle classification. Crit Care. (2013) 17(6):R293. doi: 10.1186/cc13159
6. Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, et al. Impact of minimal increases in Serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. (2008) 36(4):1129–37. doi: 10.1097/CCM.0b013e318169181a
7. Machado MN, Nakazone MA, Maia LN. Prognostic value of acute kidney injury after cardiac surgery according to kidney disease: improving global outcomes definition and staging (kdigo) criteria. PLoS One. (2014) 9(5):e98028. doi: 10.1371/journal.pone.0098028
8. Haase M, Bellomo R, Story D, Letis A, Klemz K, Matalanis G, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant. (2012) 27(1):153–60. doi: 10.1093/ndt/gfr275
9. Lannemyr L, Bragadottir G, Hjärpe A, Redfors B, Ricksten SE. Impact of cardiopulmonary bypass flow on renal oxygenation in patients undergoing cardiac operations. Ann Thorac Surg. (2019) 107(2):505–11. doi: 10.1016/j.athoracsur.2018.08.085
10. Lannemyr L, Bragadottir G, Krumbholz V, Redfors B, Sellgren J, Ricksten SE. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology. (2017) 126(2):205–13. doi: 10.1097/aln.0000000000001461
11. Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, et al. Plasma il-6 and il-10 concentrations predict aki and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol. (2015) 26(12):3123–32. doi: 10.1681/asn.2014080764
12. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Straka Z, et al. Five-Year outcomes after off-pump or on-pump coronary-artery bypass grafting. N Engl J Med. (2016) 375(24):2359–68. doi: 10.1056/NEJMoa1601564
13. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, O'Corragain OA, Edmonds PJ, et al. Comparison of renal outcomes in off-pump versus on-pump coronary artery bypass grafting: a systematic review and meta-analysis of randomized controlled trials. Nephrology (Carlton). (2015) 20(10):727–35. doi: 10.1111/nep.12506
14. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Off-Pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. (2012) 366(16):1489–97. doi: 10.1056/NEJMoa1200388
15. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. (2013) 368(13):1179–88. doi: 10.1056/NEJMoa1301228
16. Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, et al. On-Pump versus off-pump coronary-artery bypass surgery. N Engl J Med. (2009) 361(19):1827–37. doi: 10.1056/NEJMoa0902905
17. Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. (2001) 27(1):74–83. doi: 10.1007/s001340051352
18. Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. (2003) 185(5):485–91. doi: 10.1016/s0002-9610(03)00044-8
19. Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. (2009) 13(3):R90. doi: 10.1186/cc7918
20. Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. (2010) 14(1):R25. doi: 10.1186/cc8888
21. Lindsay AJ, Xu M, Sessler DI, Blackstone EH, Bashour CA. Lactate clearance time and concentration linked to morbidity and death in cardiac surgical patients. Ann Thorac Surg. (2013) 95(2):486–92. doi: 10.1016/j.athoracsur.2012.07.020
22. Hajjar LA, Almeida JP, Fukushima JT, Rhodes A, Vincent JL, Osawa EA, et al. High lactate levels are predictors of Major complications after cardiac surgery. J Thorac Cardiovasc Surg. (2013) 146(2):455–60. doi: 10.1016/j.jtcvs.2013.02.003
23. Zhang Z, Ni H. Normalized lactate load is associated with development of acute kidney injury in patients who underwent cardiopulmonary bypass surgery. PLoS One. (2015) 10(3):e0120466. doi: 10.1371/journal.pone.0120466
24. Mak NT, Iqbal S, de Varennes B, Khwaja K. Outcomes of post-cardiac surgery patients with persistent hyperlactatemia in the intensive care unit: a matched cohort study. J Cardiothorac Surg. (2016) 11:33. doi: 10.1186/s13019-016-0411-5
25. Miller D, Eagle-Hemming B, Sheikh S, Joel-David L, Adebayo A, Lai FY, et al. Urinary extracellular vesicles and micro-RNA as markers of acute kidney injury after cardiac surgery. Sci Rep. (2022) 12(1):10402. doi: 10.1038/s41598-022-13849-z
26. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate gfr without race. N Engl J Med. (2021) 385(19):1737–49. doi: 10.1056/NEJMoa2102953
27. Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between aki and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. (2014) 9(3):448–56. doi: 10.2215/cjn.02440213
28. Nadim MK, Forni LG, Bihorac A, Hobson C, Koyner JL, Shaw A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th international consensus conference of the adqi (acute disease quality initiative) group. J Am Heart Assoc. (2018) 7(11):e008834. doi: 10.1161/jaha.118.008834
29. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. (2005) 16(1):162–8. doi: 10.1681/asn.2004040331
30. Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. (2006) 114(21):2208–16. doi: 10.1161/circulationaha.106.635573
31. Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, et al. Derivation and validation of a simplified predictive Index for renal replacement therapy after cardiac surgery. Jama. (2007) 297(16):1801–9. doi: 10.1001/jama.297.16.1801
32. Birnie K, Verheyden V, Pagano D, Bhabra M, Tilling K, Sterne JA, et al. Predictive models for kidney disease: improving global outcomes (kdigo) defined acute kidney injury in UK cardiac surgery. Crit Care. (2014) 18(6):606. doi: 10.1186/s13054-014-0606-x
33. Jiang W, Teng J, Xu J, Shen B, Wang Y, Fang Y, et al. Dynamic predictive scores for cardiac surgery-associated acute kidney injury. J Am Heart Assoc. (2016) 5(8):e003754. doi: 10.1161/jaha.116.003754
34. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. (2009) 119(4):495–502. doi: 10.1161/CIRCULATIONAHA.108.786913
35. Rosner MH, Okusa MD. Acute kidney injury associated ith cardiac surgery. Clin J Am Soc Nephrol. (2006) 1(1):19–32. doi: 10.2215/CJN.00240605
36. Warren OJ, Smith AJ, Alexiou C, Rogers PL, Jawad N, Vincent C, et al. The inflammatory response to cardiopulmonary bypass: part 1–mechanisms of pathogenesis. J Cardiothorac Vasc Anesth. (2009) 23(2):223–31. doi: 10.1053/j.jvca.2008.08.007
37. Hong S, Youn YN, Yoo KJ. Metabolic syndrome as a risk factor for postoperative kidney injury after off-pump coronary artery bypass surgery. Circ J. (2010) 74(6):1121–6. doi: 10.1253/circj.cj-09-0842
38. Yoo YC, Youn YN, Shim JK, Kim JC, Kim NY, Kwak YL. Effects of renin-angiotensin system inhibitors on the occurrence of acute kidney injury following off-pump coronary artery bypass grafting. Circ J. (2010) 74(9):1852–8. doi: 10.1253/circj.cj-10-0261
39. Kim MY, Jang HR, Huh W, Kim YG, Kim DJ, Lee YT, et al. Incidence, risk factors, and prediction of acute kidney injury after off-pump coronary artery bypass grafting. Ren Fail. (2011) 33(3):316–22. doi: 10.3109/0886022X.2011.560406
40. Lee EH, Baek SH, Chin JH, Choi DK, Son HJ, Kim WJ, et al. Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med. (2012) 38(9):1478–86. doi: 10.1007/s00134-012-2599-8
41. Kumada Y, Yoshitani K, Shimabara Y, Ohnishi Y. Perioperative risk factors for acute kidney injury after off-pump coronary artery bypass grafting: a retrospective study. JA Clin Rep. (2017) 3(1):55. doi: 10.1186/s40981-017-0125-2
42. Liu W, Xi Z, Gu C, Dong R, AlHelal J, Yan Z. Impact of major bleeding on the risk of acute kidney injury in patients undergoing off-pump coronary artery bypass grafting. J Thorac Dis. (2018) 10(6):3381–9. doi: 10.21037/jtd.2018.05.98
43. Choi JS, Oh SJ, Sung YW, Moon HJ, Lee JS. Pulse wave velocity is a new predictor of acute kidney injury development after off-pump coronary artery bypass grafting. PLoS One. (2020) 15(4):e0232377. doi: 10.1371/journal.pone.0232377
44. Lee EH, Chin JH, Joung KW, Choi DK, Kim WJ, Lee JB, et al. Impact of the time of coronary angiography on acute kidney injury after elective off-pump coronary artery bypass surgery. Ann Thorac Surg. (2013) 96(5):1635–41. doi: 10.1016/j.athoracsur.2013.05.019
45. Radovic M, Bojic S, Kotur-Stevuljevic J, Lezaic V, Milicic B, Velinovic M, et al. Serum lactate as reliable biomarker of acute kidney injury in low-risk cardiac surgery patients. J Med Biochem. (2019) 38(2):118–25. doi: 10.2478/jomb-2018-0018
46. Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. (1996) 171(2):221–6. doi: 10.1016/s0002-9610(97)89552-9
47. Jansen TC, van Bommel J, Woodward R, Mulder PG, Bakker J. Association between blood lactate levels, sequential organ failure assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. (2009) 37(8):2369–74. doi: 10.1097/CCM.0b013e3181a0f919
48. Legouis D, Ricksten SE, Faivre A, Verissimo T, Gariani K, Verney C, et al. Altered proximal tubular cell glucose metabolism during acute kidney injury is associated with mortality. Nat Metab. (2020) 2(8):732–43. doi: 10.1038/s42255-020-0238-1
49. Arroyo V, García-Martinez R, Salvatella X. Human Serum albumin, systemic inflammation, and cirrhosis. J Hepatol. (2014) 61(2):396–407. doi: 10.1016/j.jhep.2014.04.012
50. Fernández J, Clària J, Amorós A, Aguilar F, Castro M, Casulleras M, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. (2019) 157(1):149–62. doi: 10.1053/j.gastro.2019.03.021
51. Fernández J. Easl clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. (2010) 53(3):397–417. doi: 10.1016/j.jhep.2010.05.004
52. Runyon BA. Introduction to the revised American association for the study of liver diseases practice guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. (2013) 57(4):1651–3. doi: 10.1002/hep.26359
53. Lee EH, Kim WJ, Kim JY, Chin JH, Choi DK, Sim JY, et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology. (2016) 124(5):1001–11. doi: 10.1097/ALN.0000000000001051
Keywords: acute kidney injury, coronary artery bypass grafting, off-pump, risk factors, arterial lactate, short-term prognosis
Citation: Yu R, Liang T, Li L, Bi Y and Meng X (2023) Predictive role of arterial lactate in acute kidney injury associated with off-pump coronary artery bypass grafting. Front. Surg. 10:1089518. doi: 10.3389/fsurg.2023.1089518
Received: 4 November 2022; Accepted: 23 February 2023;
Published: 16 March 2023.
Edited by:
Giuseppe Gatti, Azienda Sanitaria Universitaria Giuliano Isontina, ItalyReviewed by:
Alan Gallingani, University Hospital of Parma, ItalyPraveen Varma, Amrita Institute of Medical Sciences and Research Centre, India
© 2023 Yu, Liang, Li, Bi and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangbin Meng aGVucnkyMDA4bWVuZ0Bob3RtYWlsLmNvbQ==
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
 Ruiming Yu
Ruiming Yu Tingyi Liang2
Tingyi Liang2 Yanwen Bi
Yanwen Bi Xiangbin Meng
Xiangbin Meng