
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 17 April 2023
Sec. Vascular Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1086871
Background: Anticoagulant treatment is used to treat and prevent venous thromboembolism (VTE). However, the relative effectiveness of newer anticoagulants vs. warfarin has not been appraised.
Objective: The aim was to evaluate the safety and efficacy of rivaroxaban for VTE in comparison to warfarin.
Materials and methods: From January 2000 until October 2021, all related studies were collected by EMBASE, the Cochrane Library, PubMed and Web of Scienceand. During the review process, two reviewers independently analyzed the included studies, including quality evaluation, screening and data extraction. We focused on VTE events as our primary outcomes.
Results: In total, 20 trials were retrieved. These studies involved 230,320 patients, of which 74,018 received rivaroxaban and 156,302 received warfarin. Compared with warfarin, the incidence of VTE in rivaroxaban is significantly lower (risk ratio (RR) 0.71, 95% confidence interval (CI) [0.61, 0.84]; P < 0.0001, random effect model), and significantly reduced major [RR: 0.84, 95% CI (0.77, 0.91); P < 0.0001, fixed effect model] and nonmajor [RR: 0.55, 95% CI (0.41, 0.74); P < 0.0001, fixed effect model] bleeding. No significant differences in all-cause mortality between the two groups [RR: 0.68, 95% CI (0.45, 1.02); P = 0.06, fixed effect model].
Conclusion: Rivaroxaban significantly reduced the incidence of VTE compared to warfarin in this meta-analysis. In order to verify these findings, larger sample sizes are required in well-designed studies.
VTE includes deep vein thrombosis, central venous catheter-associated thrombosis, and pulmonary embolism, is a potentially life threatening condition (1, 2).
VTE can be prevented and treated with anticoagulant therapy (3); There have been several parenteral anticoagulants used historically, including low molecular weight heparin, unfractionated heparin, fondaparinux and warfarin (4). As a commonly used anticoagulant drug, warfarin has some drawbacks: the INR needs to be checks while taking the drug and certain foods increase the risk of bleeding, such as spinach, green tea and goji berries (5). Warfarin also has the potential to cause skin necrosis [Patel NB, Jain G. Warfarin induced skin necrosis(J). Postgraduate Medical Journal, 2021:postgradmedj-2021-139988]. Direct thrombin inhibitors (DTIs), as well as the anti-Factor Xa inhibitor, have been widely used in the clinical setting in recent years. Rivaroxaban is used to treat pulmonary embolism and deep vein thrombosis, and the drug is also used for secondary prevention of recurrent VTE in adults (5). This article evaluates the security and effectiveness of rivaroxaban vs. warfarin for the prevention of VTE.
We searched for studies published in EMBASE, Medline, PubMed, Web of Science, and the Cochrane Clinical Trials Database between January 2000 and October 2021, using the keywords: (1) rivaroxaban; (2) warfarin; (3) venous thromboembolism; and (4) bleeding. Combinations of keywords using the Boolean operators “and/or” were adopted for the search strategy. Two reviewers independently analyzed the literature, extracted and analyzed data.
The inclusion criteria were: (1) patients receiving rivaroxaban and warfarin; (2) primary endpoint outcome was VTE, (3) the full text of the article is written in English. The criteria for exclusion from the article were as follows: (1) Formatted as protocols, reviews, or letters; (2) articles found by duplicate search; (3) articles with unclear results.
Two reviewers independently collected articles according to predetermined standards. In case of disagreement, it was resolved by negotiate with the third reviewer. The reviewers collected the following information from articles that met the criteria: Author name, country, year of publication, type of study, patient information (number, gender, age, treatment received), follow-up, and primary outcomes.
The quality of the article literature was evaluated by the Cochrane risk of bias tool. Risk bias of non-randomized studies was evaluated using the Newcastle–Ottawa scale, which includes adequacy of comparability of studies, cohort selection, and assessment of outcomes.
The study was performed by STATA 13.0 and Revman 5.4. Risk ratios (RRs) were used for outcomes. Data heterogeneity was evaluated using I2 values: a fixed-effect model was used if I2 was <50%;and a random-effect model was used if I2 was ≥50%,; if P < 0.05 or I2 > 50%, the random effect model was used; if P ≥ 0.05 and I2 ≤ 50%, the fixed effect model was used for analysis. Sensitivity analysis was conducted by excluding merged studies individually and observing whether the result significantly changed. Publication bias was evaluated using funnel plots and Egger’s regression.
According to PRISMA guidelines, 1,438 articles were collected by screening search strategies. After the removal of duplicates, 1,278 studys were retained, and 1,183 articles were excluded after screening the abstracts and titles. After reading the study content, 75 articles were further excluded. Ultimately, 20 eligible studies were included (Figure 1).
The studies were published in the last 6 years (2017–2021) and included 1 RCT study, 1 prospective comparative study, and 18 retrospective comparative studies (Table 1). These 20 studies included a total of 230,320 patients, of which 74,018 received rivaroxaban and 156,302 received warfarin.
The Cochrane bias risk assessment or Newcastle–Ottawa Scale were used to evaluate the quality of the studies (Table 2).
A total of 18 studies with 168,247 patients reported the incidence of VTE. According to the pooled estimate, compared to the warfarin, the rivaroxaban group had a significantly lower incidence of VTE (RR: 0.71, 95% confidence interval (CI) [0.61, 0.84]; P < 0.0001, random effect model), with significant heterogeneity among the included studies (I2 = 86%, P < 0.0001) (Figure 2). Based on the results of the sensitivity analysis, ignoring any single study would not affect the final results (Figure 3).
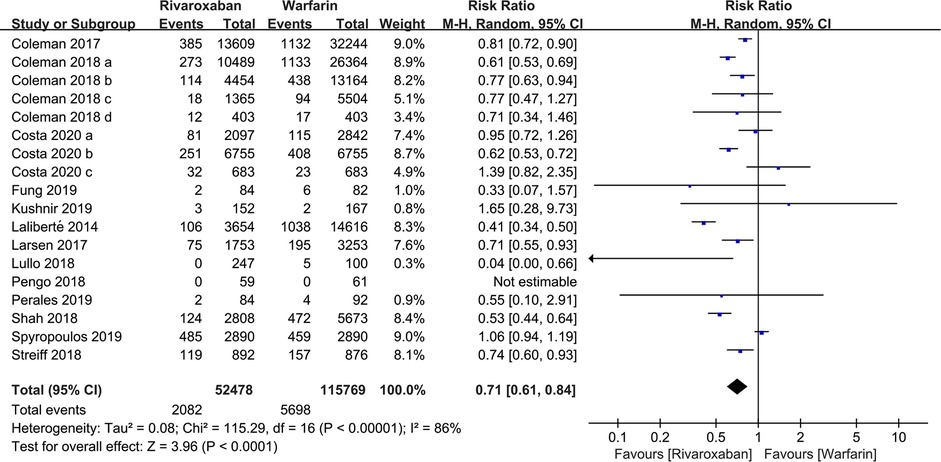
Figure 2. Forest plot for venous thromboembolism (VTE) between rivaroxaban group and warfarin group.
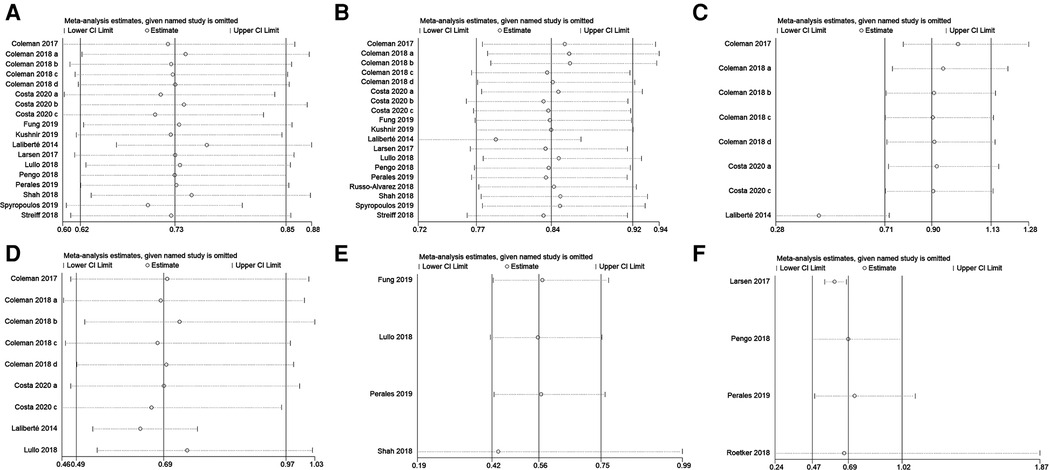
Figure 3. Sensitivity analysis of (A) VTE, (B) major bleeding, (C) intracranial hemorrhage, (D) gastrointestinal bleeding, (E) nonmajor bleeding, and (F) all-cause mortality.
169, 191 patients in 19 studies were reported major bleeding. According to the forest plot, the rivaroxaban group was lower than the warfarin group in the incidence of major bleeding [RR: 0.84, 95% CI (0.77, 0.91); P < 0.0001, fixed effect model], without significant heterogeneity among the included studies (I2 = 42%, P = 0.03) (Figure 4). The result was not changed after the sensitivity analysis (Figure 3).
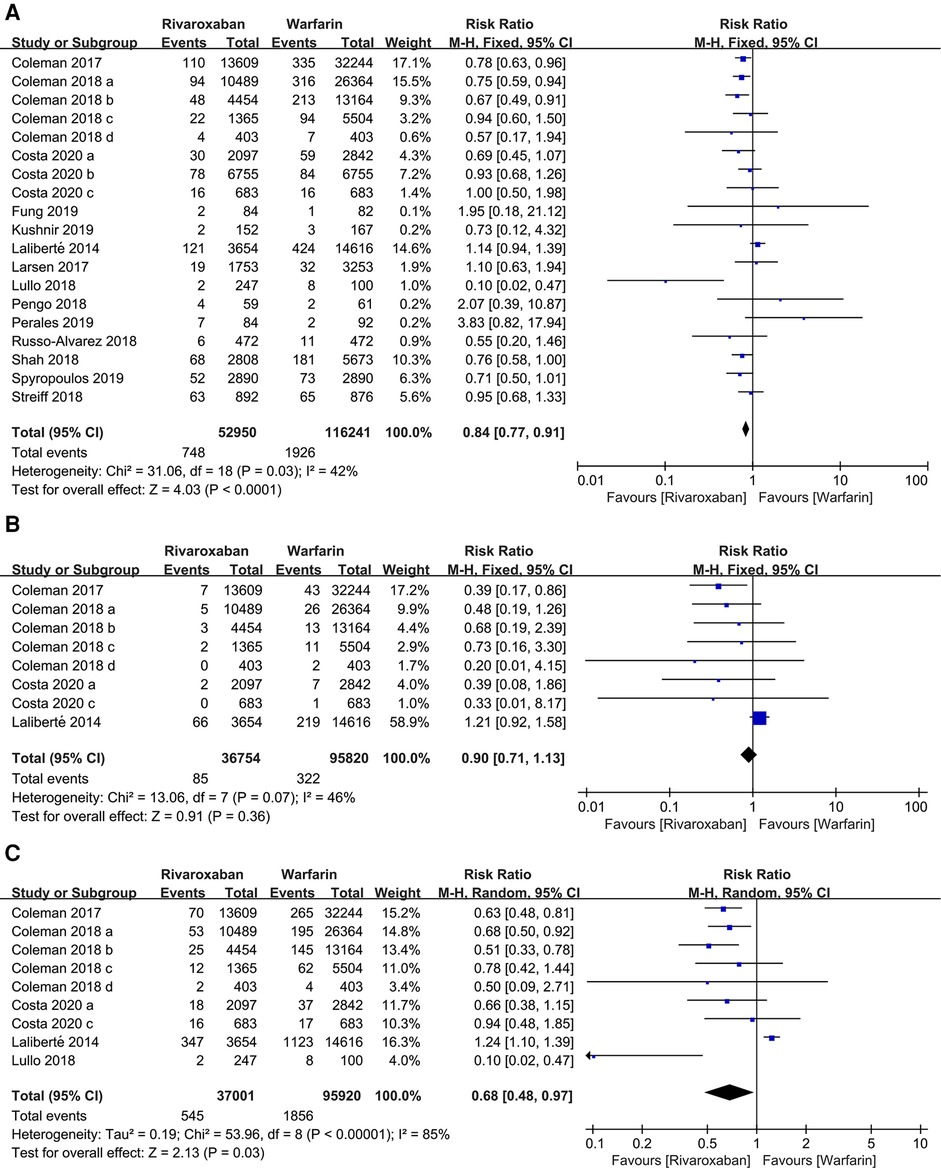
Figure 4. Forest plot for major bleeding between rivaroxaban group and warfarin group. (A) major bleeding, (B) intracranial hemorrhage, (C) gastrointestinal bleeding.
Subgroup analyses were performed according to the site of major bleeding. The pooled results of the incidence of intracranial hemorrhage and gastrointestinal bleeding are presented in Figure 4. There was no significant difference in the incidence of intracranial hemorrhage between the two groups [RR: 0.90, 95% CI (0.71, 1.13); P = 0.36, fixed effect model], while the incidence of gastrointestinal bleeding in the rivaroxaban group was significantly lower than the warfarin group [RR: 0.68, 95% CI (0.48, 0.97); P = 0.03, random effect model] (Figure 4). The sensitivity analysis showed that the study by Laliberté et al. had a significant impact on the pooled results of the incidence of intracranial hemorrhage when it was removed (6) (Figure 3).
4 studies contained information regarding nonmajor bleeding events. The results showed that rivaroxaban decreased the incidence of nonmajor bleeding events compared with warfarin [RR: 0.55, 95% CI (0.41, 0.74); P < 0.0001, fixed effect model], without significant heterogeneity (I2 = 0%, P < 0.0001) (Figure 5). The result was not changed after the sensitivity analysis (Figure 3).
Four studies mentioned all-cause mortality. A total of 1,793 out of 62,070 patients in the two groups died from various causes. These results showed no significant difference in all-cause mortality between the rivaroxaban and warfarin groups [RR: 0.68, 95% CI (0.45, 1.02); P = 0.06, fixed effect model], and no significant heterogeneity among the studies (I2 = 0%, P = 0.85) (Figure 6). The sensitivity analysis showed that the result was relatively stable (Figure 3).
There were two funnels, which are basically symmetrical (Figure 7). According to Egger's linear regression quantitative assessment, no significant publication bias in this study (VTE, P = 0.617; major bleeding, P = 0.821).
VTE is a serious complication that has a major impact on the normal of life of patients (7). Since 1941, warfarin has been a commonly used anticoagulant in the clinic (8). Warfarin decreases INR levels and increases the risk of bleeding, and frequent testing of coagulation is required while taking warfarin (9). Rivaroxaban overcomes the limitations of traditional anticoagulants in the prevention or treatment of arteriovenous thromboembolism. As a factor of Xa inhibitor, rivaroxaban can be taken orally, with a benifit of rapid onset of action and low adverse effects (10–13). This study investigates the safety and efficacy of rivaroxaban vs. warfarin in VTE prevention for the first time. According to the present study, rivaroxaban was significantly superior to warfarin in VTE prevention [RR: 0.71, 95% CI (0.61, 0.84); P < 0.0001]. Warfarin, a commonly used oral anticoagulant, is principally metabolized by CYP2C9 to form 7-hydroxywarfarin. New oral anticoagulants targeting Factor Xa, such as apixaban, rivaroxaban, and edoxaban, have been approved and will become preferred treatments for VTE. In this meta-analysis, the use of rivaroxaban resulted in a lower incidence of VTE than warfarin.
This study has implications for VTE prevention: (1) 20 studies (1 RCT, 1 prospective comparative, and 18 retrospective comparative studies) were retrieved. A large number of patients were included in this study (230,320 participants); (2) there is no previous meta-analyses of the effects of rivaroxaban vs. warfarin. Moreover, as a novel anticoagulant drug, rivaroxaban has no antagonist, so its safety comparison with traditional anticoagulant drugs is of more concern to clinicians, the ability of major or non-major bleeding to cause mortality was included in this study to evaluate the safety; (3) VTE was used to evaluate the drug efficacy, so this study has a high precision.
The limitations of this study are: (1) Only English language literatures were included in this study, so there may be a selection bias; (2) the baseline characteristics of the participants, such as chronic diseases, tumors, trauma, etc., were not considered, which might potentially affect the outcomes of the study; (3) The use of a non-major bleeding definition to count bleeding complications other than major bleeding was not specific enough, which may potentially affect the results of the study; (4) Due to the limitation in the descriptions of the cases treated in the major trials we have focussed on, no subgroup analysis of the incidence of VTE was performed in this study.
To summarize, this meta-analysis revealed that rivaroxaban is superior to warfarin in preventing VTE. Encouraging as our data analysis is there remains a need for further, more optimally designed trials to confirm the trend that is evident in the studies published todate.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Qilu Children's Hospital of Shandong University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
DS, LW: Work design. JZ, WC: Data collection. ZL: Drafting the paper. LG Making important changes to the paper. JS Approval of the final version of the paper to be published. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rodger MA, Le Gal G, Anderson DR, Schmidt J, Pernod G, Kahn SR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. Br Med J. (2017) 356:j1065. doi: 10.1136/bmj.j1065
2. Vijay A, Jha PK, Garg I, Sharma M, Ashraf MZ, Kumar B. micro-RNAs dependent regulation of DNMT and HIF1α gene expression in thrombotic disorders. Sci Rep. (2019) 9(1):4815. doi: 10.1038/s41598-018-38057-6
3. Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. (2013) 76(6):908–16. doi: 10.1111/bcp.12114
4. Steffel J, Lüscher TF. ants in clinical development: focus on factor Xa and direct thrombin inhibitors. J Cardiovasc Med. (2009) 10(8):616–23. doi: 10.2459/JCM.0b013e32832edac0
5. Tan C, Lee S. Warfarin and food, herbal or dietary supplement interactions: a systematic review. Br J Clin Pharmacol. (2021) 87(2):352–74. doi: 10.1111/bcp.14404
6. Patel NB, Jain G. Warfarin induced skin necrosis. Postgrad Med J. (2022) 98(1166):e41. doi: 10.1016/j.jaad.2008.12.039
7. Heath R. Treatment of VTE in primary care: building a new approach to patient management with rivaroxaban. Br J Cardiol. (2015) 22(2):806–9. doi: 10.5837/bjc.2015.021
8. Alberts MJ, He J, Kharat A, Ashton V. Effectiveness and safety of rivaroxaban versus warfarin among nonvalvular atrial fibrillation patients with obesity and polypharmacy. Am J Cardiovasc Drugs. (2022) 22(4):425–36. doi: 10.1016/j.jaad.2008.12.039
9. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. (2015) 12(8):464–74. doi: 10.1038/nrcardio.2015.83
10. Choonara IA, Haynes BP, Cholerton S, Breckenridge AM, Park BK. Enantiomers of warfarin and vitamin K1 metabolism. Br J Clin Pharmacol. (1986) 22(6):729–32. doi: 10.1111/j.1365-2125.1986.tb02966.x
11. Miranda M, Martinez LS, Franco R, Forte V, Barlattani A Jr, Bollero P. Differences betweenwarfarinandneworal anticoagulants indental clinical practice. Oral Implantol. (2016) 9(3):151–6. doi: 10.11138/orl/2016.9.3.151
12. Kreutz R. Pharmacokinetics and pharmacodynamics of rivaroxaban–an oral, direct factor Xa inhibitor. Curr Clin Pharmacol. (2014) 9(01):75–83. doi: 10.2174/1574884708666131111204658
Keywords: rivaroxaban, venous thromboembolism, warfarin, treatment, prevetion
Citation: Liu Z, Song D, Wang L, Wang C, Zhou J, Sun J and Guo L (2023) Rivaroxaban vs. warfarin for the treatment and prevention of venous thromboembolism: A meta-analysis. Front. Surg. 10:1086871. doi: 10.3389/fsurg.2023.1086871
Received: 1 November 2022; Accepted: 28 February 2023;
Published: 17 April 2023.
Edited by:
Konstantinos Spanos, University of Thessaly, GreeceReviewed by:
David Isenberg, University College London, United Kingdom© 2023 Liu, dan, Song Wang, Wang, Zhou, Sun and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuang Liu bGl1emh1YW5nODZAMTYzLmNvbQ== song dan c29uZ2Rhbjk5NjZAMTYzLmNvbQ==
Specialty Section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.